David S. Silver1-3, Sarah R. Markoff1, Leah Naghi4, Michael Silver5 and Lawrence May2
1Cedars Sinai Medical Center, USA
2University of California, Los Angeles School of Medicine, USA
3Targeted Medical Pharma, USA
4University of Southern California School of Medicine, USA
5University of Southern California Marshall School of Business, USA
Corresponding Author:
David S. Silver
University of California, Los Angeles School of Medicine
2934 ½ Beverly Glen Circle, #429, Los Angeles, CA 90077, USA
Tel: 310-825-6373
E-mail: silver@ptlcentral.com
Received date: January 06, 2016; Accepted date: January 20, 2016; Published date: January 27, 2016
Citation: Silver DS, Markoff SR, Naghi L, et al. Reduction in Parasympathetic Autonomic Nervous System Function in Fibromyalgia Patients. Arch Med. 2016, 8:2. doi: doi number
Keywords
Fibromyalgia syndrome, Heart rate variability, Autonomic nervous system, High Frequency
Introduction
Fibromyalgia syndrome (FMS) is characterized by chronic, widespread pain, fatigue and difficulty with sleep. Fibromyalgia patients often experience cognitive impairment, irritable bowel symptoms and posturally-mediated hypotension [1,2]. Sleep disturbances are also a common symptom of fibromyalgia syndrome. Sleep patterns are linked to biologic circadian rhythms which in turn influences the parasympathetic nervous system. Concomitant anxiety and panic disorders are common, although depression, despite common belief, is mostly seen in the severely impaired patients and appears secondary to the underlying disorder. The lack of restorative sleep in fibromyalgia patients is a particularly troubling symptom and may help to explain the daytime fatigue and many of the other symptoms described [2-6].
The diagnosis of FMS is typically made on a subjective basis. Physicians rely on the patient’s history of pain and related symptoms without another clear etiology to explain the findings.
The use of the tender point exam can be confirmatory but is not specific and totally subjective. Blood tests have proven unreliable and newer criteria proposed by the American College of Rheumatology are symptom based [7,8]. Although imaging studies such as Functional and Spectral MRI may reveal certain specific abnormalities and may even demonstrate response to treatment [9-12], availability of imaging centers that have experience with such test are limited and costs can be expensive.
Studies have indicated that autonomic nervous system (ANS) function is altered in fibromyalgia patients as revealed by objective ANS testing, such as tilt table testing. Investigators such as Buskila [13], Chervin [4] and Martinez-Levin [4,14-16] have demonstrated consistent abnormalities in the ANS function using 24-hour Holter monitor assessment of heart rate variability (HRV). Studies have suggested that the degree of ANS abnormality directly correlates with symptoms, specifically the pain intensity, indicating the importance of ANS dysfunction in FMS [17].
The studies to date looking at ANS dysfunction in FMS patients have been relatively small with well controlled patient populations [4,13,15,16]. They have excluded patients on sedative or antidepressant medications as well as with significant co-morbid conditions. The aforementioned studies may not reflect typical community based FMS patients. Larger studies without strict inclusion criteria that represent a more typical fibromyalgia population are needed.
Genetic studies suggest that FMS patients have a predilection toward dysautonomia. Studies have suggested defects in the enzyme that inactivates catecholamines, specifically catechol- O-methyl-transferase [18]. Receptor defects may be present as well. These receptors are involved in pain perception and orthostasis. HLA associations have been described in FMS, but the heterogeneity is such that no specific correlation can yet be determined [19,20].
The ANS is intimately involved in maintenance of blood pressure, heart rate, bowel function, pain perception as well as initiation and maintenance of sleep. Disruption of the normal function of the Autonomic Nervous System as seen in fibromyalgia destroys the equilibrium between the sympathetic and the parasympathetic nervous system. These abnormalities may help to explain the symptoms patients have with fibromyalgia. Heart rate variability (HRV) measures temporal differences between consecutive heart beats. One can determine the relative function of the ANS using Holter monitor to look at patterns of ANS function, particularly circadian rhythms. Decreased HRV is an independent risk factor for many disease states including myocardial infarction, asthma, diabetes and chronic renal disease [21-28].
HRV is commonly measured in time-domain by millisecond (ms), but another path to interpret the data is to convert the time to frequency (Hz). The conversion employs a method called Fast Fourier Transformation (FFT) [29,30]. Until recently, this method was constructed in such a way where the heart beats of a subject were assumed to be of equal spacing. Now that this is known as inaccurate, we used a new version of this method which eliminates the need for the assumption of equal spacing of the successive heartbeats. The frequency data resulting from FFT provides a graph showing three predominant parts: Very Low Frequency (VLF), Low Frequency (LF), and High Frequency (HF).
In the frequency domain, High Frequency (HF) activity is known to be associated with parasympathetic nervous system activity [31], which is significantly active in normal subjects from midnight to 5 am and slows down the body’s system functions for restoration during sleep. Low Frequency (LF) is associated with sympathetic nervous system activity. The increase in parasympathetic nervous system activity at night can be measured by looking at the percent change from baseline and is referred to as the Circadian index. Change in HF activity when compared to baseline can be measure in 5 minute epochs. These changes are measured between 0:00 (midnight) and 5:00 am and added together to represent the Circadian index. Decreased parasympathetic activity at night may help to explain the sleep abnormalities experienced by FMS patients. After 5:00 am, parasympathetic activity diminishes rapidly. We employed this methodology in order to understand parasympathetic and sympathetic ANS in this population while looking for a consistent pattern that might objectively separate FMS patients from patients with similar complaints who do not have FMS.
Methods
58 control patients and 329 FMS patients from a single medical practice (DS) were tested using a Holter Monitor (ECG). FMS patients were defined as meeting the 1990 American College of Rheumatology criteria, were diagnosed as mild to severe and were consecutive patients presented at the medical practice. No patients were excluded for use of medication, concomitant medical or psychiatric conditions, age, sex, or any other reason.
Control patients were randomly selected from a cohort of patients in a larger database and were characterized as having the majority of their circadian index above the normal range and having an increase over 1500% during the hours of midnight to 5:00 am, which represents the 5th percentile. The control group was compared to other historical controls from the same lab and the results were similar.
Subjects wore holter monitors for a period of 24 hours and went about their daily routines. HRV data was performed by Laboratory Services Industry. HRV, sympathetic (LF) and parasympathetic (HF) data points analyzed were: SDNN, LF (0.04 to <0.15 Hz), HF (0.15 to <0.40 Hz), Total Power, Normal LF, Normal HF, LF/HF ratio and Circadian index between the hours of 0:00 (midnight) to 5:00 am as measured by change in percentage from baseline in 5 minute epochs. The percentage changes are added together to give you the Circadian index. Normally, parasympathetic activity increases between midnight and 5:00 am and then decreases rapidly. The means of these points were compared between the control and active groups using a Two-Sample t-Test, assuming equal variances. Hypothesized mean difference was 0. QRS complexes were reviewed on a Pathfinder 710 (Reynolds Medical) by a specialized technician who censored aberrant complexes and artifacts using an algorithm based on the Lomb-Scargle method of spectral analysis to produce the standard measures of high frequency, low frequency and very low frequency (VLF, 0.003 to <0.04 Hz) spectral power, expressed in 2 msec.
Results
The FMS patients demonstrated decreased total power and parasympathetic ANS function, specifically reduction in parasympathetic nervous system function at night. The mean LF, corresponding to sympathetic function, of the 329 fibromyalgia patients was 388.99 Hz and the mean of the 58 normal patients was 470.0 Hz. (p<0.05) (Figure 1). The mean HF, corresponding to parasympathetic function, of the 329 fibromyalgia patients was 246.95 Hz and the mean of the 58 normal patients was 262.25 Hz (p=NS). The mean Total Power of ANS function for fibromyalgia patients was 1347.53 Hz and the mean of the 58 normal patients was 1625.13 Hz. (p<0.05) (Figure 2). The Total Power combines both sympathetic and parasympathetic function. The mean LF to HF ratio of the FMS patients of 1.718 and the mean of the normal patients was 1.961 (p<0.001) (Figure 3), which indicated relative sympathetic hyperreactivity compared to normals in the fibromyalgia population. The mean Circadian increase for the 329 fibromyalgia patients was 99.8% while the mean Circadian increase for the 58 normal patients was 74.54% (p<0.001) (Figure 4), demonstrating lack of parasympathetic activation at night in the fibromyalgia patients.
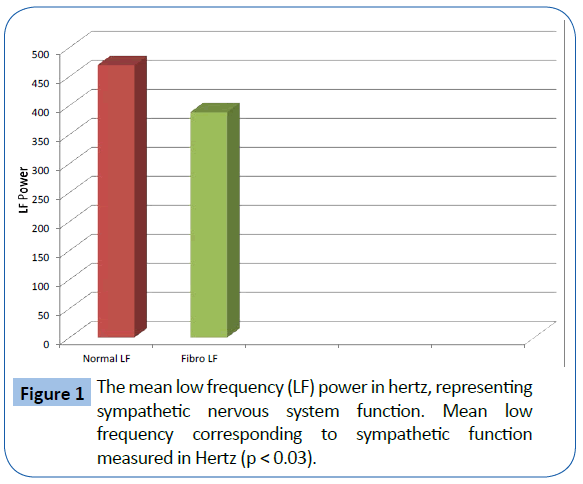
Figure 1: The mean low frequency (LF) power in hertz, representing sympathetic nervous system function. Mean low frequency corresponding to sympathetic function measured in Hertz (p < 0.03).
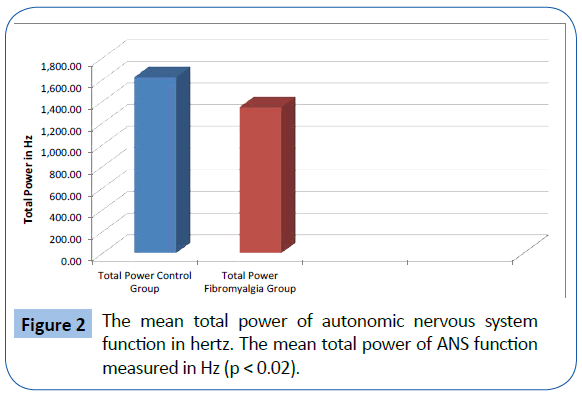
Figure 2: The mean total power of autonomic nervous system function in hertz. The mean total power of ANS function measured in Hz (p < 0.02).
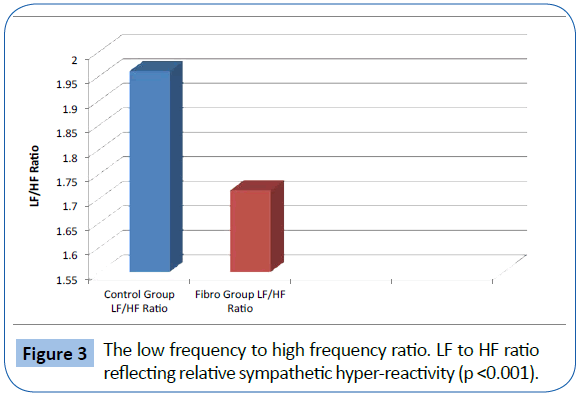
Figure 3: The low frequency to high frequency ratio. LF to HF ratio reflecting relative sympathetic hyper-reactivity (p <0.001).
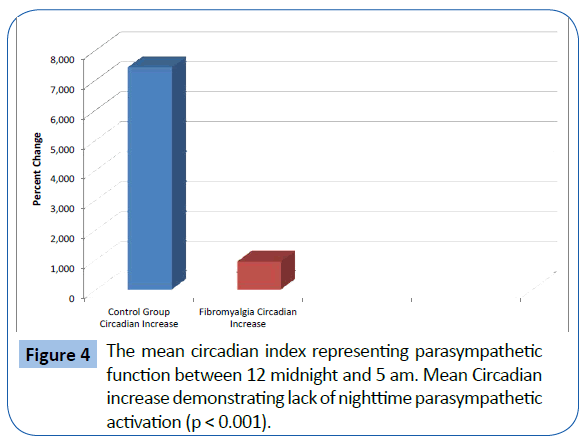
Figure 4: The mean circadian index representing parasympathetic function between 12 midnight and 5 am. Mean Circadian increase demonstrating lack of nighttime parasympathetic activation (p < 0.001).
Discussion
This study demonstrated a statistically significant reduction of parasympathetic ANS activity during the nighttime hours as measured by analysis of 24 hour continuous ECG monitoring in both the time and frequency domain. The parasympathetic ANS activity was measured by a Fast Fourier Transform in 5 minute epochs, 15 minutes apart. There was a statistically significant reduction in the high frequency band of the time domain 24 hour ECG analysis, related to reduced parasympathetic ANS activity. Several other parameters were derived from the 24 hour ECG analysis. This included a reduction of 24 hour total power, as measured in the time domain, indicating suppression of the entire ANS activity.
24 hour ECG recording is a well established methodology for assessing ANS activity. There are two fundamental methods for the 24 hour ECG recordings that include time domain and frequency domain measurements [31]. In the time domain, the interval between each successive ECG beat is recorded in milliseconds (ms). Each interval is plotted by frequency. A number of statistical measurements are made from this time domain graph. For example, the variance of the ECG beats (i.e. the range between the lowest beat per minute and the highest beat per minute) is measured by the SDNN. In normal subjects with healthy HRV, the range is from 60-120 beats per minute. In patients with certain types of chronic diseases, HRV is frequently reduced and can be as little as 5 beats per minute [32-38]. In patients with congestive heart failure, the average can be 120 beats per minute with a range of 115-125 [38,39]. However, this alteration in ANS function is not always seen in autoimmune disease and may present with different patterns in mood disorders. HRV would be a useful tool in differentiating FMS from other disease states as it has been shown to be sensitive to pain, in particular pain associated with physical and mental stressors [40].
In the frequency domain, beats per minute are converted to Hz per minute, using the mathematical technique of Fast Fourier Transform. This is a methodology less familiar to clinicians but has resulted in specific frequency bands that have specific clinical correlates. For example, the HF band, or High Frequency band, is associated with parasympathetic ANS activity [31]. The parasympathetic HF band has very little overlap with other ANS measurements and is considered specific for parasympathetic autonomic nervous system activity. Any fluctuations seen in the High Frequency band can be directly related to parasympathetic outflow. There are few other clinical or ECG methods available to measure ANS parasympathetic function.
The method used in this study utilized a new Fast Fourier Transform methodology that does not assume equal spacing between events. This methodology was originally developed for assessment of Quasars, which are similar to ECG signals. This methodology reduces both LF artifact and blurring of the other frequency bands. This is the first study that we are aware of that this high resolution FFT has been applied to patients with FMS.
It is well accepted that FMS is a disorder of the central nervous system [41-47], which clearly effects ANS function. Previous attempts to measure this have been difficult to perform and have not provided consistent data [48] except in the description and the consistency that there are significant abnormalities, specifically in the reduced parasympathetic function of the ANS.
We have demonstrated that patients with fibromyalgia have persistently reduced parasympathetic ANS activity, specifically at night. The role of parasympathetic ANS function in initiation and maintenance of sleep, which is significantly blunted in FMS patients, may help to explain some instances of sleep disturbance and daytime somnolence that patients report. In addition, patients with fibromyalgia have demonstrated reduced total power of the ANS, consistent with that seen in other studies. The ANS is closely tied to many physiologic functions that are involved in symptoms FMS patients present. These include irritable bowel syndrome, posturally-mediated hypotension, sleep disturbance, widespread pain and mood disorders such as anxiety and depression, which can be explained by the relative sympathetic hyper reactivity. The chronic nature of these conditions is often associated with long term reduction of parasympathetic activity.
Neurotransmitter deficiencies play a crucial role in chronic pain syndrome and are the target of most pharmaceutical modalities that treat FMS [49-55]. Antiepileptic drugs such as pregabalin reduces the synaptic release of several neurotransmitters, apparently by binding to α2-δ subunits, and possibly accounting for its actions in vivo to reduce neuronal excitability and seizures [56]. Duel serotonin norepinephrine reuptake inhibitors increase CNS levels of these neurotransmitters in the descending pain pathways, which are known to be diminished in chronic pain, and specifically FMS [57-59]. Tricyclic antidepressants work both on increasing serotonin and norepinephrine, while decreasing acetylcholine [60]. They often are used for their sedative effects at much lower doses than were typically used for depression. Whether this improves ANS function has yet to be determined objectively.
Another option is amino acid-based systems which provide neurotransmitter precursors that have been shown deficient in chronic pain syndromes [49-55]. These systems can potentially improve pain without significant side effects. High doses of individual amino acids precursors to specific neurotransmitters are likely not to be beneficial as the effects attenuate rather rapidly. Treatments that use small doses of several amino acids at smaller doses with other substances that improve uptake and prevent attenuation of the effects have greater promise. These therapies have been shown to provide benefit in other disease states that deplete neurotransmitters such as Gulf War Syndrome and Post Traumatic Stress Disorder and chronic back pain [61-65]. Use of Holter monitor not only to diagnose but also to monitor treatment may prove to be useful.
The use of the Holter monitor to measure ANS activity is an objective and reproducible technology. One of the challenges of FMS is finding a diagnostic test that can more objectively determine the presence or absence of the syndrome. Use of manual tender point exam is often unclear and new criteria are being established to replace the old 1990 ACR methodologies [66,67]. However, these are primarily subjective tests and therefore have significant limitations.
The use of 24-hour Holter monitoring measuring HRV may be the first straightforward method that can objectively determine FMS in patients who have the clinical symptoms consistent with the diagnosis. Although the method is not limited strictly to FMS diagnosis, there is a high rate of sensitivity with patients showing evidence of a marked impairment of parasympathetic activation at night. If the study can be reproduced, it will likely represent the first objective test for FMS and would be useful in the following ways:
1. In confirming diagnosis of patients who meet all clinical criteria; and
2. In patients who have confounding symptoms that may limit the effectiveness of objective evaluation, there would be a high value in negative testing (i.e. severe depression or somatization symptoms).
This study has limitations. First, the clinical diagnosis of FMS may be considered subjective and vary between examiners. Control groups need to be better defined, although the profound decrease in parasympathetic ANS function from midnight to 5 am makes it unlikely that a changing control group will alter this data. Medication usage, exercise and dietary routines were not controlled for in this study as patients were derived from a regular practice setting.
Parasympathetic ANS function is clearly suppressed in FMS patients, especially from the hours of midnight to 5 am. This finding, if confirmed, may finally provide clinicians with an opportunity to have an objective test to confirm or refute the diagnosis of fibromyalgia in individual patients.
Conclusion
Patients with fibromyalgia have persistently reduced parasympathetic ANS activity, specifically at night. The role of parasympathetic ANS function in initiation and maintenance of sleep may help to explain the sleep disturbance and daytime somnolence patients report. The ANS is closely tied to many physiologic functions that are involved in symptoms FMS patients experience including irritable bowel syndrome, posturally mediated hypotension, sleep disturbance, widespread pain and anxiety, which can be explained by the relative sympathetic hyperreactivity. Holter monitor may represent an objective diagnostic tool for FMS and therapies that target abnormalities in ANS function may represent a new therapeutic option for FMS patients.
Acknowledgments
Elizabeth Charuvastra, RN was instrumental in using this technology to help patients to help diagnose and treat patients. She passed away before this manuscript was completed. She is missed by all who had the privilege of knowing her.
9124
References
- Silver DS, Wallace DJ (2002) The management of fibromyalgia-associated syndromes. Rheum Dis Clin North Am 28: 405-417.
- Silverman S, Sadosky A, Evans C, Yeh Y, AlvirJM, et al. (2010) Toward characterization and definition of fibromyalgia severity. BMC MusculoskeletDisord 11: 66.
- Lerma C, Martinez A, Ruiz N, Vargas A, Infante O, et al. (2011) Nocturnal heart rate variability parameters as potential fibromyalgia biomarker: correlation with symptoms severity. Arthritis Res Ther 13: R185.
- Moldofsky H (2009) The significance of dysfunctions of the sleeping/waking brain to the pathogenesis and treatment of fibromyalgia syndrome. Rheum Dis Clin North Am 35: 275-283.
- Moldofsky H (2008) The significance of the sleeping-waking brain for the understanding of widespread musculoskeletal pain and fatigue in fibromyalgia syndrome and allied syndromes. Joint Bone Spine 75: 397-402.
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, et al. (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33: 160-172.
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, et al. (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 62: 600-610.
- López-Solà M, Pujol J, Wager TD (2014) Altered functional magnetic resonance imaging responses to nonpainful sensory stimulation in fibromyalgia patients. Arthritis Rheumatol 66: 3200-3209.
- Kim JY, Kim SH, Seo J, Kim SH, Han SW, et al. (2013) Increased power spectral density in resting-state pain-related brain networks in fibromyalgia. Pain 154: 1792-1797.
- Napadow V, Kim J, Clauw DJ, Harris RE (2012) Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum 64: 2398-2403.
- Kim J, Loggia ML, Cahalan CM (2015) The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheum 67: 1395-1405.
- Cohen H, Neumann L, Shore M, Amir M, Cassuto Y, et al. (2000) Autonomic dysfunction in patients with fibromyalgia: application of power spectral analysis of heart rate variability. Semin Arthritis Rheum 29: 217-227.
- Chervin RD, Teodorescu M, Kushwaha R, Deline AM, Brucksch CB, et al. (2009) Objective measures of disordered sleep in fibromyalgia. J Rheumatol 36: 2009-2016.
- Martínez-Lavín M, Hermosillo AG, Rosas M, Soto ME (1998) Circadian studies of autonomic nervous balance in patients with fibromyalgia: a heart rate variability analysis. Arthritis Rheum 41: 1966-1971.
- Kooh M, Martínez-Lavín M, Meza S, Martín-del-Campo A, Hermosillo AG, et al. (2003) Simultaneous heart rate variability and polysomnographic analyses in fibromyalgia. ClinExpRheumatol 21: 529-530.
- Martínez-Lavín M, Hermosillo AG (2000) Autonomic nervous system dysfunction may explain the multisystem features of fibromyalgia. Semin Arthritis Rheum 29: 197-199.
- Lee YH, Kim JH, Song GG (2015) Association between the COMTVal158Met polymorphism and fibromyalgia susceptibility and fibromyalgia impact questionnaire score: a meta-analysis. RheumatolInt 35: 159-166.
- Buskila D, Sarzi-Puttini P (2006) Biology and therapy of fibromyalgia. Genetic aspects of fibromyalgia syndrome. Arthritis Res Ther 8: 218.
- Yunus MB (1998) Genetic factors in fibromyalgia syndrome. Z Rheumatol 57 Suppl 2: 61-62.
- Hejjel L, Gál I (2001) Heart rate variability analysis. ActaPhysiol Hung 88: 219-230.
- Lucini D, Milani RV, Costantino G, Lavie CJ, Porta A, et al. (2002) Effects of cardiac rehabilitation and exercise training on autonomic regulation in patients with coronary artery disease. Am Heart J 143: 977-983.
- Du J, He J, Wang Y (2001) [A study of heart rate variability in asthma]. ZhonghuaJie He He Hu Xi ZaZhi 24: 744-745.
- Sucharita S, Bantwal G, Idiculla J, Ayyar V, Vaz M (2011) Autonomic nervous system function in type 2 diabetes using conventional clinical autonomic tests, heart rate and blood pressure variability measures. Indian J EndocrinolMetab 15: 198-203.
- Yun WH, Min SW, Huh J, Ro YJ, Kim CS (2010) Autonomic changes in preoperative uncomplicated diabetic patients with postural changes. J Int Med Res 38: 1764-1771.
- Rodrigues TC, Ehrlich J, Hunter CM, Kinney GL, Rewers M, et al. (2010) Reduced heart rate variability predicts progression of coronary artery calcification in adults with type diabetes and controls without diabetes. Diabetes TechnolTher 12: 963-969.
- Karayaylali I, San M, Kudaiberdieva G (2003) Heart rate variability, left ventricular functions, and cardiac autonomic neuropathy in patients undergoing chronic hemodialysis. Ren Fail 25: 845-853.
- Laaksonen S, Voipio-Pulkki L, Erkinjuntti M, Asola M, Falck B (2000) Does dialysis therapy improve autonomic and peripheral nervous system abnormalities in chronic uraemia? J Intern Med 248: 21-26.
- Ellis RJ, Sollers Iii JJ, Edelstein EA, Thayer JF (2008) Data transforms for spectral analyses of heart rate variability. Biomed SciInstrum 44: 392-397.
- CowanMJ (1995) Measurement of heart rate variability. West J Nurs Res 17: 32-48.
- Malik M, CammAJ (1995) Heart Rate Variability. Futura Publishing Compnay, USA.
- Press WH, Rybicki GB (1989) Fast algorithm for spectral analysis of unevenly sampled data. Astrophysical J 338: 277-280.
- Gerritsen J, Dekker JM, TenVoorde BJ (2001) Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care 24: 1793-1798.
- Pagani M, Lucini D (2001) Autonomic dysregulation in essential hypertension: insight from heart rate and arterial pressure variability. AutonNeurosci 90: 76-82.
- Konrady AO, RudomanovOG, YacovlevaOI, ShlyakhtoEV (2001) Power spectral components of heart rate variability in different types of cardiac remodelling in hypertensive patients. Med SciMonit 7: 58-63.
- Singh JP, Larson MG, Tsuji H, Evans JC, O'Donnell CJ, et al. (1998) Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension 32: 293-297.
- Baselli G, Cerutti S, Civardi S, Lombardi F, Malliani A, et al. (1987) Heart rate variability signal processing: a quantitative approach as an aid to diagnosis in cardiovascular pathologies. Int J Biomed Comput 20: 51-70.
- Ho YL, Lin C, Lin YH, Lo MT (2011) The prognostic value of non-linear analysis of heart rate variability in patients with congestive heart failure--a pilot study of multiscale entropy. PLoS One 6: e18699.
- Wu GQ, Arzeno NM, Shen LL, Tang DK, Zheng DA, et al. (2009) Chaotic signatures of heart rate variability and its power spectrum in health, aging and heart failure. PLoS One 4: e4323.
- Haley RW, Charuvastra EH (2012) Cholinergic Autonomic Dysfunction in Veterans with Gulf War Illness: Confirmation in a Population-Based Sample.
- Staud R (2011) Brain imaging in fibromyalgia syndrome. ClinExpRheumatol 29: S109-117.
- Henry DE, Chiodo AE, Yang W (2011) Central nervous system reorganization in a variety of chronic pain states: a review. PM R 3: 1116-1125.
- Clauw DJ, Arnold LM, McCarbergBH; FibroCollaborative (2011) The science of fibromyalgia. Mayo ClinProc 86: 907-911.
- Petersel DL, Dror V, Cheung R (2011) Central amplification and fibromyalgia: disorder of pain processing. J Neurosci Res 89: 29-34.
- Tang S, Calkins H, Petri M (2004) Neurally mediated hypotension in systemic lupus erythematosus patients with fibromyalgia. Rheumatology (Oxford) 43: 609-614.
- Naschitz JE, Rozenbaum M, Rosner I, Sabo E, PriselacRM, et al. (2001) Cardiovascular response to upright tilt in fibromyalgia differs from that in chronic fatigue syndrome. J Rheumatol 28: 1356-1360.
- Bou-Holaigah I, Calkins H, Flynn JA, Tunin C, Chang HC, et al. (1997) Provocation of hypotension and pain during upright tilt table testing in adults with fibromyalgia. ClinExpRheumatol 15: 239-246.
- Raj SR, Brouillard D, Simpson CS, HopmanWM, Abdollah H (2000) Dysautonomia among patients with fibromyalgia: a noninvasive assessment. J Rheumatol 27: 2660-2665.
- Schwarz MJ, Offenbaecher M, Neumeister A, Ackenheil M (2003) Experimental evaluation of an altered tryptophan metabolism in fibromyalgia. AdvExp Med Biol 527: 265-275.
- Jasmin L, Boudah A, Ohara PT (2003) Long-term effects of decreased noradrenergic central nervous system innervation on pain behavior and opioid antinociception. J Comp Neurol 460: 38-55.
- Larson AA, Giovengo SL, Russell IJ, Michalek JE (2000) Changes in the concentrations of amino acids in the cerebrospinal fluid that correlate with pain in patients with fibromyalgia: implications for nitric oxide pathways. Pain 87: 201-211.
- Budai D, Larson AA (1998) The involvement of metabotropic glutamate receptors in sensory transmission in dorsal horn of the rat spinal cord. Neuroscience 83: 571-580.
- GoettlVM, Larson AA (1996) Nitric oxide mediates long-term hyperalgesic and antinociceptive effects of the N-terminus of substance P in the formalin assay in mice. Pain 67: 435-441.
- Alexander GM, Reichenberger E, Peterlin BL, PerreaultMJ, Grothusen JR, et al. (2013) Plasma amino acids changes in complex regional pain syndrome. Pain Res Treat 2013: 742407.
- Fernstrom JD (1977) Effects on the diet on brain neurotransmitters. Metabolism 26: 207-223.
- Choi S, DiSilvio B, Fernstrom MH, Fernstrom JD (2011) Effect of chronic protein ingestion on tyrosine and tryptophan levels and catecholamine and serotonin synthesis in rat brain. NutrNeurosci 14: 260-267.
- Arnold LM, Russell IJ, DiriEW, DuanWR, Young JP Jr, et al. (2008) A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain 9: 792-805.
- Arnold LM, Rosen A, Pritchett YL, D'Souza DN, Goldstein DJ, et al. (2005) A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain 119: 5-15.
- Clauw DJ, Mease P, Palmer RH, GendreauRM, Wang Y (2008) Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. ClinTher 30: 1988-2004.
- MeasePJ, Clauw DJ, GendreauRM, RaoSG, Kranzler J, et al. (2009) The efficacy and safety of milnacipran for treatment of fibromyalgia. a randomized, double-blind, placebo-controlled trial. J Rheumatol 36: 398-409.
- Rico-Villademoros F, Slim M, Calandre EP (2015) Amitriptyline for the treatment of fibromyalgia: a comprehensive review. Expert Rev Neurother 15: 1123-1150.
- Shell WE, Charuvastra EH, DeWood MA, May LA, Bullias DH, et al. (2012) A double-blind controlled trial of a single dose naproxen and an amino acid medical food theramine for the treatment of low back pain. Am J Ther 19: 108-114.
- Shell WE, Pavlik S, Roth B, Silver M, Breitstein ML, et al. (2014) Reduction in Pain and Inflammation Associated With Chronic Low Back Pain With the Use of the Medical Food Theramine. Am J Ther .
- Shell WE, May LA, Bullias DH, Pavlik SL, Silver DS (2012) Sentra PM (a Medical Food) and Trazodone in the Management of Sleep Disorders. J Cent NervSyst Dis 4: 65-72.
- Shell WE, Charuvastra M, Breitstein M, Pavlik S, Charuvastra A, et al. (2014) Administration of an Amino Acid-Based Regimen for the Management of Autonomic Nervous System Dysfunction Related to Combat Induced Illness. Journal of Central Nervous System Disease 6: 93-98.
- Yunus MB, AldagJC (2012) The concept of incomplete fibromyalgia syndrome: comparison of incomplete fibromyalgia syndrome with fibromyalgia syndrome by 1990 ACR classification criteria and its implications for newer criteria and clinical practice. J ClinRheumatol 18: 71-75.
- McBeth J, MulveyMR (2012) Fibromyalgia: mechanisms and potential impact of the ACR 2010 classification criteria. Nat Rev Rheumatol 8: 108-116.










