Introduction
Breast conserving surgery with radiotherapy has replaced mastectomy as the standard care for early-stage breast cancer in the last few decades. Angiosarcoma (AS) arising in the irradiated breast after breast-conserving therapy (BCT) is being reported in the literature with increasing frequency of 1-5. As more women undergo BCT, the incidence can be expected to increase upto 6. The risks of developing AS of the breast have been attributed to multiple risk factors in our literature review; this includes trauma, radiation, lymphoedema, and breast implants 7-10. However at present, there are no concrete or definitive data to support a causal link between breast AS and either radiotherapy or trauma.
In our study, we have reviewed cases of breast AS at our institution that have occurred in the last two years – we report the clinical presentation, suspected cause of AS, histopathology, treatment and their management outcome.
Methods
Patient with angiosarcoma of breast in patients who had breast cancer surgery followed by radiation were identified during the breast multi-disciplinary meeting in a single breast screening unit from 2013-2015. The clinical records were analyzed for all breast clinic correspondence, radiology investigations, pathology results and relevant referrals to specialist centers and patient outcome.
A thorough literature search was performed using Pub Med database and the search engines Google/Google Scholar using keywords with the varying combination of the search terms breast, angiosarcoma, radiotherapy, trauma, secondary, primary, metastasis. Searches were screened and those suitable articles with full text versions were retrieved.
Results
Case 1
A 59 year old female presented to one stop breast clinic with a 4 week history of a right breast lump. She had a history of previous right breast cancer 8 years ago and underwent breast conserving surgery (BCS) in the form of wide local excision (WLE) and axillary node clearance followed by radiotherapy and hormonal treatment (tamoxifen) for 5 years. She remained well and was discharged from regular follow up until her recent presentation. She was found to have a small lump in the lower inner quadrant of the same breast surrounded by an area of induration and bruising.
The 2 view mammogram revealed no abnormality apart from reported diffuse skin thickening consistent with previous surgery (M1). Ultrasound of her left breast demonstrated a hypoechoic lesion within the skin measuring 11 × 7 mm representing a possible atypical cystic lesion (U3).
For histological diagnosis a punch biopsy of the lesion was performed. The histology revealed that the deep dermis was almost entirely occupied by a tumour which was composed of irregular dissecting vascular channels lined by pleomorphic, mitotically active cells with prominent, eosinophilic nucleoli (Figure 1).
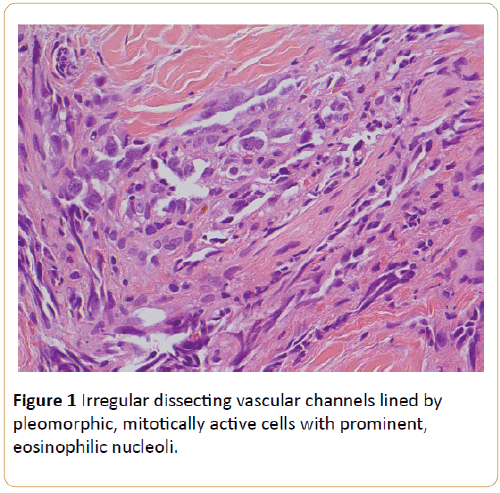
Figure 1: Irregular dissecting vascular channels lined by pleomorphic, mitotically active cells with prominent, eosinophilic nucleoli.
Immunohistochemistry confirmed that the tumour cells expressed CD34 with weak, minimal expression of Factor VIII. The cells did not express pan-cytokeratin AE1/AE3. The tumour infiltrated to all margins of the specimen and there was associated red cell extravasation; the features were consistent with that of an angiosarcoma related to radiation treatment.
She underwent an MRI of both breasts which revealed a 37 × 12 mm enhancing lesion within the skin, with no infiltration into breast tissue and no evidence of recurrence of her breast cancer. She also underwent a staging CT scan of the chest abdomen and pelvis suggesting no evidence of metastatic spread in these sites.
The patient’s case was discussed at the local MDT and it was decided to refer her case to the regional centre for sarcomas. The patient proceeded to having a radical mastectomy and latissimus dorsi flap reconstruction. At this stage she was not given any adjuvant chemotherapy or radiotherapy treatment. Histology of the breast specimen excised showed a tumour within the dermis and invading the superficial subcutis. The tumour was seen to be composed of complex blood-filled slitlike spaces, sheets and possible papillary structures of spindle cells with moderately pleomorphic vesicular nuclei and small nucleoli. Mitotic figures were difficult to see due to poor cellular preservation, 6 mitoses were seen in 10 high power fields but as the Ki67 proliferation index was very high (>60%). The appearances were those of a cutaneous AS which was likely to be radiation-induced.
The patient remains well with no evidence of local or distant recurrence in the last 6 months. She is currently under clinical follow up by the regional center for sarcomas and plastic surgery.
Case 2
A 56 year old female with previous right wide local excision axillary node clearance (June 2010) for grade 3 invasive ductal carcinoma which was oestrogen receptor ER + and HER 2 +ve. She was successfully treated with adjuvant chemotherapy, Herceptin and 3 weeks of 15 Gy radiotherapy. CT prior to adjuvant chemotherapy showed no metastases. She was discharged after surveillance of breast cancer for 5 years and had a normal mammogram before her discharge.
This lady was referred back to our clinic 3 months later for a progressing bruise on the right breast which she felt got triggered by the mammogram. This is shown in Figures 2 and 3.
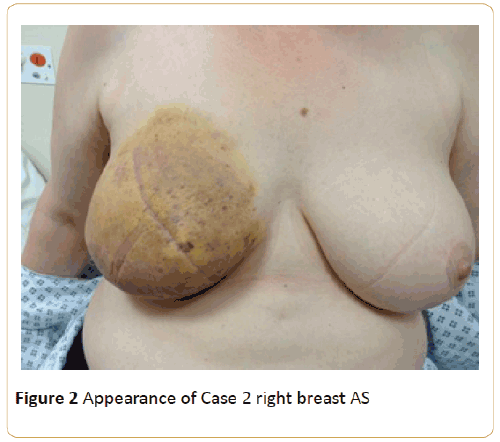
Figure 2: Appearance of Case 2 right breast AS
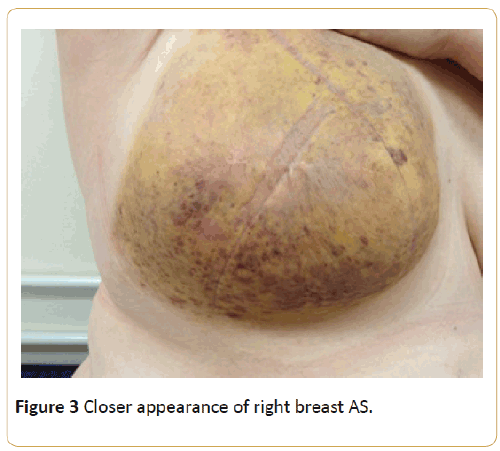
Figure 3: Closer appearance of right breast AS.
On clinical examination, there was a widespread right breast skin discolouration with red and blue blotches and nonspecific nodularity and skin thickening.
A punch biopsy of the skin at a couple of suspicious sites was performed. Histology revealed a proliferation of atypical endothelium within the dermis forming small dissecting vascular structures (Figure 4).
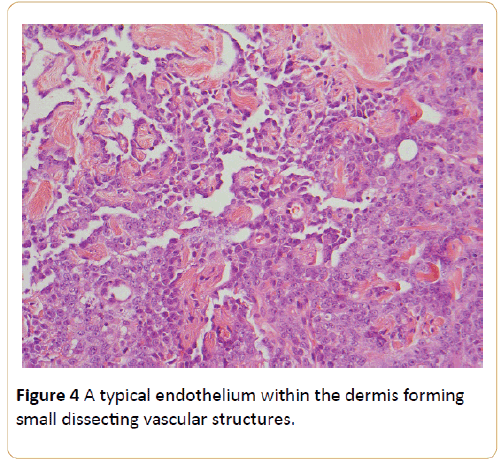
Figure 4: A typical endothelium within the dermis forming small dissecting vascular structures.
There was nuclear pleomorphism in the specimen. Immunohistochemictry of this tissue confirmed the endothelial nature of this proliferation (factor VIII related antigen positive, CD 34 positive). There was a very high proliferation rate on Ki67 staining. The histological features were those of angiosarcoma. A repeat CT scan confirmed a new right breast mass suspicious of malignancy, indeterminate mediastinal lymph nodes and indeterminate lung and liver lesions. An MRI scan of the breast corroborated the above and confirmed a likely malignant mass of the right breast which was not clearly evident on the last surveillance mammogram done 3 months prior to this.
This lady was referred to the specialist regional sarcoma team and plastics for further management and underwent a radical mastectomy including excision of pectoralis major muscle and to fill this gap she had latissimus dorsi flap reconstruction and skin grafting. No adjuvant chemotherapy or radiotherapy was administered at the time.
3 months later she developed a vascular lesion on the right upper abdominal wall and enlarged right supraclavicular node. Both these areas were sampled and unfortunately confirmed metastatic angiosarcoma.
Figure 5 below illustrates the small round lesion that was excised. Also demonstrated is the scarring from previous radical mastectomy and latissimus dorsi flap and skin split grafts.
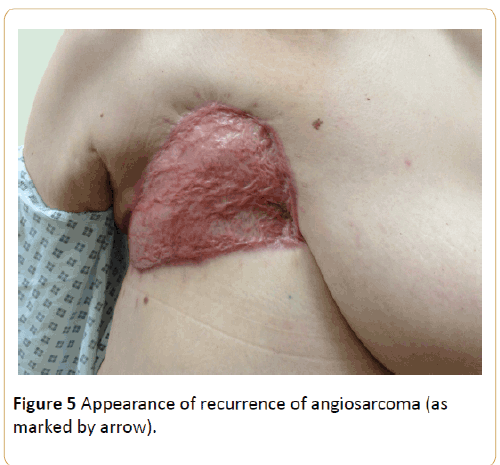
Figure 5: Appearance of recurrence of angiosarcoma (as marked by arrow).
Due to the recurrence of the problem a staging CT scan was performed which revealed multiple pulmonary metastases, increasing volume of hilar and mediastinal lymphadenopathy along with new enlarging lymph nodes on the diaphragm consistent with disease progression. In addition there was growing soft tissue nodule close to the left adrenal gland (19 × 13 mm) and sclerotic focus within the left iliac bone. She was referred to oncology for chemotherapy and currently is receiving weekly Paclitaxel for her disseminated disease.
Case 3
A 79-year-old female underwent right breast wide local excision (1999) and axillary node dissection followed by radiotherapy and 5 years of Tamoxifen. She presented 15 years after treatment with a 2 week history of a lump in the upper inner right breast medial to the nipple areola complex associated with itchiness. On examination, a hard 3 cm mass with overlying inflammatory erythema was felt in the right breast. She had a mammogram which showed a new 10 mm rounded opacity in the inner half of the right breast (graded M3) and also an ultrasound of the breast which showed a 10 mm lesion, the nature of which was indeterminate (U3). A fine needle aspiration of this lesion showed plentiful small discohesive cells displaying atypia which were suspicious of cancer (C4). She also had a core biopsy of this area which identified that the tissue was infiltrated by a tumour composed of atypical, mitotically active, spindled cells with vesicular channels dissecting into the adipose tissue with surrounding red cell extravasation (Figure 6).

Figure 6: Adipose tissue with surrounding red cell extravasation.
Immunohistochemistry revealed that the cells expressed factor VIII with patchy expression of CD34 but there was no expression of cytokeratins (MNF 116). The proliferation index was measured with Ki67 was high, with expression by more than 50% of the atypical cells. The imunoprofile and morphology were consistent with angiosarcoma.
CT scan showed some skin thickening surrounding a ‘nodule’ in the right breast highly suspicious of malignancy. There was no evidence of metastases seen on the scan. She underwent an excision of right chest wall angiosarcoma this was followed by radiotherapy but no chemotherapy was administered. The patient is currently under regular follow up by the sarcoma team and plastic surgeons and remains well and free of recurrence.
Discussion
AS of the breast after BCT with radiation is a rare entity but seems to be on the rise. With increased use of radiation in the conservative surgical strategies for breast cancer, a larger number of cases are sure to arise. This makes it a very important issue despite its rarity as it poses significant challenges to healthcare professionals due to its poor prognosis, ill-understood sequalae and causative factors [1-11]. The annual incidence of breast AS is 4.6 cases per million women. It accounts for less than 0.04% of all malignant breast tumors [3]. Mammary sarcomas are a heterogeneous group of malignant neoplasms that arise from the mammary stroma. Angiosarcoma originates from endothelial cells that line blood vessel walls [3].
We have reported three different cases of secondary AS. They all had breast conserving surgery and radiotherapy 5-15 years prior to their presentation. The median latency period to presentation after radiotherapy from the literature was 6.13 years (range 4 to 13 years) [1-3,11-14]. There was no reported latency period of longer than 13 years in the literature. One of our cases, had a latency period of over 15 years however this is expected in a rare entity and good insight that the latency period can stretch out as much as that.
In contrast to primary AS, secondary AS presents in older women (median age 65-71) following a median of 10.5 years after radiotherapy for breast cancer [12,13,15-17]. Two of our patients are significantly younger (aged 56 and 59) than the median age quoted by the majority of authors in the literature. The youngest, 56 years of age had a very atypical and abrupt presentation. She had a normal surveillance mammogram 3 months prior to her diagnosis and had noticed a bruise on the breast which she attributes to trauma due to mammogram. It is unclear if there was any link with the trauma sustained from mammogram as reported by Cao Y et al. [8] published a case report of breast AS related to trauma. They reported a 54 year old lady with strong family history of cancer who developed right breast AS 4 months following a skiing accident where she sustained significant trauma to her right breast. She had a past history of several benign breast surgeries for correction of fibrotic capsulation around the implant. A punch biopsy showed findings suggestive of chronic inflammation and malignancy. MRI of the breast revealed hematoma associated with skin thickening.
The first case of breast AS was presented in 1907 by Borrman [18] and the first case of secondary AS was described in 1987 by Body et al. [2]. Since then, attempts have been to understand this condition better in terms of clinical pattern recognition, earlier recognition, understanding disease origin and progression and to correlate histological features and patterns of growth with outcome.
Secondary AS has been reported to present as painless bruising that is frequently multifocal but can present with a mass 3, 6 and 8. There can be a delay in diagnosis as it is often neglected because of its seemingly innocent appearance. Our first case presented with a progressive growing painless lump over 4 weeks with associated bruising in the lower inner quadrant of the right breast, away from the surgical site, similar to our third case. Our second case presented with entire bruising/rash of the right breast as shown in Figures 1 and 2. Approximately 2% of the patients may present with diffuse enlargement of the breast as our second case. A bluish red discoloration of the overlying skin may be present.
The histological features of AS of the breast are classified into grades I, II and III. Angiosarcomas have a wide range of histological appearances from well-differentiated grade I tumours consisting infiltrating bland vascular channels to poorly differentiated grade III tumours with a sarcomatous spindle cell pattern. In cases of secondary AS, skin punch and incisional biopsy have been recommended [2-6,15].
On pathological analysis the lesions are notable for irregular vascular formations evident on with hyperchromatic and irregular nuclei. The diagnosis can be clarified by immunohistologic staining for the endothelial marker CD31, the most sensitive and specific indicator of angiogenic proliferation; however, the lesions will also stain positive for the vascular markers. Factor VIII, Fli 1, will usually at least be weakly positive for CD34. The most useful antibodies to demonstrate endothelial cell differentiation are factor VIII, CD31, and CD34, but they can be absent in less differentiated angiosarcomas. As carcinomas do not usually stain for endothelial markers, the combined use of epithelial and endothelial antibodies is important. All 3 of our cases had expression of CD34 and a high proliferation rate on Ki67 staining. Factor VIII related antigen was also uniformly positive. A panel of antibodies for vascular differentiation should be used to ensure a diagnosis isn’t missed.
From our reported cases, only Case 2 developed pulmonary and nodal metastases following breast angiosarcoma diagnosis. This patient had a radical mastectomy and latissimus dorsi reconstruction and a CT scan at the time showed no clear evidence of metastases. However, only 2 months later, a follow up CT scan showed disease progression and metastatic spread to the lungs and nodes (mediastinal, hilar and diaphragmatic) demonstrating the rapid progressive and aggressive nature of this disease [19-22].
There was very little in the literature that commented on secondary breast angiosarcoma metastasis similar to our case. Silverman et al. [23] reported a remarkable case with a 30-year survival of a patient with metastatic angiosarcoma of the breast who achieved remission after treatment. This is the longest reported survival with documented metastatic angiosarcoma of the breast. Silverman et al. went on to report that patients with poorly differentiated angiosarcoma of the breast who had adjuvant chemotherapy had a statistically significant improved outcome compared to no adjuvant treatment. This was in contrast to the inconclusive finding in well differentiated angiosrcoma patients. Our patient (Case 2) had poorly differentiated angiosarcoma and was started on chemotherapy and her response will be monitored. More recently, Wang et al. [24] reported a further case of angiosarcoma metastasis, but it was a primary angiosarcoma diagnosis as opposed to our cases of secondary angiosarcoma reported here.
Mammogram and ultrasound do not have pathognomonic characteristics in angiosarcoma which are seen with adenocarcinoma or DCIS. Our case reviews highlighted the nature of how equivocal imaging can be in diagnosing AS.
AS of the breast is very rare, there is no established standard treatment that is evidence based. Mastectomy however is the mainstay of treatment [21]. Although some individuals seem to benefit from chemotherapy, it is of minimal benefit for patients with disseminated disease. This should be managed in a tertiary centre in close cooperation with surgeons, medical oncologists, radiologists and plastic surgeons in consideration for latissimus dorsi flap. All our cases were discussed in the breast MDT and referred to the tertiary specialist centre for multi-disciplinary management.
Conclusion
We conclude that with increasing use of radiotherapy for breast conserving surgery will result in a growing number of cases of secondary angiosarcoma for which early detection is important and requires a high index of clinical and pathological suspicion. Patient education is the key and they need to be advised to remain vigilant of any skin changes for early detection and prompt treatment for this condition.
9396
References
- Morgan EA, Kozono DE, Wang Q (2012) Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol 19: 3801-3808.
- Seinen JM, Styring E, Verstappen V (2012) Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol 19: 2700-6
- Arora TK, Terracina KP, Soong J (2014) Primary and secondary angiosarcoma of the breast. Gland Surg 3:28–34.
- Billings SD, McKenney JK, Folpe AL (2004) Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol 28: 781-788.
- Kim MK, Huh SJ, Kim DY (1998) Secondary angiosarcoma following irradiation--case report and review of the literature. Radiat Med 16: 55-60.
- Hodgson NC, Bowen-Wells C, Moffat F (2007) Angiosarcomas of the breast: a review of 70 cases. Am J Clin Oncol 30: 570-573.
- Hui A, Henderson M, Speakman D (2012) Angiosarcoma of the breast: a difficult surgical challenge. Breast 21: 584-589.
- Cao Y, Panos L, Graham L (2012) Primary cutaneous angiosarcoma of the breast after breast trauma. Proc Bayl Univ Med Cent 25: 70–72
- Cuesta-Mejías T, de León-Bojorge B, Abel de la Pena J (2002) Breast angiosarcoma in a patient with multiple surgical procedures and breast implant. Report of a case. Ginecol Obstet Mex 70: 76-81.
- Kirova YM, Vilcoq JR, Asselain B (2005) Radiation-induced sarcomas after radiotherapy for breast carcinoma: a large-scale single-institution review. Cancer 104:856-863
- Strobbe LJ, Peterse HL, van Tinteren H (1998) Angiosarcoma of the breast after conservation therapy for invasive cancer, the incidence and outcome. An unforseensequela. Breast Cancer Res Treat 47: 101-109
- Taghian A, de Vathaire F, Terrier P (1991) Long-term risk of sarcoma following radiation treatment for breast cancer. Int J Radiat Oncol Biol Phys 21: 361-367
- Marchal C, Weber B, de Lafontan B (1999) Nine breast angiosarcomas after conservative treatment for breast carcinoma: a survey from French comprehensive Cancer Centers. Int J Radiat Oncol Biol Phys 44: 113-119.
- Yap J, Chuba PJ, Thomas R (2002) Sarcoma as a second malignancy after treatment for breast cancer. Int J Radiat Oncol Biol Phys 52: 1231-1237.
- Zahir ST, Sharahjin NS, Rahmani K (2010) Primary breast angiosarcoma: Pathological and radiological diagnosis. Malays J Med Sciv 21.
- Bhosale SJ, Kshirsagar AY, Patil MV, Wader JV, Nangare N, et al. (2013) Primary angiosarcoma of breast: A case report. Int J Surg Case Rep 4: 362–364.
- Scow JS, Reynolds CA, Degnim AC (2010) Primary and secondary angiosarcoma of the breast: the Mayo Clinic experience. J Surg Oncol 101: 401-407
- Borrman R (1907) Metastasenbildung bei histologish gutartigen geschwulsten: Fall von Metastasierendem Angiom. Beitr Pathol Anat 40: 372–393.
- Velidedeolu M, Bilgin IA, Karaduman Z (2014) A case report of primary breast angiosarcoma causing hemorrhagic shock in pregnancy. Ulus Cerrahi Derg 30: 54–56.
- Kaklamanos IG, Birbas K, Syrigos KN (2011) Breast angiosarcoma that is not related to radiation exposure: a comprehensive review of the literature. Surg Today 41: 163-168.
- Fodor J, Orosz Z, Szabó E (2006) Angiosarcoma after conservation treatment for breast carcinoma: our experience and a review of the literature. J Am Acad Dermatol 54: 499-504.
- Bennani A, Chbani L, Lamchahab M (2013) Primary angiosarcoma of the breast: a case report. Diagn Pathol 8: 66.
- Silverman LR, Deligdisch L, Mandeli J (1994) Chemotherapy for angiosarcoma of the breast: case report of 30-year survival and analysis of the literature. Cancer Invest 12:145-155.
- Wang L, Huang C, Yang X (2015) Primary Angiosarcoma of the Breast with Pulmonary Metastasis. Breast J21: 435-437.











