Helena Mörse1, Ingrid Øra1, Aleksandra Turkiewicz2, Claus Yding Andersen3, Charlotte Becker4, Anders Isaksson4 and Maria Elfving5
Department of Pediatrics, Pediatric Oncology and Hematology, Clinical Sciences, Lund University, Sweden
Clinical Epidemiology Unit, Orthopedics, Clinical Sciences Lund, Lund University, Sweden
Laboratory of Reproductive Biology, Rigshospitalet, University of Copenhagen, Denmark
Department of Laboratory Medicine, Skåne University Hospital, Lund University, Malmö, Sweden
Infectious diseases department, National Medical Center "La Raza", IMSS, Mexico City, Mexico
*Corresponding Author:
Helena Morse
Department of Pediatrics, Pediatric Oncology and Hematology
Clinical Sciences, Lund University, Lasarettsgatan 40, SE-221 85 Lund
Sweden
Tel: +46 46 178281
Fax: +46 46 130573
E-mail: helena.morse@med.lu.se
Received date: January 29, 2016; Accepted date: February 15, 2016; Published date: February 18, 2016
Citation: Mörse H, Ora I, Turkiewicz A, et al. Reliability of AMH in Serum after Long-term Storage at -80°C and an Extended Thawing Episode. Ann Clin Lab Res. 2016, 4:1.
Keywords
Anti-Müllerian hormone, Immunoassay, Sample storage, Thawing, Freezing
Abbreviations
AMH: Anti-Müllerian Hormone, FSH: Follicle Stimulating Hormone, LH: Luteinizing Hormone, ELISA: Enzyme-Linked Immunosorbent Assay
Introduction
Anti-Müllerian hormone (AMH) is produced by granulosa cells of small growing ovarian follicles and has been shown to affect follicular growth and development as well as production of steroid hormones [1-3]. Serum AMH levels reflect the number of small growing follicles, and several studies have shown that AMH correlates well with antral follicle count assessed via transvaginal ultrasound [4]. During recent years, it has become routine practice to measure circulating anti-Müllerian hormone (AMH) levels to follow the ovarian reserve.
Measurement of AMH levels was facilitated by the development of sensitive enzyme-linked immunosorbent assays, ELISAs [5-7], where the first commercially available assay had a lower limit of detection of 0.1 ng/mL (IOT assay, merchandized by Beckman Coulter) [8]. Subsequently, two more sensitive assays from Diagnostic System Lab (DSL) and an improved ELISA assay from Beckman Coulter (AMH Gen II, using the same antibodies as DSL) were introduced. In 2012 a fourth commercially available AMH assay was introduced; the AMH ELISA, AL-105-I, Ansh Labs Houston, TX, USA with a lower limit of detection of 0.023 ng/mL.
Due to lack of standard reference material for AMH, the AMH assays show a consistent difference in results. The Gen II assay gave 22-40% higher results than the DSL assay did in two studies [9,10], whereas another study showed 20% lower values for the Gen II assay versus the DSL assay [11]. The correlation, however, was very good in both assays. These findings questioned the robustness of the AMH assays in different settings. Furthermore, it has been suggested that AMH assays may exhibit pre-analytical variability with probable influence on sample handling in the laboratory and perhaps also on long-term stability [12]. Until now, few studies have been conducted on the impact of longterm freezer storage on AMH results [12]. The influence on AMH results after intermittent thawing-which is often required in long-term studies-has also been discussed, as analytical methods improve and change over time [13,14].
Our own experience of a mishap with a freezer led us to conduct the current study. The aim of this study was to test the robustness of AMH levels in a clinical setting after long-term storage of serum samples at -80°C and to investigate the potential influence of a period of thawing.
Material and Methods
Serum samples from 35 young female patients (0.3-16.5 years) were prospectively collected. All females were diagnosed with cancer or hematological disease during 2007-2010 and treated at the Department of Pediatric Oncology and Hematology, Skåne University Hospital, Sweden. Blood from a central venous catheter was collected for both serum and Li-heparin plasma at diagnosis and approximately once every three months during, and regularly up to three years after cessation of treatment. The samples were transported to the laboratory, centrifuged, aliquoted and stored at -80°C. The first samples were collected in February 2007 and the last in December 2010. Altogether 188 samples were available, i.e. an average of 5 serum samples from each patient.
The 188 serum samples were analyzed for the first time in May 2011. Samples were assayed with the DSL-10-14400, Diagnostic System Laboratories Inc., Webster, TX, USA, (DSL-AMH) according to the manufacturer’s instructions. The lower limit of detection was 0.04 ng/mL [15].
After storage at -80°C, most of the samples were exposed to an accidental period of thawing up to 11°C for a period of up to 21 days and then refrozen again in April 2013. The same 188 serum samples (new aliquots) were again analyzed for AMH in May 2014. At that time, as the DSL-AMH method was no longer available, the samples were reanalyzed with AMH ELISA, AL-105-I, Ansh Labs Houston, TX, USA (Ansh-AMH), also according to the manufacturer’s instructions and with a lower limit of detection of 0.023 ng/mL. Figure 1 shows the flowchart of these events.
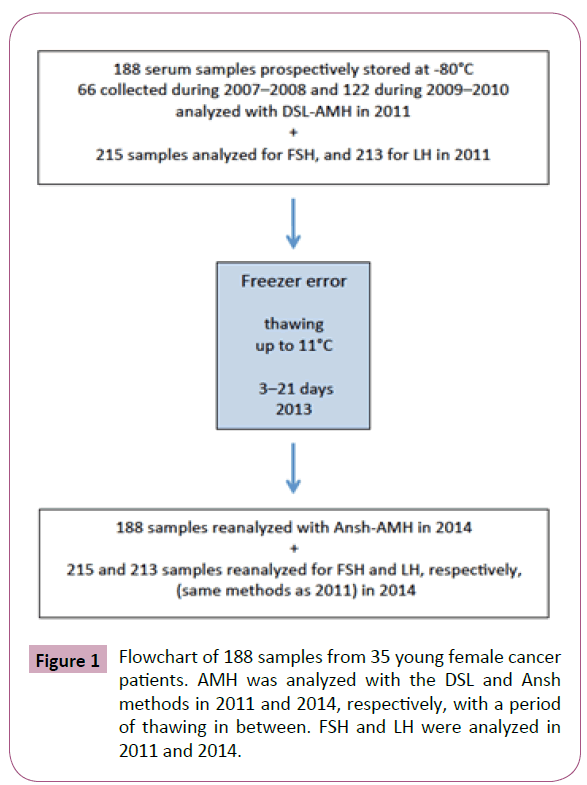
Figure 1: Flowchart of 188 samples from 35 young female cancer patients. AMH was analyzed with the DSL and Ansh methods in 2011 and 2014, respectively, with a period of thawing in between. FSH and LH were analyzed in 2011 and 2014.
All AMH analyses were performed at the Laboratory of Reproductive Biology, Copenhagen, Denmark. Interassay coefficients of variation of two independent samples were 4.6% and 8.0% (N=8).
Written informed consent was obtained from each patient and/ or her parents prior to inclusion in the study. The study was approved by the Regional Ethics Committee, Medical Faculty, Lund University, Sweden (approval number 211/2006).
To provide additional data for comparison on long-term stability, FSH and LH were analyzed in 215 and 213 paired samples, respectively. One aliquot was analyzed in 2011 and the other in 2014 at the Department of Clinical Chemistry, Skåne University Hospital, Lund, Sweden, all with the above thawing episode in between. The assays used for FSH and LH (Roche Diagnostics, Mannheim, Germany) had a lower limit of detection in both assays of 0.1 IU/L. All analyses were performed on thawed plasma/serum samples.
A thawing experiment on serum samples from 10 pre- or postmenopausal female patients was conducted. All patients had detectable AMH values. Six same-sample aliquots per patient were placed in a -80°C freezer. Five aliquots from each patient were thawed up to 11°C for 1, 3, 7, 14 and 21 days, respectively, and then refrozen again prior to analysis with the Ansh-AMH assay.
All statistical analyses were performed in STATA version 13, and the figures were created in GraphPad Prism version 5.01. We calculated the correlation, agreement, and limits of agreement for the AMH values of the 188 serum samples before and after the thawing episode, in the thawing experiment, and for the FSH and LH measurements [16]. In the analysis of agreement we used logarithmic transformation to stabilize variance. We excluded the values under the detection level from the analysis of agreement, and present those separately. The correlation coefficient was calculated using Spearman’s correlation coefficient.
Results
Altogether 188 serum samples were analyzed for AMH at two time points, in 2011 and in 2014. The analysis showed that 75 samples had undetectable AMH values when measured at both time points with DSL-AMH and Ansh-AMH, respectively. Undetectable values are explained by the toxic effect on the ovaries from treatment with chemotherapy and/or radiotherapy. Nine serum samples had a value above the detection limit with one of the methods but not with the other, which mainly reflects the different detection levels of the assays.
In 104 serum samples with detectable AMH levels with both methods, the Ansh-AMH analyzed in 2014 showed average values 1.6 times higher than the DSL-AMH analysis in 2011 (limits of agreement 0.9-3.0) and r=0.96 as shown in Figure 2A. Similar results were obtained for the 36 serum samples from 2007-2008 stored two years longer compared with the 68 serum samples stored in 2009-2010 (1.7 times higher, limits of agreement 0.9- 2.9, compared with 1.6 times higher, limits of agreement 0.9-3.0, respectively). The correlation coefficient was r=0.97 between the Ansh-AMH and the DSL-AMH in the 2007-2008 samples, and 0.96 in the 2009-2010 samples (Figure 2B and 2C).
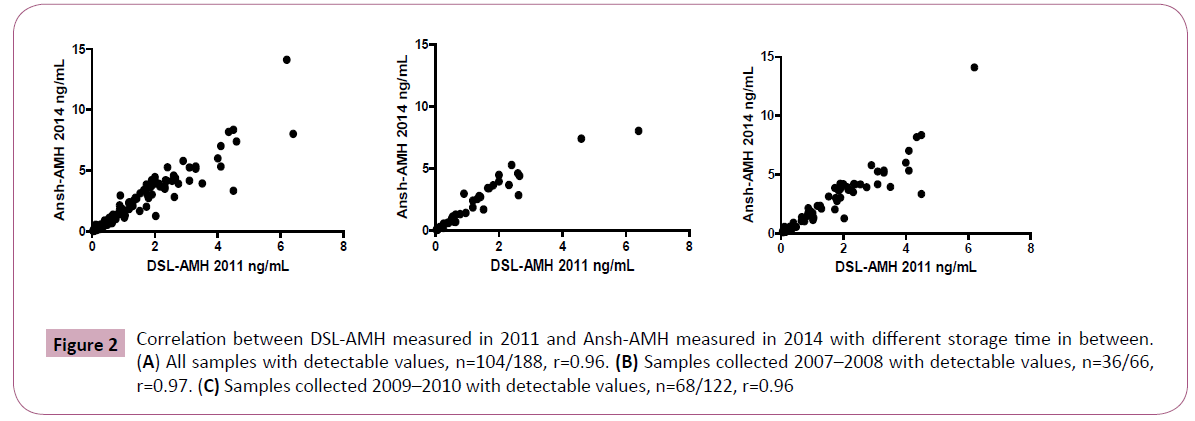
Figure 2: Correlation between DSL-AMH measured in 2011 and Ansh-AMH measured in 2014 with different storage time in between. (A) All samples with detectable values, n=104/188, r=0.96. (B) Samples collected 2007–2008 with detectable values, n=36/66, r=0.97. (C) Samples collected 2009–2010 with detectable values, n=68/122, r=0.96
For comparison, we studied levels of FSH and LH in 215 and 213 paired samples, respectively, that were analyzed at the same time points, 2011 and 2014, i.e. before and after the freezer error with the same analytical methods (Figure 1). We found good overall agreement for FSH and LH values between the two time points, 0.97 (limits of agreement 0.85 to 1.10) and 0.95 (limits of agreement 0.79 to 1.10), with a correlation coefficient of r=0.98 and 0.94, respectively (Figure 3A and 3B). In the experiment with serum samples exposed to thawing for up to 11°C for 1-21 days, the AMH levels remained unaffected (Figure 4). The limits of agreement with the reference sample (no thawing) were all close to 1 (between 0.97 and 1.04).
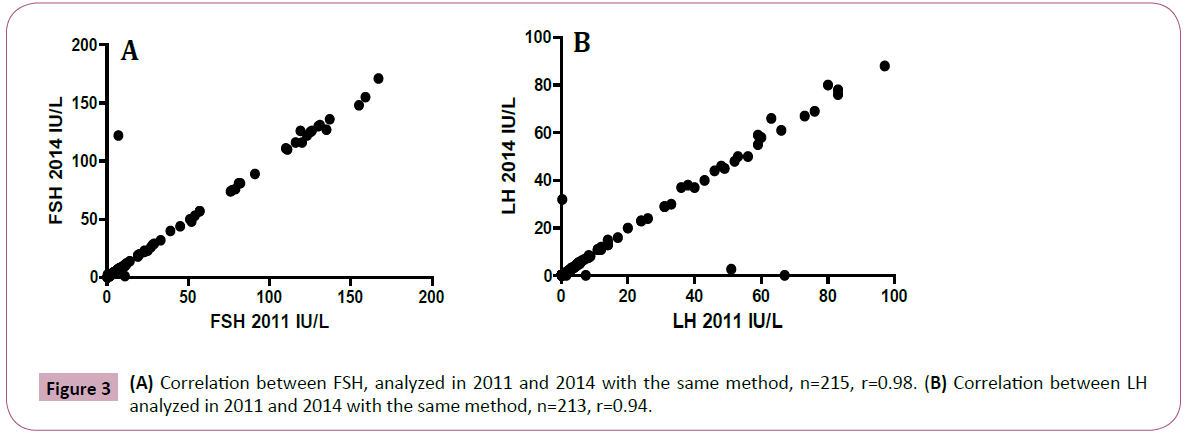
Figure 3: (A) Correlation between FSH, analyzed in 2011 and 2014 with the same method, n=215, r=0.98. (B) Correlation between LH analyzed in 2011 and 2014 with the same method, n=213, r=0.94.
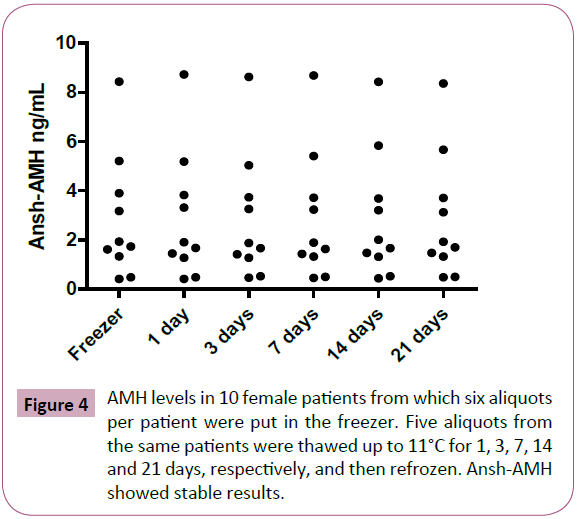
Figure 4: AMH levels in 10 female patients from which six aliquots per patient were put in the freezer. Five aliquots from the same patients were thawed up to 11°C for 1, 3, 7, 14 and 21 days, respectively, and then refrozen. Ansh-AMH showed stable results.
Discussion
Longitudinal clinical laboratory studies may encounter challenges such as change of analysis methods and sometimes repeated freeze-thaw cycles. A period of thawing for up to 21 days did not affect the AMH results in our own experimental study. In our longitudinal study we observed negligible effects on AMH levels when long-term storage at -80°C was extended for an additional two years. A change of AMH assays during the investigation period constitutes a special challenge as an international AMH standard is lacking.
This study is one of few dealing with AMH reliability after longterm storage in a freezer. Rey et al. found in a study from 1993 that AMH values analyzed with an “in-house assay” increased by 230% after a few weeks in a -20°C freezer, probably due to proteolysis [17]. In 2013, Beckman Coulter reported in a Field Safety Notice (FSN-20434-3) that complement factors in serum can influence measured levels of AMH due to interference. Later, two studies of short-term storage at -20 and - 80°C, respectively, showed fair stability with the Gen II AMH assay [18,19]. In 2014, Gassner et al showed reliable results with an automated AMH assay after long-term storage for up to nine months at -20°C and -80°C [14].
In the current study we compared the results from analyses with two different AMH methods on serum samples stored at -80°C subjected to a period of thawing to 11°C and with an extended storage period of two years. A similar conversion factor was observed even if the samples were stored two years longer, with 1.6-1.7 times higher values using the Ansh-AMH method compared with the DSL-AMH method. However, as the limits of agreement were wide, we cannot exclude the possibility that an extended storage time may result in a minor drift of the AMH measurements. The difference between the two AMH methods was in agreement with other studies comparing Ansh-AMH and GenII-AMH (the latter method using the same antibody as DSL-AMH), which resulted in 1.4-1.8 times higher values with Ansh-AMH [20,21]. No previous studies report conversion factors between Ansh-AMH and DSL-AMH, and as there is no international AMH standard, comparison between the different assays is difficult. Our observations of negligible influence on the AMH results using either the DSL-AMH or Ansh-AMH method after long-term storage at -80°C are in line with those of Fleming and co-workers, who reported stable levels of AMH (Gen II) even after a storage period of more than three years at -20°C [22].
Regarding long-term storage, freezer temperature might also be of importance as it was demonstrated that after storage at -20°C for five days, the AMH (Gen II) levels increased 23%, but were unchanged after storage at -80°C [11]. The influence of different freezing temperatures was not observed in the study by Gassner, although the freezing time was extended to up to nine months [14]. Whereas this might suggest that recent AMH assays are more robust, further studies are needed.
The effect of thawing on the AMH results, especially after repeated freeze-thaw cycles, was also recently discussed in the review by Rustamov et al. [12]. Few studies have been published on this matter, although the publications dealing with AMH levels in different clinical settings have increased enormously over the past 10-15 years since AMH methods became clinically available. In longitudinal studies, it is not unusual to repeat thawing and refreezing as analytical methods change and improve over time. In one study, Al-Qahtani et al demonstrated unchanged immunoreactivity after repeated freeze-thaw cycles at -80°C after short-term storage for 4 days with an in-house AMH method [13]. In another study [18], Gen II-AMH increased by 15% in serum and 5% in plasma after three cycles of freeze-thawing. In the study by Gassner in 2014, no change in AMH was demonstrated after up to three freeze-thaw cycles with an automated assay [14]. Our samples were exposed to a thawing period of up to 21 days due to a freezer error. Nevertheless, as shown by the current experiment with unchanged AMH levels for 10 serum samples thawed for up to 21 days, we conclude that the measured AMH levels in our longitudinal study were reliable. Thus, an extended thawing episode of up to 3 weeks up to 11°C had no impact on the Ansh-AMH results in our laboratory.
Sample handling procedures, such as time from collection to freezing, should also be considered, as they can differ and might influence the AMH results obtained.
Several studies examined AMH stability in unfrozen serum samples both at room temperature and in a fridge. In one study, there was an average increase of 58% in the Gen II MH levels after sample storage at room temperature for 7 days [11] in another study, the increase in Gen II AMH levels was 31% after storage at 20°C for 90 hours [23]. Kumar et al., [18] used GenII AMH analysis to demonstrate that unfrozen serum and plasma samples stored at 2°- 8°C for up to 7 days showed a 4% variation compared with samples analyzed immediately. It has been pointed out that detailed recommendations for AMH sample handling, transportation, and storage are needed [11,14]. The samples in our study were exposed to room temperature during transportation to the local laboratory and after preparation they were stored at -80°C within a few hours. This sample handling has with current knowledge probably negligible influence on the AMH results.
Conclusion
In conclusion, AMH values are robust with the assays employed in the current study, even after long-term storage and a prolonged episode of thawing. Whether the storage time is two or four years has no significant influence on the results. These data are reassuring and enable the planning of longitudinal studies that simultaneously analyze AMH for all collected serum samples.
Acknowledgement
Research Nurse Ingrid Hagelin is gratefully acknowledged for continuous and skillful assistance and co-administration of the study
Funding
The Swedish Childhood Cancer Foundation, The Swedish Cancer Society, Skåne University Hospital Donation Fund, the Regional and Governmental funds for clinical research (FoU, ALF), the Danish Cancer Society (DP05112/ R2-A41-09-S2) and the Danish Medical Research Council (271-07-0452; 09-072265) have supported the study. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
8646
References
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, et al. (2014) The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update 20: 370-385.
- Eilso Nielsen M, Rasmussen IA, Fukuda M, Westergaard LG, Yding Andersen C (2010) Concentrations of anti-Mullerian hormone in fluid from small human antral follicles show a negative correlation with CYP19 mRNA expression in the corresponding granulosa cells. MolHum Rep 16:637-643.
- Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, et al. (2013) Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. MolHumRep19:519-527.
- Hansen KR, Hodnett GM, Knowlton N, Craig LB (2011) Correlation of ovarian reserve tests with histologically determined primordial follicle number. FerSter95:170-175.
- Baker ML, Metcalfe SA, Hutson JM (1990) Serum levels of mullerian inhibiting substance in boys from birth to 18 years, as determined by enzyme immunoassay. Clin Endo Met 70:11-15.
- Hudson PL, Dougas I, Donahoe PK, Cate RL, Epstein J, et al. (1990) An immunoassay to detect human mullerian inhibiting substance in males and females during normal development. Clin Endo Met70: 16-22.
- Josso N, Legeai L, Forest MG, Chaussain JL, Brauner R (1990) An enzyme linked immunoassay for anti-mullerian hormone: a new tool for the evaluation of testicular function in infants and children. Clin Endo Met70:23-27.
- Long WQ, Ranchin V, Pautier P, Belville C, Denizot P, et al. (2000) Detection of minimal levels of serum anti-Mullerian hormone during follow-up of patients with ovarian granulosa cell tumor by means of a highly sensitive enzyme-linked immunosorbent assay. Clin Endo Met85: 540-544.
- Wallace AM, Faye SA, Fleming R, Nelson SM (2011) A multicentre evaluation of the new Beckman Coulter anti-Mullerian hormone immunoassay (AMH Gen II). Ann Clin Bio48:370-373.
- Li HW, Ng EH, Wong BP, Anderson RA, Ho PC, et al. (2012) Correlation between three assay systems for anti-Mullerian hormone (AMH) determination. Ass RepGen 29:1443-1446.
- Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, et al. (2012) Anti-Mullerian hormone: poor assay reproducibility in a large cohort of subjects suggests sample instability. Hum Rep 27:3085-3091.
- Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, et al. (2014) The measurement of anti-M├╝llerian hormone: a critical appraisal. J ClinEndocrinolMetab 99: 723-732.
- Al-Qahtani A, Muttukrishna S, Appasamy M, Johns J, Cranfield M, et al. (2005) Development of a sensitive enzyme immunoassay for anti-Mullerian hormone and the evaluation of potential clinical applications in males and females. ClinEndo 63:267-273.
- Gassner D, Jung R (2014) First fully automated immunoassay for anti-M├╝llerian hormone. ClinChem Lab Med 52: 1143-1152.
- Morse H, Elfving M, Lindgren A, Wolner-Hanssen P, Andersen CY, et al. (2013) Acute onset of ovarian dysfunction in young females after start of cancer treatment. Pedcan 60:676-681.
- Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8: 135-160.
- Rey R, Lordereau-Richard I, Carel JC, Barbet P, Cate RL, et al. (1993) Anti-mullerian hormone and testosterone serum levels are inversely during normal and precocious pubertal development. ClinEndo Met 77:1220-1226.
- Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE (2010) Development of a second generation anti-Mullerian hormone (AMH) ELISA. Imm Meth 362:51-59.
- Han X, McShane M, Sahertian R, White C, Ledger W (2014) Pre-mixing serum samples with assay buffer is a prerequisite for reproducible anti-Mullerian hormone measurement using the Beckman Coulter Gen II assay. Hum Repr29:1042-1048.
- Su HI, Sammel MD, Homer MV, Bui K, Haunschild C, et al. (2014) Comparability of antimullerian hormone levels among commercially available immunoassays. FerSter101:1766-1772.
- Welsh P, Smith K, Nelson SM (2014) A single-centre evaluation of two new anti-Mullerian hormone assays and comparison with the current clinical standard assay. Hum Rep 29:1035-1041.
- Fleming R, Nelson SM (2012) Reproducibility of AMH. Hum Reprod 27: 3639-3641.
- Fleming R, Fairbairn C, Blaney C, Lucas D, Gaudoin M (2013) Stability of AMH measurement in blood and avoidance of proteolytic changes. Reprod Biomed Online 26: 130-132.










