Keywords
Remifentanil; CPB; Inflammation; Cardiac surgery
Introduction
The obligatory systemic inflammatory response to cardiac surgery with cardiopulmonary bypass (CPB) has been associated with significant perioperative and long-term morbidity and mortality. Cardiac surgery with CPB initiates a profound systemic inflammatory response, characterized by increased level of inflammatory mediators and oxidative stress mediators which have been shown to be correlated with the incidence of organ dysfunction and adverse clinical outcome [1]. The proinflammatory mediators such as tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 are associated with anti-inflammatory mediators such as IL-10 and TGF-β [2]. However, the net effect of these circulating inflammatory mediators seems to be distorted as inhibition of innate immune cells, the molecular and cellular mechanisms responsible for suppression of the immune system after cardiac surgery with CPB [3]. In addition, generation of reactive oxygen species (ROS) such as hydrogen peroxide, superoxide and malondialdehyde occurs upon reperfusion following CPB and these may be important contributors to tissue injury [4]. Furthermore, post-CPB coronary endothelial dysfunction appears to be partially mediated by ROS [5]. Opioids have been widely used as anesthetic agents for various types of surgery including cardiac surgery. Several studies found opioid preconditioning had a protective effect on the postischemic heart [6-8]. Also, exogenous activation of μ-opioid receptor has been shown to ameliorate inflammation in experimental colitis [9], supporting the concept that μ-opioid receptor agonists might act as regulatory modulators of gut inflammatory processes. However, no study has examined the direct role of opioids in the expression of pro-inflammatory mediators (including IL-6, and IL- 8) and malondialdehyde (MDA) in cardiac surgery with CPB. These observations provided the background for our hypothesis that exogenous opioids might attenuate the inflammatory response induced by cardiac surgery with CPB. To test our hypothesis, we used fentanyl and remifentanil as anesthetic agents for cardiac surgery with CPB. The aim of our study was to investigate the effect of exogenous administration of opioids on the systemic inflammatory response induced by cardiac surgery with CPB. We assessed the changes of pro-inflammatory mediators including IL-6, and IL-8, oxidative stress mediator (MDA) and myocardial damage markers such as cardiac troponin T and creatine kinase MB in the patients undergoing cardiac surgery with CPB.
Materials and Methods
Patients and study protocol
The study was approved by Chonnam National University Hospital’s Institutional Review Board and written informed consent was obtained. This prospective, randomized study was performed on 60 patients undergoing elective valve replacement using CPB. Patients with ASA classification more than 4, coronary disease requiring surgical revascularization, unstable cardiac function with the need for medical or mechanical inotropic supports, severe hepatic or renal disease, malignancy, preexisting lung parenchymal disease and acute inflammatory response were excluded. Patients were randomly divided into either the fentanyl group (n=30) who received fentanyl for anesthetic induction (3-10 μg/kg) and maintenance (0.03-0.1 μg/kg/min), and the remifentanil group (n=30) who received remifentanil for anesthetic induction (0.5-1.0 μg/kg) and maintenance (0.05-0.1 μg/kg/min). Preanesthetic medication included midazolam (0.1 mg/kg, PO) and famotidine (0.3 mg/kg, IV). Anesthesia was induced with midazolam (0.05-0.15 mg/kg, IV), fentanyl or remifentanil and tracheal intubation were facilitated with rocuronium (0.8 mg/kg). Patients were mechanically ventilated with 50% oxygen with air to maintained normocarbic (PaCO2 35 ± 5 mmHg). Anesthesia was maintained with sevoflurane (0.5-1 vol%), and fentanyl or remifentanil. The cardiac surgeon and anesthesiologists were blinded to group assignment. CPB was established using a twostage venous drainage and ascending aortic return. After administration of heparin (300 IU/kg), standard CPB was started with the priming volume. Body temperature was maintained under mild hypothermia (32-33) with cold blood cardioplegic solution. Pump flow rate was maintained at 2.0-2.5 L/min/m2 of body surface area with mean arterial blood pressure of 50-80 mmHg using non-pulsatile flow. All patients underwent continuous monitoring with radial artery and pulmonary artery catheters about hemodynamic variables such as mean arterial blood pressure, heart rate, mean pulmonary artery pressure, central venous pressure, pulmonary capillary wedge pressure, systemic vascular resistance and cardiac index were measured continuously. Blood was sampled from the radial artery at the following points: preinduction (T1), just before aortic clamping (T2), just before aortic declamping (T3), 5 (T4), 30 (T5), and 60 (T6) minutes (min) after aortic declamping. Myocardial cell damage as assessed by plasma level of troponin T and creatine kinase-MB (CK-MB) were measured before and 24 hours after surgery.
Determination of cytokines
Sampled blood was centrifuged at 3000 g for 10 min, and the serum was separated and stored at -80 until assayed. In each sample, immunoreactive IL-6, and IL-8 were quantified using commercially available enzyme-linked immunoabsorbant assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) in accordance with the manufacturer’s instructions and as described previously [10].
Determination of malondialdehyde (MDA)
Serum MDA levels, referred to as thiobarbituric acid-reactive substance (TBARS), were measured according to the method described previously [11]. A volume sample of 250 μl of plasma was added to 35 μl of D/W and the color reaction was initiated by the addition of 25 μl of sodium dodecyl sulfate (SDS) (8.1%, W/V) and 190 μl of acetic acid (20%) in thiobarbituric acid (0.8%, W/V). The mixture was heated in a boiling water bath for 40 min, until adducts were formed. After the samples were cooled, the TBARS (pink complex color) were extracted with 0.6 ml of nbutanol: Prydine (15:1). Butanal phase was separated by centrifugation at 12000 rpm for 10 min. Aliquots of the nbutanol phase were placed in a 96 well plate and read at 532 nm in a microplate spectrophotometer reader.
Determination of troponin T and creatine kinase with muscle and brain subunits (CK-MB)
Serum troponin T concentration was determined by the Enzyme Immunoassay Method (Enzyme UN-Test Troponin T; Roche Diagnostics, Tokyo, Japan). Normal values were < 0.25 ng/mL. The limit of detection was 0.11 ng/mL. Serum isoenzyme of CK-MB concentration was determined by the ultraviolet absorption spectrophotometry method (Merck auto CK-MB; Kanto Chemical, Tokyo, Japan). Normal values were <25 IU/L. The limit of detection was 0 IU/L.
Statistical analysis
Sample size was calculated using cardiac troponin levels at 24 hours after surgery as the primary outcome. A minimum difference of 0.5 ng/mL between groups was considered clinically significant. Based on the institutional result for patients who underwent cardiac surgery, the standard deviation of troponin level was 0.5. A sample size of at least 27 in each group was required to achieve a power 0.95 and a 2-side α error of 0.05. Considering the 10% of drop-out rate, 30 patients in each group were enrolled. All statistical analyses were performed using SPSS (SPSS Inc., Chicago, IL, USA). Data were presented as the number of patients or the mean ± SD. Normal distribution of the data was determined by Shapiro-Wilk test. Demographic and surgical data were analyzed by Mann-Whitney test. Change of cytokines, MDA, cardiac enzymes and hemodynamic variables at different time points were evaluated using repeated measure ANOVA with Tukey post hoc test for normally distributed variables and Friedman test with Duncan post hoc test for nonnormally distributed variables. P<0.05 was considered statistically significant.
Results
Demographic and surgical data were not different between the two groups (Table 1). The hemodynamic variables such as MAP, HR, CI, PCWP and SVRI were similar in two groups during the study period (Table 2). The required amount of inotropics, vasopressors and the number of units of blood products transfused per transfused patients were not statistically different between groups (not shown).
| |
Fentanyl (n=30) |
Remifentanil (n=30) |
| Sex (M/F) |
14/16 |
15/15 |
| Age (yr) |
55 ± 12 |
57 ± 11 |
| Body weight (kg) |
64.4 ± 10.7 |
60.1 ± 12.1 |
| Height (cm) |
163.5 ± 8.7 |
168.1 ± 7.1 |
| Diabetes mellitus (n) |
5 |
7 |
| Hypertension (n) |
11 |
12 |
| Operation |
| MVR |
16 |
12 |
| AVR |
11 |
14 |
| DVR |
3 |
2 |
| Pre-operative LVEF (%) |
|
|
| Medications |
| ACE inhibitors (n) |
10 |
11 |
| Beta blocker (n) |
5 |
5 |
| Calcium channel blocker (n) |
11 |
12 |
| Diuretics (n) |
17 |
18 |
| Statins (n) |
13 |
15 |
| Duration of CPB (min) |
122 ± 28.2 |
112 ± 36.5 |
| Duration ACC (min) |
84 ± 44.8 |
74 ± 41.7 |
Values are means ± SD or absolute numbers. MVR: mitral valve replacement; AVR: aortic valve replacement; DVR: double valve replacement; BW: body weight; LVEF: left ventricular ejection fraction; CPB: cardiopulmonary bypass; ACC: aortic cross clamp.
Table 1: Demographic and surgical data.
| |
T1 |
T2 |
T3 |
T4 |
T5 |
T6 |
| MAP (mmHg) |
F |
86.2 ± 16.6 |
69.7 ± 9.2 |
69.7 ± 7.3 |
75.9 ± 16.5 |
67.7 ± 6.8 |
62.8 ± 4.3 |
| R |
93.4 ± 13.3 |
77.7 ± 15.6 |
62.5 ± 8.3 |
65.3 ± 12.7 |
75.3 ± 11.4 |
73.9 ± 17.5 |
| HR (beats/min) |
F |
84.5 ± 21.3 |
78 ± 15.5 |
- |
104.8 ± 34.7 |
101.3 ± 20.2 |
107.6 ± 12.4 |
| R |
81.8 ± 16.3 |
83.6 ± 16.8 |
- |
111.3 ± 33.8 |
106.9 ± 25.1 |
108.3 ± 21.8 |
| CI (L/m2/min) |
F |
3.2 ± 0.9 |
3.1 ± 0.8 |
- |
2.9 ± 0.7 |
3 ± 0.9 |
2.9 ± 0.7 |
| R |
3.1 ± 0.8 |
3.3 ± 0.9 |
- |
3.2 ± 0.8 |
3.1 ± 0.8 |
3 ± 0.6 |
| PCWP (mmHg) |
F |
24.1 ± 9.9 |
23.7 ± 1.8 |
- |
22.9 ± 2.2 |
20 ± 2.1 |
22.5 ± 2.2 |
| R |
23.3 ± 3.1 |
25.5 ± 3.5 |
- |
25 ± 4.4 |
22.2 ± 3.1 |
19.3 ± 2.1 |
| SVRI (dynes.sec/cm5/m2) |
F |
2980 ± 546 |
2664 ± 401 |
- |
2484 ± 372 |
2571 ± 394 |
2762 ± 443 |
| R |
2957 ± 356 |
2872 ± 338 |
- |
2379 ± 315 |
2487 ± 414 |
2581 ± 455 |
Values represent means ± SD. F: fentanyl group (n=30); R: remifentanil group (n=30); MAP: mean arterial pressure; HR: heart rate; CI: cardiac index; PCWP: pulmonary capillary wedge pressure; SVRI: systemic vascular resistance index; T1: preinduction; T2: just before aortic clamping; T3: just before aortic declamping; T4: 5 min after aortic declamping; T5: 30 min after aortic declamping; T6: 60 min after aortic clamping. *: p<0.05 versus T1 values, **: p<0.05 versus Fentayl.
Table 2: Hemodynamic data.
The pattern of change in the serum IL-6, IL-8 and MDA level within both groups was similar in the point of significant increase just before aortic declamping compared with preinduction (P<0.001). There were significant differences between both groups just before aortic declamping: In the remifentanil group, the serum IL-6, IL-8 and MDA level were measured at significantly lower levels compared with the fentanyl group (P<0.05; Table 3, Figure 1). The serum Troponin T and CK-MB levels within both groups were significantly higher at the 24 hours after surgery compared with the preoperative baseline level (P<0.001), and were significantly lower in the remifentanil group than fentanyl group (P<0.05; Table 4).
| |
T1 |
T2 |
T3 |
T4 |
T5 |
T6 |
IL-6
(pg/ml) |
F |
1.2 ± 0.6 |
6.8 ± 1.7 |
120.9 ± 20.2* |
179.3 ± 22.1* |
234.4 ± 30.6* |
255.9 ± 31.0* |
| R |
0.7 ± 0.2 |
4.9 ± 0.7 |
14.7 ± 2.5*† |
98.6 ± 15.3*† |
158.4 ± 17.8*† |
162.3 ± 17.7*† |
IL-8
(pg/ml) |
F |
0.8 ± 0.1 |
4.9 ± 1.7 |
42.7 ± 10.9* |
54.5 ± 10.0* |
66.2 ± 10.5* |
113.4 ± 17.3* |
| R |
0.7 ± 0.4 |
1.6 ± 0.5 |
7.5 ± 2.2*† |
21.8 ± 4.9*† |
37.7 ± 4.5*† |
56.0 ± 10.8*† |
MDA
(nmol/mg) |
F |
1.2 ± 0.4 |
1.8 ± 0.5 |
2.5 ± 0.7* |
2.6 ± 0.4* |
2.6 ± 0.8* |
2.7 ± 1.0* |
| R |
1.2 ± 0.2 |
1.8 ± 0.8 |
2.0 ± 0.3*† |
2.2 ± 0.2*† |
2.2 ± 0.3*† |
2.0 ± 0.3*† |
Values are means ± SD. F: fentanyl group (n=30), R: remifentanil group (n=30), IL: interleukin, MDA: malondialdehyde. T1: preinduction, T2: just before aortic clamping, T3: just before aortic declamping, T4: 5 min after aortic declamping, T5: 30 min after aortic declamping, T6: 60 min after aortic declamping. *: p<0.05 versus T1 values, †: p<0.05 versus Fentanyl.
Table 3: The change of serum cytokine and MDA.
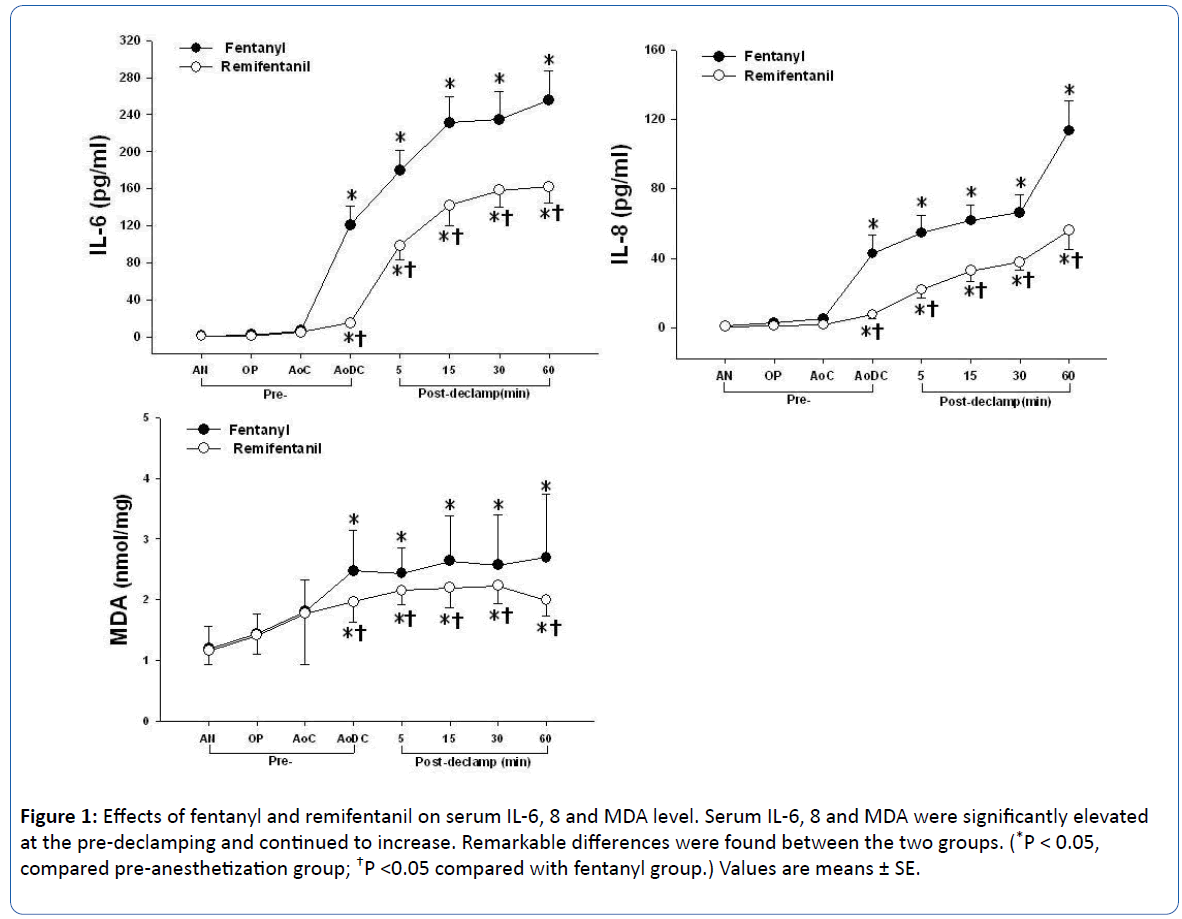
Figure 1: Effects of fentanyl and remifentanil on serum IL-6, 8 and MDA level. Serum IL-6, 8 and MDA were significantly elevated at the pre-declamping and continued to increase. Remarkable differences were found between the two groups. (*P < 0.05, compared pre-anesthetization group; †P <0.05 compared with fentanyl group.) Values are means ± SE.
| |
Fentanyl (n = 30) |
Remifentanil (n = 30) |
| Troponine T (ng/ml) |
| Pre-OP. |
0.05 ± 0.0 |
0.05 ± 0.0 |
| Post-OP. |
1.01 ± 0.97* |
0.4 ± 0.31*† |
| CK-MB (U/L) |
| Pre-OP. |
9.0 ± 1.3 |
8.0 ± 1.5 |
| Post-OP. |
50.0 ± 1.6* |
37.0 ± 13.7*† |
Values are means ± SD. CK-MB: creatine kinase with muscle and brain subunits.
*: p< 0.05 versus Pre-Op. †: p < 0.05 versus Fentanyl.
Table 4: The change of serum troponin T and CK-MB.
Discussion
This study evaluated the effects of remifentanil and fentanyl anesthesia on pro-inflammatory cytokines, oxidative stress mediator and myocardial damage markers induced by cardiac surgery using CPB. The remifentanil ameliorated IL-6, IL-8, MDA, troponin T and CK-MB more than provided by fentanyl.
Our study showed that the levels of cytokines and oxidative stress mediator such as MDA during CPB and after aortic declamping (reperfusion) were significantly higher than those pre inductions. The heart is inevitably faced with ischemia/ reperfusion injury during CPB and after aortic declamping. In addition, inflammatory reaction occurs as a result of neutrophil accumulation in the myocardium. The neutrophils that migrate to the tissue as a result of the inflammatory process initiate the tissue damage by triggering several reactions [12]. Neutrophils that accumulate in the tissue secrete myeloperoxidase, which produces hydroxychloride (HOCl). HOCl has a direct cytotoxic effect and by inactivating a 1-protease inhibitor, it participates in the production of collagenases and elastases from the neutrophils [13,14]. The neutrophils in this environment favor the secretion of proinflammatory cytokines such as IL-6, IL-8, and TNF-α [14].
Cytokines are intracellular signaling molecules and elicit important defense mechanisms. However, when the heart was exposed to inflammation and I/R injury during cardiac surgery, cytokines that enhance neutrophil accumulation in the critical organs are excessively produced, and tissues are damaged when elastase and oxygen free radicals are released from neutrophils. Consequently, oxygen free radicals and various cytokines are produced, thus exacerbating microcirculation and tissue damage [15,16]. Excessive inflammatory cytokine production activates neutrophils and enhances the expression of adhesion molecules to exacerbate microcirculation and tissue damage [17]. Therefore, it is important to remove excess amounts of cytokines and active oxygen radicals. In the present study, remifentanil inhibited the increase of IL-6, IL-8 and MDA after aortic declamping more than those of fentanyl. Although fentanyl has been previously reported to suppress oxygen free radicals and modulate pro inflammatory cytokines [18], the present study is clinically significant because it is the first to clarify the anticytokine and antioxidant activity of remifentanil in a clinical situation. The results may be attributed that remifentanil may be potentially useful therapeutic adjuncts more than fentanyl regard to anti-inflammatory response and free radical scavengers and believed to be an ideal anesthetic that can be used safely.
Opioids have been widely used as anesthetic agents for various types of surgery, including cardiac surgery. The primary actions of opioids are analgesia and sedation. In addition to their analgesic and sedative effects, opioids modulate the immune response via opioid receptors expressed directly on the immune cells themselves. Recently, it has been suggested that the kappa opioid receptor system has a modulatory role in various inflammatory diseases. The finding that opioid receptors are expressed in numerous types of immune cells [19,20] gave the first indication that opioids may have a direct effect on the immune system. Also, both endogenous and exogenous opioids alter antibody response, cell-mediated immunity, phagocytic activity, chemotaxis, and respiratory burst responses of neutrophils and mononuclear phagocytes [21,22]. Opioidinduced cardioprotection and ischemic preconditioning (IPC) seem to share a common pathway including protein kinase C and mitochondrial adenosine triphosphate–sensitive potassium (KATP) channels [23-25]. Remifentanil, an ultrashort-acting opioiod, has been shown to trigger both immediate and delayed cardiac preconditioning in the rat heart [26]. Our study demonstrated that remifentanil has more effective action than fentanyl on the decrease of cytokines and oxidative stress in the cardiac surgery with CPB. The dosages of drugs, fentanyl and remifentanil were used on the basis of clinical cardiac anesthesia [27].
The elevation of cardiac biomarkers such as CK-MB and cardiac troponin T after cardiac surgery has been shown to be a good predictor of clinical outcomes. Most patients undergoing cardiac procedures have some degree of postprocedural cardiac biomarker elevation, however, only significant elevations greater than the normal range (i.e., 10-fold) have been associated with poor outcomes [28]. In this study, both remifentanil and fentanyl attenuated the elevation of troponin T and CK-MB after cardiac surgery and the levels of postoperative cardiac enzymes in the remifentanil group were kept significantly lower compared with the fentanyl group. Similarly, Winterhalter et al. [29] reported there was a trend towards lower CK-MB level in the remifentanil group and statistically lower CK-MB and a trend towards lower troponin T level.
There are several limitations to this study. First, it was not possible to investigate control group which did not use opioid because the use of opioid is standard anesthesia in cardiac surgery. Therefore, only fentanyl and remifentanil groups were compared. Second, clinically, postoperative outcomes such as mortality, length of intensive care unit and postoperative complications were not investigated. This study was not primarily designed to assess these parameters. The intraoperative laboratory examination and hemodynamic data only were measured. It is imaginable that the benefit of laboratory data as the meaning of lower myocardial damage will be translated into better clinical outcome. Therefore, it will be important to investigate clinical possibility. Third, in the design of the experimental protocols, TNF-α was designed to be measured at the same time when cytokines were measured. But TNF-α was not found overall in some unexplained reasons.
Conclusion
In conclusion, in addition to its analgesic and sedative effects, remifentanil attenuated inflammatory cytokine (IL-6, and IL-8), oxidative stress mediator (MDA) and myocardial damage markers (Troponin T and CK-MB) and appears to be a promising and useful anesthetic agent for cardioprotection during cardiac surgery with CPB.
Financial support
This study was supported by a grant (CRI14025-1) Chonnam National University Hospital research institute of clinical medicine.
20829
References
- Mekontso-Dessap A, Houel R, Soustelle C, Kirsch M, Thebert D, et al. (2001) Risk factors for post-cardiopulmonary bypass vasoplegia in patients with preserved left ventricular function. Ann Thorac Surg 71: 1428-1432.
- Franke A, Lante W, Fackeldey V, Becker HP, Thode C, et al. (2002) Proinflammatory and antiinflammatory cytokines after cardiac operation: Different cellular sources at different times. Ann Thorac Surg 74: 363-370.
- Wilhelm W, Grundmann U, Rensing H, Werth M, Langemeyer J, et al. (1992) Monocyte deactivation in severe human sepsis or following cardiopulmonary bypass. Shock 17: 354-360.
- Barta E, Pechan I, Cornak V, Luknarova O, Rendekova V, et al. (1991) Protective effect of alpha-tocopherol and L-ascorbic acid against the ischemic-reperfusion injury in patients during open-heart surgery. Bratisl Lek Listy 92: 174-183.
- Sellke FW, Shafique T, Ely DL, Weintraub RM (1993) Coronary endothelial injury after cardiopulmonary bypass and ischemic cardioplegia is mediated by oxygen-derived free radicals. Circulation 88: 395-400.
- Kato R, Foex P (2000) Fentanyl reduces infarction but not stunning via delta-opioid receptors and protein kinase C in rats. Br J Anaesth 84: 608-614.
- Kato R, Ross S, Foex P (2000) Fentanyl protects the heart against ischaemic injury via opioid receptors, adenosine A1 receptors and KATP channel linked mechanisms in rats. Br J Anaesth 84: 204-214.
- Zhang Y, Irwin MG, Wong TM (2004) Remifentanil preconditioning protects against ischemic injury in the intact rat heart. Anesthesiology 101: 918-923.
- Philippe D, Dubuquoy L, Groux H, Brun V, Chuoi-Mariot MT, et al. (2003) Gaveriaux-Ruff C, Colombel JF, Kieffer BL, Desreumaux P. Antiinflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest 111: 1329-1338.
- Yum HK, Arcaroli J, Kupfner J, Shenkar R, Penninger JM, et al. (2001) Involvement of phosphoinositide 3-kinases in neutrophil activation and the development of acute lung injury. J Immunol 167: 6601-6608.
- Kikugawa K, Kojima T, Yamaki S, Kosugi H (1992) Interpretation of the thiobarbituric acid reactivity of rat liver and brain homogenates in the presence of ferric ion and ethylenediaminetetraacetic acid. Anal Biochem 202: 249-255.
- Hayashi Y, Sawa Y, Nishimura M, Tojo SJ, Ichikawa H, et al. (2000) P-selectin monoclonal antibody may attenuate the whole body inflammatory response induced by cardiopulmonary bypass. Asaio J 46: 334-337.
- Ossanna PJ, Test ST, Matheson NR, Regiani S, Weiss SJ (1986) Oxidative regulation of neutrophil elastase-alpha-1-proteinase inhibitor interactions. J Clin Invest 77: 1939-1951.
- Sullivan GW, Carper HT, Novick WJ, Jr., Mandell GL (1988) Inhibition of the inflammatory action of interleukin-1 and tumor necrosis factor (alpha) on neutrophil function by pentoxifylline. Infect Immun 56: 1722-1729.
- Kraemer R, Seligmann B, Mullane KM (1990) Polymorphonuclear leukocytes reduce cardiac function in vitro by release of H2O2. Am J Physiol 258: H1847-H1855.
- Suematsu M, DeLano FA, Poole D, Engler RL, Miyasaka M, et al. (1994) Spatial and temporal correlation between leukocyte behavior and cell injury in postischemic rat skeletal muscle microcirculation. Lab Invest 70: 684-695.
- Paccaud JP, Schifferli JA, Baggiolini M (1990) NAP-1/IL-8 induces up-regulation of CR1 receptors in human neutrophil leukocytes. Biochem Biophys Res Commun 166: 187-192.
- Raut A, Ratka A (2009) Oxidative damage and sensitivity to nociceptive stimulus and opioids in aging rats. Neurobiol Aging 30: 910-919.
- Doerschuk CM, Tasaka S, Wang Q (2000) CD11/CD18-dependent and -independent neutrophil emigration in the lungs: how do neutrophils know which route to take? Am J Respir Cell Mol Biol 23: 133-136.
- Rane MJ, Carrithers SL, Arthur JM, Klein JB, McLeish KR (1997) Formyl peptide receptors are coupled to multiple mitogen-activated protein kinase cascades by distinct signal transduction pathways: role in activation of reduced nicotinamide adenine dinucleotide oxidase. J Immunol 159: 5070-5078.
- Parkhill AL, Bidlack JM (2006) Reduction of lipopolysaccharide-induced interleukin-6 production by the kappa opioid U50, 488 in a mouse monocyte-like cell line. Int Immunopharmacol 6: 1013-1019.
- Rogers TJ, Peterson PK (2003) Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol 24: 116-121.
- Gross GJ (2003) Role of opioids in acute and delayed preconditioning. J Mol Cell Cardiol 35: 709-718.
- Schultz JE, Gross GJ (2001) Opioids and cardioprotection. Pharmacol Ther 89: 123-137.
- Zhang Y, Chen ZW, Girwin M, Wong TM (2005) Remifentanil mimics cardioprotective effect of ischemic preconditioning via protein kinase C activation in open chest of rats. Acta Pharmacol Sin 26: 546-550.
- Yu CK, Li YH, Wong GT, Wong TM, Irwin MG (2007) Remifentanil preconditioning confers delayed cardioprotection in the rat. Br J Anaesth 99: 632-638.
- Mehta AR, Romanoff ME, Licina MG (2013) Anesthetic Management in the Precardiopulmonary Bypass Period. In: Hensley FA, ed. A Practical Approach to Cardiac Anesthesia. 5th edn., Lippincott Williams & Wilkins, Philadelphia, pp: 179-191.
- Feldman DN, Kim L, Rene AG, Minutello RM, Bergman G, et al. (2011) Prognostic value of cardiac troponin-I or troponin-T elevation following nonemergent percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc Interv 77: 20-30.
- Winterhalter M, Brandl K, Rahe-Meyer N, Osthaus A, Hecker H, et al. (2008) Endocrine stress response and inflammatory activation during CABG surgery. A randomized trial comparing remifentanil infusion to intermittent fentanyl. Eur J Anaesthesiol 25: 326-335.







