Keywords
Microbial contaminants, recovery and detection, indicator pathogens, microbial limit tests, non-sterile pharmaceuticals
Introduction
Microbial contamination of pharmaceutical preparations is a common problem which has been reported for several non-sterile medicaments [1]. It is perhaps a little surprising that the problem of microbial contamination in non-sterile medicines received detailed attention only recently. Contamination of Pharmaceuticals with micro-organisms can bring about changes in their physical characteristics, including the breaking of emulsions, the thinning of creams, fermentation of syrups, and appearance of turbidity or deposit, besides producing possible off odors and color changes [2]. These changes will not only make the product aesthetically unacceptable but can also affect the therapeutic potency and dosage delivery [3]. The presence of microbial contaminants in pharmaceutical products was also proved to be a potential health hazard to the consumer. The extent of the hazard will vary from product to product and patient to patient, depending on the types and numbers of organisms present, the route of administration, and the resistance of the patient to infection [3]. The majority of contaminants of pharmaceutical products and ingredients are bacteria, yeast and filamentous fungi (mould). Some of these contaminants may be pathogenic while others grow as commensals even in the presence of preservatives and spoil products [4]. Nonsterile preparations, although not required by most pharmacopeia to be sterile, are, none the less, required to pass tests for the absence of certain specified micro-organisms (Escherichia coli, Salmonella spp., Pseudomonns aeruginosa, Staphylococcus aureus and Candida albicans), and microbial bioburden tests (tests for total aerobic microbial count, TAMC). Specified organisms are also referred to as “indicator” organisms. In developing countries such as Egypt, drug-borne infections may have serious debilitating effects on patients because of averagelow socio-economic lifestyle. This problem may be compounded by the fact that pharmaceutical preparations are frequently stored under uncontrolled conditions and may be dispensed in non-protective packaging. The warm and slightly humid climatic conditions that prevail in Egypt would tend to support the survival and growth of many microorganisms. Therefore, there is the need to identify and evaluate the presence of microbial contaminations in non-sterile pharmaceutical preparations. Reports of microbial quality evaluations of cosmetics and toiletries have mainly been from temperate countries [5-8], however few studies have been carried out in Egypt [9,10]. Moreover, Ashour et al. [11], conducted a study on the microbiological environmental monitoring in pharmaceutical facility and revealed the isolation of a number of bacterial and fungal isolates from different locations which may eventually influence the microbiological quality of the final products leading to severe financial losses for the pharmaceutical company. Thus a systematic approach is required by manufacturers of nonsterile pharmaceuticals to evaluate the significance of microbial isolates taking into account the type of the isolated pathogen, the number of organisms present, the type of dosage form, and the potential hazard to the user [10]. Consequently, the objective of this study was to test conditions and challenges for microbial contaminants recovery and detection in some non-sterile products in the Egyptian market.
Materials and Methods
Collection of pharmaceutical test samples
A total of 280 possibly contaminated non-sterile pharmaceutical samples were tested. The samples were either obtained from different pharmaceutical companies or purchased sporadically from various retail pharmacies in Egypt. The samples comprised 165 preparations for oral use and 115 preparations for topical use, all of which were locally manufactured. The oral pharmaceutical preparations included syrups, tablets, capsules and powders whereas the topical preparations included creams, ointments, lotions and gels.
Preparation of Pharmaceutical samples
Sample preparation was conducted according to the United States Pharmacopeia (USP 31) [12]. The method for sample preparation depended on the physical characteristics of the product to be tested The outside surfaces of all containers were swabbed with 70% v/v ethanol before opening. In general, not less than 1 g (solids or semisolids) or 1 ml (liquids) samples were tested for each product and 1:10 sample dilutions of the pharmaceutical products in each of sterile Trypticase Soy Broth (TSB) and Sabouraud Dextrose Broth (SDB) were prepared.
Method suitability verification
The validity of the test methods used for microbial limit testing rests largely upon the adequacy of a demonstration that the examined products do not, of themselves, inhibit the multiplication of the microorganisms that may possibly be present. Therefore, method suitability verification, using a standard test microorganism (Staphylococcus aureus ATCC 433001), was conducted according to the USP. Test acceptance criteria necessitates that the average numbers of Colony Forming Units (CFU) recovered from the test articles should be not less than 50% of the inoculum control [12]. Adequate recovery of the test organism confirms the suitability of the test method. Reduction of the growth by a factor greater than,two indicates antimicrobial activity and invalidates that portion of examination, thus necessitating a modification of the procedure. The modification procedures used to neutralize the activity of antimicrobial agents included dilution (1:100 dilution of the product in TSB) and the use of Dey-Engley neutralizing broth (samples of the product were suspended in Dey-Engley neutralizing broth at appropriate dilutions not exceeding 1 g or 1 ml %). The choice of the neutralization technique depended on the nature of the inhibitory substances present in the test preparations.Upon using one of the neutralizing techniques, it is part of the method suitability test to demonstrate the efficacy and absence of toxicity for microorganisms by the chosen neutralization method. The test design used to evaluate neutralizer efficacy and its toxicity was conducted according to the method described by Clontz [4] and involved three treatment groups, test group, control group and viability group. To show adequate neutralizer efficacy, the count from test group must not be less than a factor of two when compared to the count from the control group. To show lack of neutralizer toxicity, the count from the control group must not be less than a factor of 2 when compared to the count from viability group [4].
Microbial enumeration tests: Total viable aerobic count
Microbial enumeration tests were conducted according to the United States Pharmacopeia (USP 31) [12], using the spread plate technique. From the tested sample dilutions, aliquots were transferred onto Trypticase Soy Agar (TSA) plates suitable for the cultivation of bacteria or Sabouraud Dextrose Agar (SDA) plates, suitable for the cultivation of fungi. Triplicate plates of each culture medium were done for each test dilution. For samples having antimicrobial properties, such properties were eliminated prior to conducting enumeration tests, using methods that have been shown to be suitable as described in method suitability verification. At the end of the incubation period, the recovered colonies from each plate were enumerated and the arithmetic mean count was used for calculating the viable count of the test sample (CFU/ml) with each culture medium.
Isolation of specified microbial contaminants
For monitoring the safety of non-sterile pharmaceutical preparations, the USP microbial limit tests require the absence of 5 specified microbial indicators in pharmaceutical preparations; Salmonella spp., Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa, Escherichia coli (E. coli) and Candida albicans. Therefore screening described hereafter, is mainly focused on the detection of these particular contaminants.
Tests for specified microbial contaminants were conducted according to the United Stated Pharmacopeia (USP 31) [12] with minor modifications. Sample dilutions (1:10) of the pharmaceutical products were prepared in TSB and SDB as described earlier. Aliquots of the TSB cultures were sub-cultured on Mannitol Salt agar, Cetrimide agar and MacConkey agar for the detection of S. aureus, Pseudomonas aeruginosa and E. coli, respectively. Additional 1 ml aliquots of the TSB cultures were transferred into 9 ml sterile fluid tetrathionate broth to detect Salmonella spp. which after incubation was further subcultured on the surface of brilliant green agar. On the other hand, loopfuls of the SDB cultures (for the detection of Candida albicans), were streaked on the surface of SDA plates. From the growth obtained on the different solid culture media, morphologically different isolated colonies were separately streaked for purification onto the surface of TSA plates for bacteria and SDA plates for fungi. The isolated colonies were then gram stained and identified by biochemical tests. Tests for identification of Gram negative isolates included growth on Eosin methylene blue agar, Triple Sugar Iron agar, oxidase production test, urease production test and API 20E strip (Bio Merieux, France) while those for identification of Gram positive isolates included catalase test, coagulase tests, starch hydrolysis, citrate utilization and novobiocin sensitivity.
Results
Recovery of microbial contaminants from the collected pharmaceutical samples
Verification tests conducted on the 280 collected samples revealed the following; 255 preparations showed valid recovery count (> 50% of the initial inoculums used) of the test organism, however in 25 preparations, the average numbers of CFU of the test organism recovered were less than 50% of the control preparation (test acceptance recovery criteria should be not less than 50%). Suitable neutralization procedures (dilution, Dey Engley neutralizing broth) for microbial recovery from these 25 preparations were therefore applied and the microbial recovery was evaluated and achieved. For another six preparations, after several testing attempts, no suitable neutralizing method was found to demonstrate valid recovery of the test organism. It is thus assumed that the inherent microbicidal activity of these products prevents contamination by the given microbial species (USP 31) [12]. Accordingly, these six preparations were omitted from further testing.
After verification, microbial testing revealed the following; a total of 60 bacterial and 31 fungal isolates were recovered from 280 different collected non-sterile pharmaceutical preparations. Of these isolates, 41 bacterial and 26 fungal isolates were recovered from 160 oral preparations whilst 19 bacterial and 5 fungal isolates were recovered from 115 topical preparations. Table 1 summarizes the microbial contaminants recovered from the different collected dosage forms. The results presented in the table reveal that the preparations from which microbial contaminants were recovered are of low number although the tested preparations were validated and treated for successful microbial recovery. The different contaminants were recovered from 76 out of 275 preparations with a percentage of 27.6%. No contaminants were recovered from 5 tested powder preparations (data not presented in the table). Interestingly, it was observed that a single microbial contaminant was isolated from each contaminated preparation in all cases with few exceptions. These exceptions included the recovery of two bacterial contaminants from 5 out of 275 preparations and one bacterial plus one fungal contaminant were recovered from 10 out of 275 preparations. Also the results in table 1 reveal that fungi constitute an important concern as a contaminant for pharmaceutical preparations since it comprised about 50% of bacterial contaminated preparations.
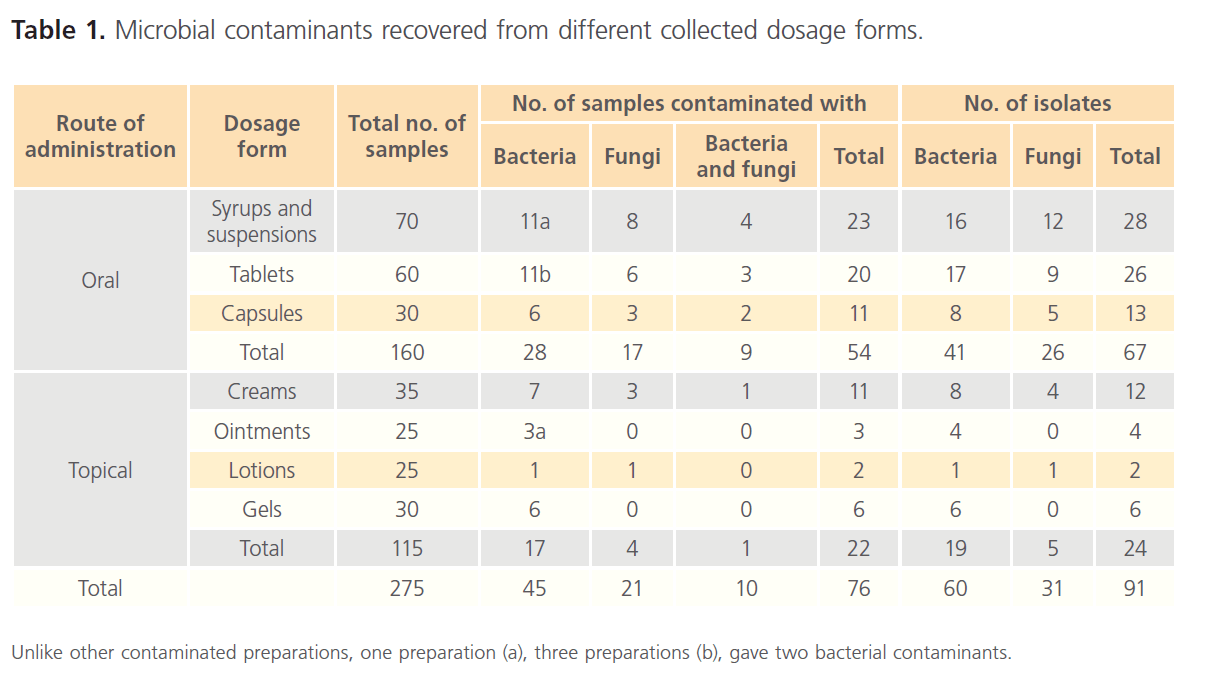
Table 1: Microbial contaminants recovered from different collected dosage forms.
Bioburden level of the collected pharmaceutical preparations
Viable aerobic microbial count using the spread plate technique was evaluated and used for microbial enumeration. Aerobic microbial counts were made on TSA plates whilst counts of yeasts and moulds were made on SDA plates. The distribution of bacterial and fungal counts in different dosage forms is summarized in tables 2 and 3. For bacteria, over 95% of the items tested contained fewer than 2 X 102 CFU/ml. The viable bacteria were not recovered from about 80% of the items examined and 2.2% of the products contained 2 X 102 - 2 X 103CFU/ml whilst only 0.73% contained more than 2 X 103 CFU/ml. As shown in table 2, bacterial counts indicate that only tablets had counts > 2 x 103 CFU/g; other dosage forms did not show counts in this range. USP 31 specifies an acceptance criterion of a total viable count of not more than 2 x 103 CFU/g or ml for non-aqueous preparations for oral use and not more than 2 x 102 CFU/g or ml for aqueous preparations for oral use and topical preparations. Accordingly, 6 products (3 syrups, 2 tablets and 1 cream) were found to exceed the specified limits.

Table 1: Microbial contaminants recovered from different collected dosage forms.
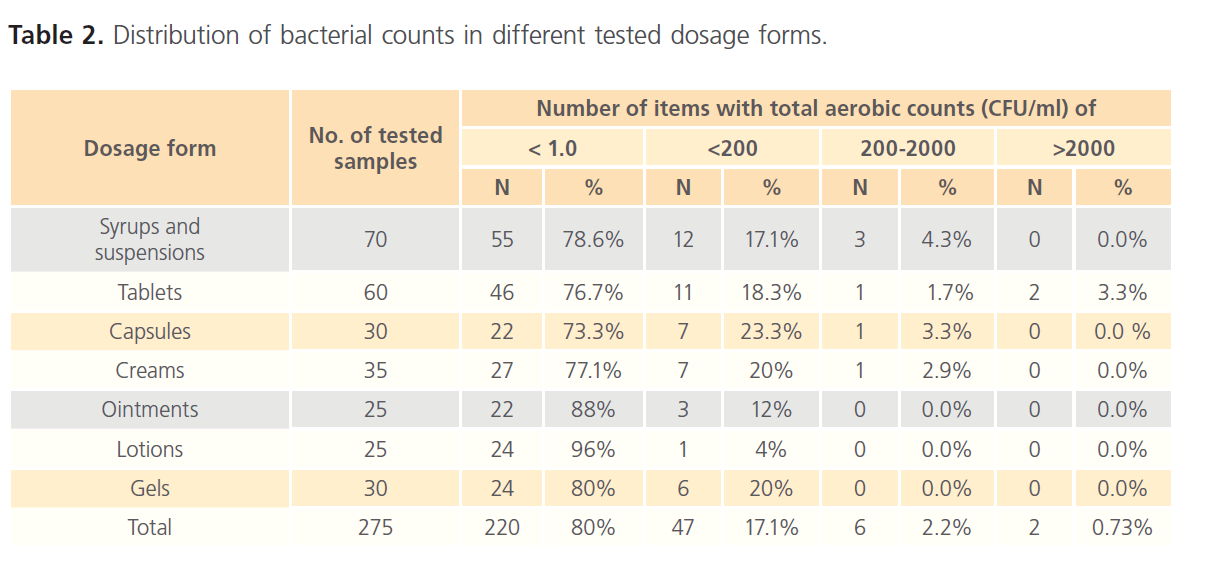
Table 2: Distribution of bacterial counts in different tested dosage forms.
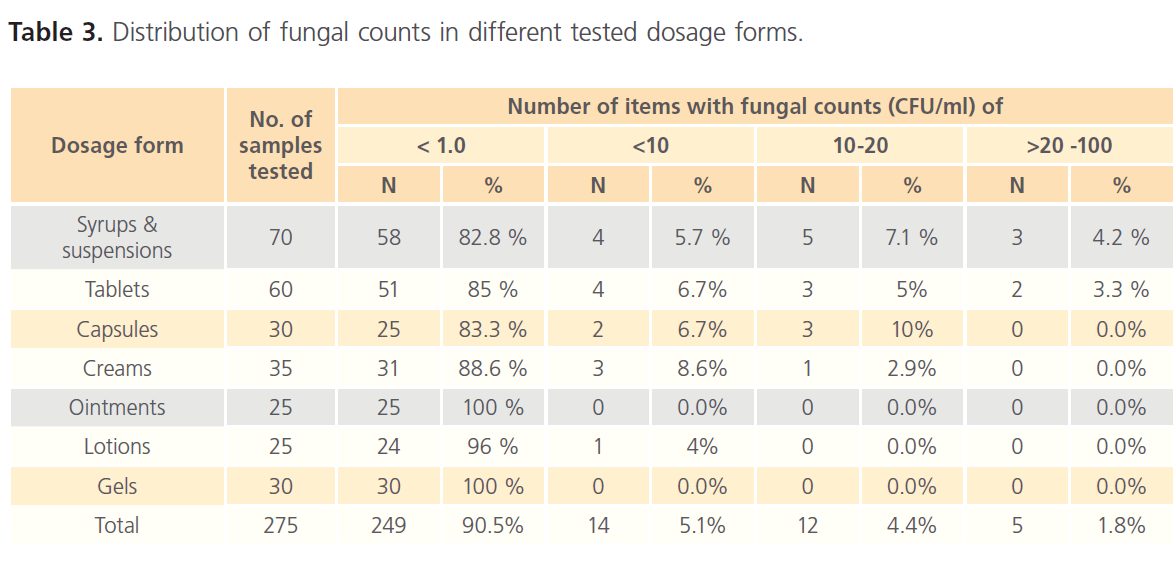
Table 3: Distribution of fungal counts in different tested dosage forms.
Likewise, for fungi, about 95% of the items tested contained fewer than 10 CFU/ml. Viable fungi were not recovered from about 90% of the items examined and 4.4% of the products contained 10- 20 CFU/ml whilst only 1.8% contained more than 20 CFU/ml. Fungal counts indicate that only few tested tablets and syrups had counts >20 CFU/ml; other dosage forms did not show counts in this range. The USP 31 specifies acceptance criteria of not more than 2 x 102 CFU yeast and mold/g or ml for non-aqueous oral preparations and tablets and not more than 2 x 10 CFU yeast and mold/g or ml for aqueous oral and topical preparations. Accordingly, only three oral preparations (syrups) exceeded the specified limits.
Detection and identification of the specified recovered microbial contaminants from the tested dosage forms
Streaking of the broths onto nonselective and selective media for isolation of pathogens revealed the following; primarily 60 bacterial and 31 fungal isolates were recovered. Identification of the recovered microbial contaminants was carried out through studying their microscopical and biochemical characteristics. According to the results obtained, the recovered isolates were categorized as shown in figure 1. Gram staining and microscopical examination of the purified bacterial isolates revealed that 44 isolates were Gram positive organisms and 16 isolates were Gram negative rods. Depending upon the results of microscopical examination and culture characteristics on some relevant selective/diagnostic media, a number of confirmatory biochemical reactions were conducted as well as API 20E identification kits. The results are shown in figure 1. For fungal isolates, some tested targeted identification of Candida albicans were carried out and the results revealed the identity of one fungal isolate belong to this species, isolate 9S, figure 1.
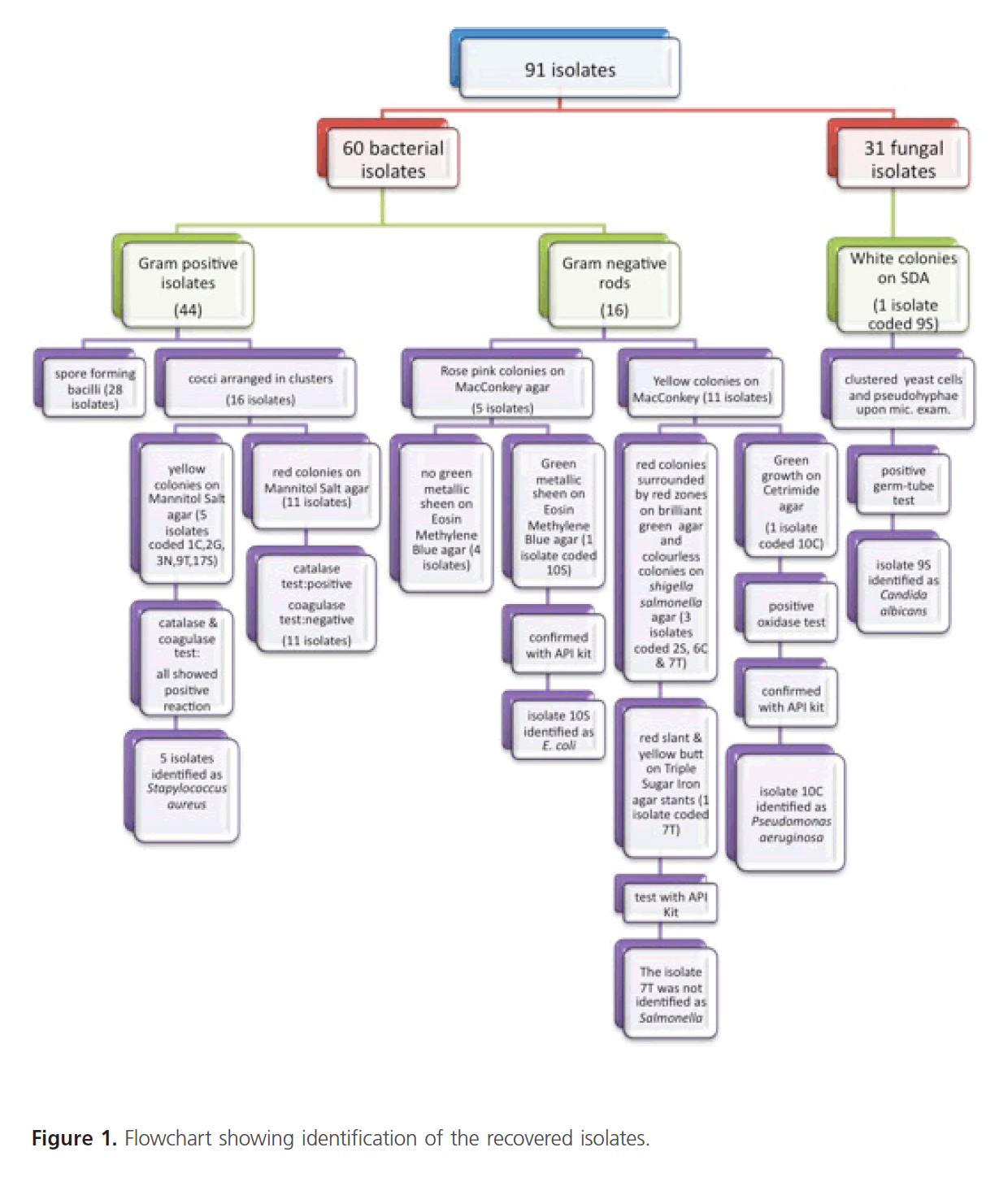
Figure 1: Flowchart showing identification of the recovered isolates.
From the identification results, the USP indicator pathogens could be recovered and identified as follows: one Escherichia coli isolate, coded 10S, was recovered from a syrup preparation whilst no Salmonella spp. could be recovered from any tested preparation. Five S. aureus isolates were recovered; S. aureus isolate, coded 1C, was recovered from a cream, S. aureus isolate, coded 2G, was recovered from a gel, S. aureus isolate, coded 3N, was recovered from an ointment, S. aureus isolate, coded 9T, was recovered from a tablet whilst S. aureus isolate, coded 17S, was recovered from a syrup. One Pseudomonas aeruginosa isolate coded 10C, was recovered from a capsule preparation whilst one Candida albicans isolate, coded 9S, and was recovered from a syrup.
The different specified isolated contaminants classified as microbial categories or identified species and the dosage forms from which they were recovered are summarized in table 4. Table 4 shows the prevalence of different microbial species in various tested dosage forms. The different dosage forms were found to be contaminated with various microbial species of bacteria and fungi among which the USP indicator pathogens were found. Regarding recovered bacterial species and tested dosage forms, Gram positive bacilli (sporulating species) accounted for the majority of isolates (30.8%). Coagulase-negative Gram positive cocci and Gram negative rods (other than the indicator pathogens) were frequently detected (12% and 15% respectively). In case of dosage forms contaminated with fungi, molds constituted the majority of contaminated dosage forms compared to yeast contaminated products.
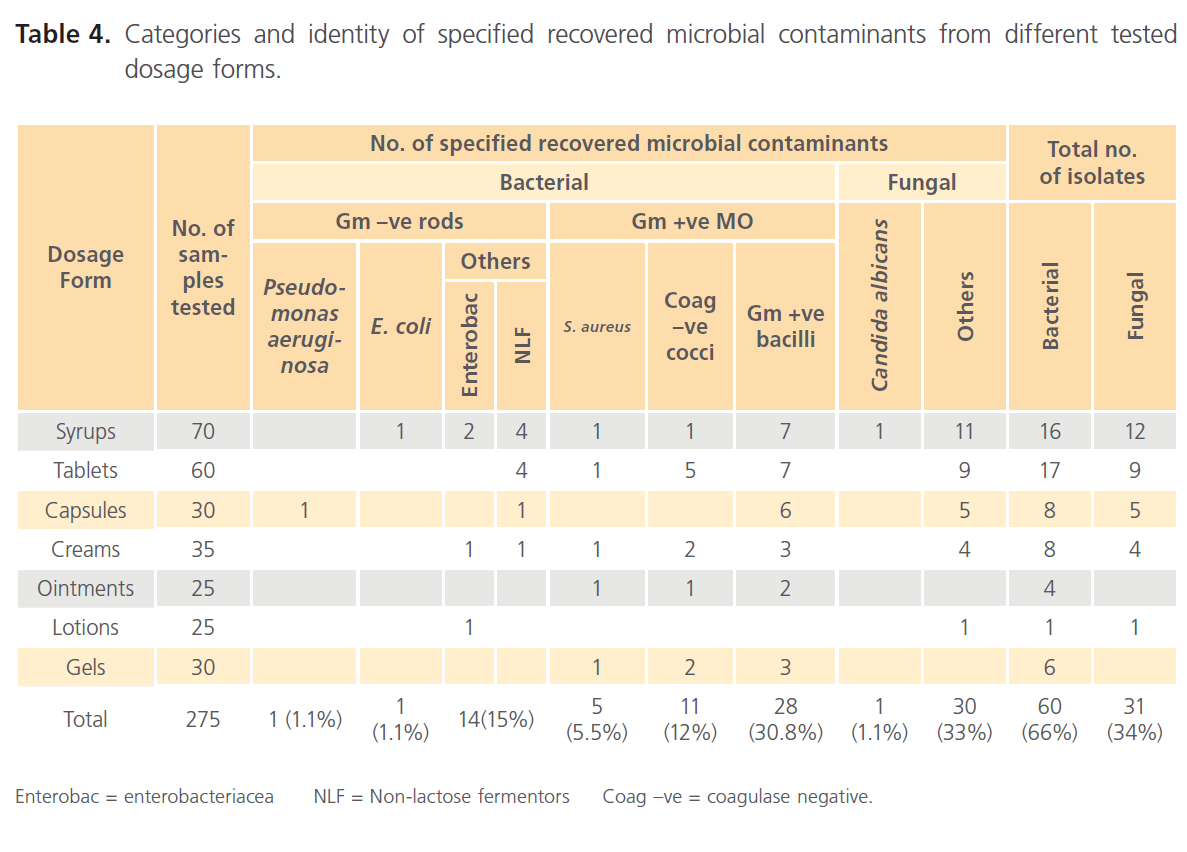
Table 4: Categories and identity of specified recovered microbial contaminants from different tested dosage forms.
Discussion
A total of 280 different collected pharmaceutical samples were screened for microbial contamination after verification of the recovery process. Results revealed that 27.6% of the products were contaminated and that the bioburden levels were generally adequate, with the exception of few cases. The percentage of contaminated samples in the present study is close to that previously reported in a similar study, where an incidence of contamination of 32% was reported [6]. On the other hand, a much lower incidence of contamination was documented by Onurdag et al. [13], where only 13.8 % of the items examined were contaminated. Similarily, Jarvis et al. [5], after testing products purchased from retail outlets throughout England and Wales reported that viable microorganisms were not recovered from over 50 % of the items tested.
The level of fungal contamination in this study was less than that of bacteria (11.3% and 20% respectively), however it was observed that fungal contamination comprised 50% of bacterial contamination. Ashour and Abdelaziz [9], have shown similar incidences of contamination where level of fungal contamination in the tested products was less than that of bacteria and more than 50% of the examined samples showed no recovery of viable fungi. Higher incidence rates were reported by Muhammed et al. [14] and Anar et al. [15], where in the latter study, frequencies of bacterial and fungal contamination of 53.47% and 35.64%, respectively were reported. Moreover, several other studies conducted on different pharmaceutical preparations revealed even higher contamination rates where it was observed that out of all the tested products, none failed to grow microorganisms [16-19]. Interestingly, it has been observed that on several occasions, more than one isolate was recovered from the same product resulting in a total recovery of 60 bacterial and 31 fungal isolates from 76 contaminated samples. Similar observations have been reported previously [6,10,16,20]. Moreover, the tested oral preparations in this study showed a higher incidence of contamination (33.75%) in comparison to the topical preparations (19.1%). A similar study conducted in Iraq investigating a number of oral and topical non-sterile pharmaceutical preparations revealed, in contrast to our results, that topical medicaments showed high proportion of contaminated samples (17.6 %) and more bacterial genera than oral ones [21].
There are a number of explanations for the differing results from the surveys on the incidence of contamination in non-sterile products. Different types of product have been sampled; certain products, particularly aqueous products, are known to be more susceptible to contamination than others [6]. In some surveys only one type of product has been sampled [16,19]. Methods of sampling and cultivation have also varied; some have involved direct culture of the product, whilst others have used enrichment techniques. In the case of the latter, higher contamination rates have generally been found. Neutralization of antibacterial agents has also varied from survey to survey, or may have been overlooked. Furthermore, the results from all surveys, including this one, are based on the examination of a limited number of samples, mostly less than 300 products. Interpretation of results, expressed as percentages, is therefore restricted, but these results may be used for comparative purposes [6].
As for the bioburden level of collected pharmaceutical preparations, the number of isolated microorganisms in this study is generally less than that reported earlier by other authors [5,10]. This may be due to the introduction of better adherence to ‘Good Manufacturing Practices’ by pharmaceutical manufacturers in recent years. The result of the microbiological examination of non-sterile pharmaceutical samples showed some considerable variations in microbial load. For bacteria, with the exception of six products (3 syrups, 2 tablets and 1 cream), the samples tested had satisfactory microbial levels compared to the USP specification of 2 x 102 cells per ml for aqueous preparations for oral use and topical preparations and 2 x 103 CFU per ml for non aqueous preparations for oral use (2.2% of the products). As for fungal contamination, results revealed that with the exception of three syrups (1.1% of the products), the samples tested had satisfactory microbial levels compared to the USP specification of not more than 2 x 102 CFU yeast and mold/g or ml for non-aqueous oral preparations and tablets and not more than 2 x 10 CFU yeast and mold/g or ml for aqueous oral and topical preparations. The proportion of the products containing viable aerobic microbial count > 2 x 103 CFU per ml or g was small (0.73%) which indicates that the microbiological quality of the examined products was, in general, adequate and, in most cases, excellent. The low microbial count recorded for pharmaceutical products tested in this study may be attributed to several factors; the sugar content of the syrups provide high osmotic pressure that is inhibitory to many micro-organisms and the low water activity in solid dosage forms and oily preparations has the potential to reduce microbial growth and spoilage.
Different levels were reported by several authors; Onurdag et al. [13], reported that 6.8% of the tested products were off limits, Gad et al. [10], reported that 7.5% of the contaminated products contain > 10³ CFU/ml whilst Nawas et al. [18], reported that 21% of the items tested were found not to conform to the microbial limit standards and to be heavily contaminated. Lower levels were reported by Arkele et al. [16] and Mwambete et al. [22] where in all samples tested, the aerobic mesophilic bacteria and fungi were within the standard numerical limits for non-sterile preparations.
A variety of contaminants were isolated from different types of products (Table 4). Sixty bacterial and 31 fungal isolate were detected. The contamination of pharmaceuticals is mediated by different factors such as poor personal hygiene, low efficiency of preservatives or antimicrobial agents employed, post production contamination and changes in production standards. The organisms also may originate from water and equipment used [23]. Microbiological specifications for non-sterile pharmaceuticals require the absence of 5 indicator pathogens; S. aureus, Pseudomonas aeruginosa, Salmonella spp., E. coli and Candida albicans. Our results revealed that 9% of the recovered isolates were of the indicator pathogens; Five S. aureus isolates (5.5% of isolates) were recovered from a cream, a gel, an ointment, a tablet and a syrup; one isolate of fecal indicator, Escherichia coli, (1.1%) was recovered from a syrup; one Pseudomonas aeruginosa isolate (1.1%) was recovered from a capsule and one Candida albicans isolate (1.1%) was recovered from a syrup. However, in common with other studies [5,10,14], none of the tested samples showed presence of Salmonella spp. Previous reports on the isolation of these indicator pathogens have been published. Al-Charrakh [21] reported the isolation of E. coli (5.7%), S. aureus (20.8%) and Pseudomonas aeruginosa (1.9%), from a range of pharmaceutical products while Onurdag et al. [13], reported the isolation of Candida spp., Staphylococcus aureus and E. coli. Similar results have been reported in several other studies [18,24,25]. The presence of S. aureus as a contaminant reflects contamination of processing unit and/or raw material. The organisms being normal floral of the body easily contaminate products during handling and processing by personnel. Moreover, the heat resistance of Staphylococcus aureus and their ability to thrive well in fairly high concentration of sugar contributes to their survival in processed products [14]. Even though the USP specifies the absence of S. aureus in topical preparations, yet their isolation from oral products has health implications as the enterotoxin-producing species of the organisms are implicated in food poisoning [23]. The occurrence of E. coli is another source of great concern with respect to hygienic practices. This is because E. coli has the human colon as a natural habitat and its presence in products is a strong indication of fecal contamination. Presence of Eschericha coli indicates poor hygiene practices, lack of adequate handling of the products and suggests the route of contamination is possibly water [19]. Isolation of Pseudomonas aeruginosa is an indictment of the raw materials used as well as the conditions prevalent in the environment in which the products are manufactured and packaged [26]. The absence of Pseudomonas aeruginosa in topical preparations has been specified by the USP due to their pathogenic effects, yet evidence suggests that their presence in medicaments for oral use may also be undesirable since septic infection with Pseudomonas aeruginosa may result from autoinfections with the bacteria from the patient’s own bowel [27]. The occurrence of fungal isolates (esp. Candida albicans) in pharmaceutical products cannot be overlooked. Most of the fungal contaminants isolated in this study were molds however, only one preparation contained yeast, Candida albicans. Nahata [28] stated that fungal contamination of products constituted a public health hazard while Riederer et al. [29] reported that opportunistic Candida infections constitute one of the commonest disorders (> 50%) following HIV infections [24]. The presence of certain molds is harmful since they produce metabolites that may be toxic to consumers and cause rapid deterioration of the product due to the biodegradation of the different components of formulations arising from the production of toxins [10]. Even though most molds fall under the category of opportunistic pathogens and are generally harmless yet they can become pathogenic in immunocomprised patients and when present in large quantities may cause serious health problems. Therefore, it important to note that any excessive mold growth needs to be taken care of, regardless of the species; since they can spell to increased allergies and toxicity [30].
In addition to the indicator pathogens, several other species of bacteria were isolated in the current study. The use of the five pathogen indicator bacteria does not mean that the presence of other bacteria might not be a problem during quality evaluations. However, route of application and intended use of a given product, nature of the product, and potential risk to the consumer will determine if there is a risk involved when these other microorganisms are present [31]. Gram-positive rods (spore-bearers) were the most commonly isolated bacteria from oral and topical medicaments (30.8 %) followed by Gram-positive cocci and Gram-negative rods (17.6% each). Our findings are consistent with those of previous studies, where the majority of microbial contaminants in non-sterile pharmaceuticals are Bacillus spp. [16,21,32]. This could be explained by the fact that these bacteria produce spores which are resistant to harsh processing, elevated heat and dry conditions and therefore they can survive for a long time in the product in a dormant state [7]. Bacillus spp. are ubiquitous and considered harmless, though undesirable because of their spoilage potential. Their presence in product suggests poor environmental hygiene during processing or badly contaminated or adulterated raw materials [33]. A significant number of the microorganisms isolated from the samples were normal human flora, which are widely distributed in nature. This suggests that these medicines were microbiologically contaminated as a result of improper handling, poor hygienic procedures during repackaging into smaller packs, and dispensing of medicines. Moreover, some products were found to be contaminated with enterobacteriaceae. The contamination of any preparation with gram negative organisms is not desirable and constitutes a public health concern [34]. Generally, the presence of potentially pathogenic opportunistic microbes, cannot be overemphasized, because they may cause a significant deterioration in the health status of patients, particularly in elderly, debilitated and chronically sick patients, those who are immunologically compromised, and of infants with an immature immune system [33].
The importance of and potential for microbial contamination of pharmaceuticals is now widely recognized in the pharmaceutical industry and attempts to safeguard products from contamination include, among others, good manufacturing practices such as raw material testing, equipment sanitization and automation, microbiological testing and validation of water systems, monitoring of the environment, training of personnel, wearing of gloves, masks, hats, and laboratory uniforms, and packaging of products in individual waterproof, tamperproof wrappings [24,31]. Effective preservatives should also be employed [34]. The failure of strict observation of good manufacturing practice at any stage of production may greatly affect the microbiologic quality of the end products [33].
8778
References
- Na’was, TE., Salem, MS., N, AH. Microbial contamination and preservation efficacy of cough preparations. J Clin Pharm Ther. 1990; 15: 365-369.
- Shaikh, D., Jamshed, TA., Shaikh, R. Microbial contamination of pharmaceutical preparations. Pak J Pharm Sci. 1988; 1: 61-66.
- Bloomfield, FH. Microbial contamination: Spoilage and Hazard. Denyer, SP., Baird, RRM., (eds.). Guide To Microbiological Control In Pharmaceuticals And Medical Devices. 2nd ed. Boca Raton: Taylor & Francis Group. 2007. pp. 23-50.
- Clontz, L. Microbial Limit and Bioburden Tests: Validation Approaches and Global Requirements. Boca Raton: Taylor & Francis. 2010.
- Jarvis, B., Rhodes, AC., Armstrong, M. A survey of microbiological contamination in cosmetics and toiletries in the U.K. J Soc Cosmet Chem 1974; 25: 563-575.
- Baird, RM. Microbial contamination of cosmetic products. J Soc Cosmet Chem 1977; 28: 17-20.
- Stevic, T., Stankovic, S., Savikin, K. Pathogenic Microorganisms of Medicinal Herbal Drugs. Arch Biol Sci. 2012; 64: 49-58.
- Becks, VE., Lorenzoni, NM. Pseudomonas aeruginosa outbreak in a neonatal intensive care unit: A possible link to contaminated hand lotion. Am J Infect Control. 1995; 23: 396-398.
- Abdelaziz, AA., Ashour, MSE., Hefni, H., El-Tayeb, OM. Microbial contamination of cosmetics and personal care items in Egypt-shaving creams and shampoos. J Clin Pharm Ther. 1989; 14: 29- 34.
- Gad Gamal, FM., Ashour, MS. Microbial Evaluation of Some Non-sterile Pharmaceutical Preparations Commonly Used in the Egyptian Market. Trop J Pharm Res. 2011; 10: 437-445.
- Ashour, MS., Mansy, MS. Microbiological Environmental Monitoring in Pharmaceutical Facility. Egypt Acad J biolog Sci. 2011; 3: 63-74.
- United States Pharmacopeial Convention. The United States Pharmacopeia : USP 31: The National Formulary: NF 26. United States Pharmacopeial convention, Inc. 2008.
- Onurdag, FK., Ozgen, S., Abbasoglu, D. Microbiological Investigation of Used Cosmetic Samples. Hacettepe Univ J Fac Pharm. 2010; 30: 1-16.
- Muhammed, A. and Umoh, V. J. Incidence and effects of microorganisms on the quality of some pharmaceutical mixtures in Zaria – Nigeria. Nig J Pharm Sci. 2009; 8: 126-134.
- Anar et al. [Microbiological examination of some cosmetics]. Izmir: Ege University, Institute of Health Sciences.Turkish. 1977.
- Akerele, JO and Ukoh, GC. Aspects of microbial contamination of tablets dispensed in hospitals and community pharmacies in Benin City, Nigeria. Trop J Pharm Res. 2002; 1: 23-28.
- Hugbo, PG., Igwe, I. Microbial contamination and preservative capacity of some brands of cosmetic creams. Trop J Pharm Res. 2003; 2: 229-234.
- Na’was, T., Alkofahi, A. Microbial contamination and preservative efficacy of topical creams. J Clin Pharm Ther. 1994; 19: 41-46.
- Obi, CN NU. Microbiological analyses of drug tablets from selected outlets in Umuahia, Abia state, Nigeria. Niger Res J pharm. 2010; 4: 31-37.
- Adeola Anifowoshe, R., Opara, MI., Adeleye Isaac, A. Microbial quality of some non-sterile pharmaceutical products sourced from some retail pharmacies in Lagos, Nigeria. Afr J Microbiol Res. 2012; 6: 4903-4907.
- Al-Charrakh, AH. Frequency and antimicrobial resistance of bacteria isolated from oral and topical medicaments from Hilla, Iraq. J Infect Dev Ctries 2012; 6: 489-494.
- Mwambete, KD., Fazleabbas, FS. Microbiological Assessment of Commercially Available Quinine Syrup and Water for Injections in Dar Es Salaam, Tanzania. Trop J Pharm Res. 2009; 8: 441-447.
- Ogbulie, JN., Ibe, I., Nguma, CC. The Microbial Associates Of Unexpired And Expired Paediatric Syrups. Niger J Microbiol. 2009; 23: 1817-1822.
- Obuekwe, IF., Obuekwe, CO. Microbial contamination of pharmaceutical products in a tropical environment. Pakistan J Sci Ind Res. 2002; 45: 340-344.
- Okeke, IN., Lamikanra, AA. Bacteriological quality of skin moisturizing creams and lotions distributed in a tropical developing country. J Appl Microbiol. 2001; 91: 922-928.
- Osungunna, MO., Adetuyi, A. Bacteriological and Antibiotic Sensitivity Patterns of Bacterial Isolates from Creams and Lotions Hawked in Sagamu, Ogun State. Pak J Nutr. 2010; 9: 773-775.
- Parker, MT. The clinical significance of the presence of micro-organismins in pharmaceutical and cosmetic preparations. J Soc Cosmet Chem. 1972; 23: 415-426.
- Nahata, MC., Allen, LJ. Extemporaneous Drugs Formulations. Clin Ther. 2008; 30: 2112-2119.
- Riederer, AP., Grein, GO., Bogner, JR. High prevalence of opportunistic infections in the head and neck related to human immunodeficiency virus. A prospective study of the distribution of otorhinolaryngologic disorders in 250 patients. Infection 1996; 24: 440-446.
- Mwambete, KD. Incidence of fungal contamination of tablets available in Dar Es Salaam market-Tanzania. J Pharm Res. 2011; 4: 868-870.
- Jimenez, L. Microbial Limits. Microbial Contamination Control in the Pharmaceutical Industry: CRC Press. 2004. pp. 15-44.
- Mwambete, SA. Microbiological quality and preservative capacity of commonly available cosmetics in Dar es Salaam, Tanzania. East Cent Afri J Pharm Sci. 2010; 13: 3-11.
- Mugoyela, V., Mwambete, KD. Microbial contamination of nonsterile pharmaceuticals in public hospital settings. Ther Clin Risk Manag. 2010; 6: 443-448.
- Udeze, AO., Jayeoba, T., Innocent-Adiele, HC., Okerentugba, PO., Nwanze, JC. et al. Bacteriological Assessment Of Some Selected Antacid Suspension Products In Nigeria. N Y Sci J. 2012; 5.










