Keywords
Retinoids; Central nervous system; Alzheimer's disease; Amyloid beta; Retinolbinding proteins; Cytosolic retinol-binding proteins.
Abbreviations
AD: Alzheimer's Disease; ATRA: All-trans Retinoic Acid; RBP: Retinol- Binding Proteins; Aβ: Amyloid Beta; CNS: Central Nervous System; RA: Retinoic Acid; LTP/ LTD: Long-Term Potentiation/Depression; IL-6: Interleukin; ChAT: Choline Acetyltransferase; SOD: Superoxide Dismutase; NF-Κb: Signaling Pathway In Inflammation; CRABP1: Cytosolic Retinol-Binding Proteins 1; RXR: Retinoid X Receptor; CRABP2: Cytosolic Retinol-Binding Proteins Cytosolic 2; Fos: Proto-Oncogene; LXRs: Liver X Receptors; PPARs: Peroxisome Proliferator-Activated Receptors; OGD: Oxygen Glucose Deprivation; PC12: Model for Neural Differentiation.
Introduction
Recent studies revealed that main cause of many neurological diseases such as AD occurrence and progression is unregulated inflammatory responses [1]. Nerve Inflammation which is known as neuroinflammation can be mediated by neurons, invading microbes such as viruses, bacteria and Amyloid beta peptides.
These processes, via microglia activation, increases bloodbrain- barrier cells permeability and immune cells chemotactic, in cerebral cortex. Of course, these processes are not only affected by microglia, but also by astrocytes, neurons, endothelial cells, cerebral blood vessels, and T cells. Retinoic acid (RA) have significant effects in numerous neuroplasticity features. Neuroplasticity is fundamental process in new memories creation, which indorsed by RA, which are long-term potentiation/depression (LTP/LTD), neurogenesis, and neuritis [2]. As RA provisions neuronal survival and neuroplasticity, is indispensable for learning and memory (Figure 1).
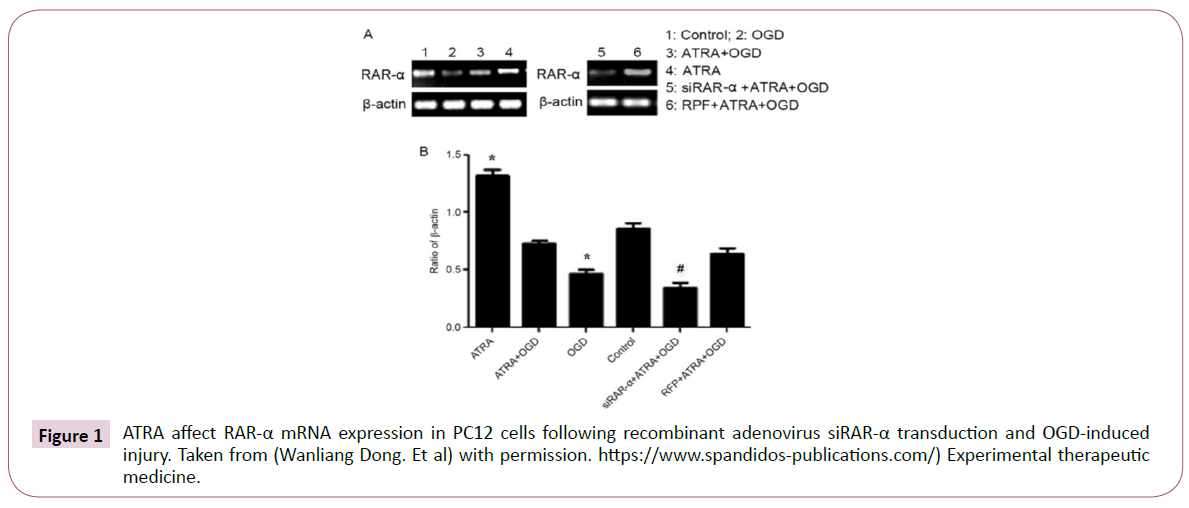
Figure 1: ATRA affect RAR-α mRNA expression in PC12 cells following recombinant adenovirus siRAR-α transduction and OGD-induced injury. Taken from (Wanliang Dong. Et al) with permission. https://www.spandidos-publications.com/) Experimental therapeutic medicine.
Studies revealed that RA decreases amyloid-β (Aβ) neurotoxicity. Moreover, there are evidences which vitamin A-deficient diet in rodent’s cause RA signaling system interruption and Aβ deposition in cerebral blood vessels, and these modifications may be conversed by RA consumption and led to cytokines synthetize repression, such as interleukin- 6 which comprised in various age related diseases and inflammatory responses. Lnterleukin 6 mRNA Levels intensified primary in the Tg2576 AD model mice hippocampus. RA also suppress microglia main functions, such as tumor necrosis factor alpha synthesis and inducible nitric oxide. These RA anti-inflammatory properties will be constructive in neurodegenerative diseases therapy.
Biochemistry of Retinoids
Retinoids are structurally and functionally analogous to vitamin A. Retinoids are also specific modulators for proliferation, differentiation and morphogenesis in vertebrates cells [3]. Vitamin A is a fat-soluble vitamin that is indispensable for health, development and epithelial tissue integrity as well as for vision, especially in low light [4].
Vitamin A has two sources: its plant origin is β- carotene and its animal origin is in the form of fatty acid ester or retinol. β- Carotene and retinoid compounds have antioxidants and anticancer properties [5].
The term retinoid consist of vitamin A-dependent compounds include retinol and its biological precursor, carotenoids, Retinoic acid that activates alpha, beta and gamma RAR receptors [6]. Metabolites that activate alpha, beta and gamma RXR receptor and Compounds that alter retinoic acid activity by affecting metabolism, biosynthesis, or other functional pathways on cofactors deprived of binding to RAR and RXR [7]. All-trans retinoic acid (ATRA), which is constituents of retinoid group, is metabolized to 9-cis retinoids and 13-cis-retinoids (Figure 2).
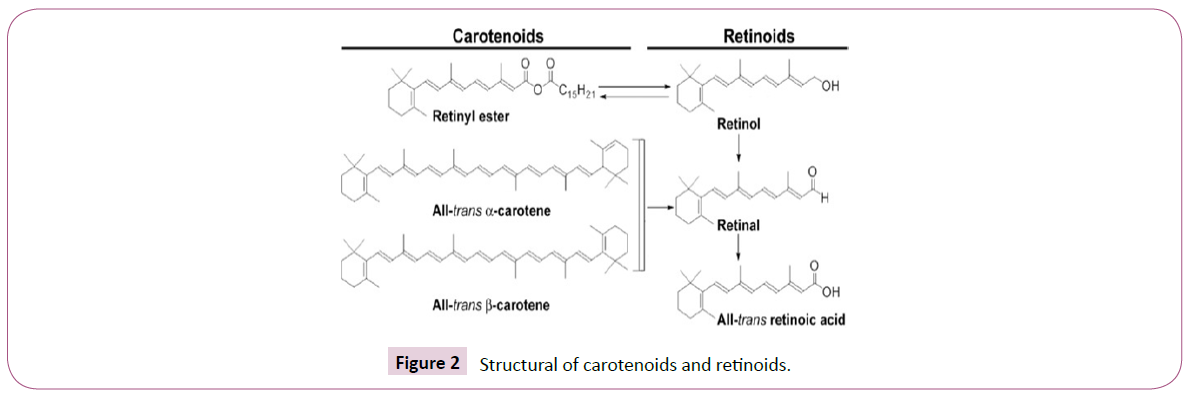
Figure 2: Structural of carotenoids and retinoids.
All-trans retinoic acid, which is one of the constituents of the retinoid group, is metabolized to 9-cis retinoids and 13-cisretinoids in the human body [8]. They are transcribed and are effective in regulating the growth of epithelial cells, their differentiation and proliferation [9].
Retinoic acid is the active form of retinoids, that couples and activates RAR alpha, beta, and gamma (Figure 2). The 13-cisretinoic acid isomer also form of retinoid but is a lesser amount than retinoic acid [10].
9-Cis-retinoic acid is composed of retinoic acid and interacts to RAR and RXR alpha, beta, gamma and activates them.
The 13-cis-retinoic acid isomer can likewise be an internal form of retinoid, nevertheless its frequency is fewer than that of retinoic acid. Connects and activates them [11].
Retinoids Metabolis
Most carotenoid vitamins A and pro-vitamins are transformed to all-trans retinoic acid by several reactions in intestinal lumen and mucosa. Retinoids are metabolized in liver (Figure 3) [12]. It is transferred by retinol-binding proteins (RBP). In many target cells, retinol crosses the plasma membrane freely. Retinol transported by retinol-binding protein in 4: 4 ratio of trans-tyrotine [13].
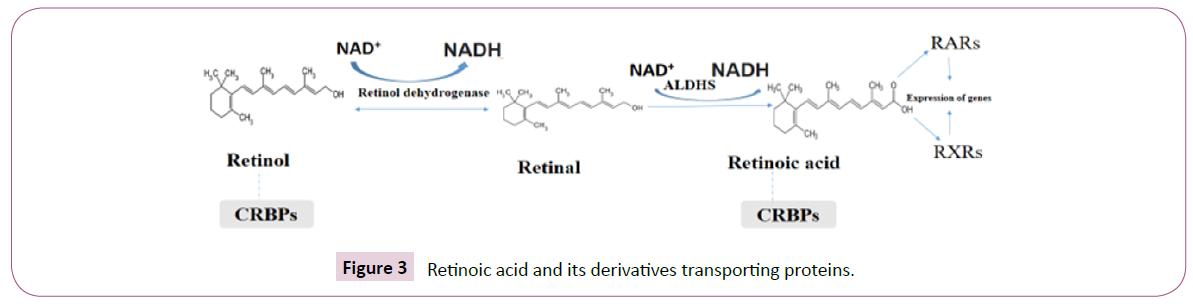
Figure 3: Retinoic acid and its derivatives transporting proteins.
Retinoic Acid Receptors
Retinol metabolites activity is initially mediated by nuclear retinol receptor proteins called retinoic acid receptors RAR1 (alpha, beta, and gamma) and retinoid X receptors RXR (alpha, beta, and gamma) [14].
These receptors are members of steroid receptor family and function like transcription factors [6]. Each of the alpha, beta, and gamma subspecies is encoded by diverse genes which have various promoters. Transcripted peptides undergo different splicing consequence in multiple receptor isoforms (e.g. RAR beta 4, RAR beta 2, RAR beta 3, RAR beta 1) [15]. Different receptors can be associated; For example, RAR forms heterodimers with RXR, and RXRs can synthesis heterodimers through a large group of nuclear receptor proteins. RARs and RXRs, as they bind to nuclear receptors, include a ligand binding region and a DNA binding region. Specific ligands of these receptors are may be different. RAR associated with all-trans-retinoic acid with high affinity and with 13-cis retinoic acid with less affinity, while RXR combines with 9-cis-retinoic acid [16].
It is possible that vitamin A and its metabolites which effect gene transcription is widespread due to binding of all-trans retinoic acid with RAR. Since in vivo cells have no 9-cis-retinoic acid. RXRRAR heterodimer Stimulation is regulated by RAR since selected RXR proteins do not affect gene transcription; (A phenomenon called "RAR dominance"). If a RAR ligand is attached, RXR ligands can upregulate transcription RXR-RAR heterodimer efficacy. Recent research shows that RAR is essence in cell nucleus; They can transfer amid nuclear receptors [17]. It is unrevealed about 20% of RAR be cytoplasmic, but transported to the nucleus by coupling to all trans retinoic acid. The intracellular distribution of RAR in adult neurons has not been studied. However, ligand binding of RAR to the nucleus could affect gene expression, suggesting a new approach to affect cellular function by RAR and other nuclear receptors.
Retinol has several metabolic pathways. Retinol is initially accumulated intracellularly in vitamin A esters formula. Retinol be able to then modification to its biologically active derivatives (all-trans retinoic acid) [18]. The all-trans retinoic acid stands the most widely studied vitamin A metabolites. Gene transcription promotes cell growth and differentiation.
Along with the all-trans retinoic acid / RAR study pathway, there are several relatively new pathways for bio activation of the 4- retinoids. For instance, retinol can be altered to all-trans-4-oxoretinol. 4-Oxo retinol can induce transcription of genes by RAR in a mechanism comparable to all-trans retinoic acid. Furthermore, retinol be able to transformed to retortinoids, that regulate cell growth and differentiation, regardless of retinoid receptors. In adult brain, these derived metabolites are indescribable. Biosynthesis of All-trans retinoic acid is as a result of two consecutive oxidations [19]. The primary reaction is achieved by retinol dehydrogenase 5 or cytosolic alcohol 6 dehydrogenase and retinol is converted to retinal (retinaldehyde).
Retinal is then fully oxidized to trans-retinoic acid by retinal dehydrogenase. Retinal dehydrogenases are alcohol dehydrogenases members and are also known as alcohol dehydrogenases. Different forms of each of these enzymes are expressed in vivo in specific adult brain segments, indicating retinoic acid synthesis occurs throughout the trans. All-trans retinoic acid creation has been shown in rabbits, rats and adult mice brain tissue [20]. All trans retinoic acid synthesis in brain, cerebellum and meninges, and rat liver were measured and its revealed the presence of retinal dehydrogenase in the pelvis and meninges was greater [21].
Retinoid-Binding Proteins
Retinols are transported by binding to retinoid-binding proteins, but there are also cytosolic retinoid-binding proteins which have retinoids relatrd high affinity for. Cytosolic retinol-binding proteins 1( CRABP1)bind to retinol, retinol-binding proteins cytosolic 2(CRABP2) bind to retinol and retinal, and retinoic acidbinding proteins cytosolic 1 and 2 bind to all-trans retinoic acid [22].
These are members of the superfamily of proteins that bind to fatty acids. CRABP1are ubiquitous, facilitating retinol entrance into target cells and directing intracellular metabolism of retinol into its stock form, the retinal ester (or active metabolite) of alltrans retinoic acid [23].
In contrast, CRABP2are expressed only in enterocytes, where retinol metabolism is directed to the retinal ester to release chylomicron. Both retinoic acid binding protein 1 and 2 are existent in cytosol and cell nucleus [24]. It is unrevealed that they can regulate all-trans retinoic acid binding ability to its receptors, by this means altering gene transcription. In the elderly, CRABP1 are ubiquitous, but CRABP2 are expressed only in skin, uterus, ovaries, choroid, some cholinergic neurons, and soft tissue. Studies show that proteins which bind to CRABP1are passive carriers and their activities depend on concentration. It seems that CRABP1suppress cellular response to all- trans retinoic acid by catalysing its degradation and thereby reducing intracellular concentration of all- trans retinoic acid [25].
In contrast CRABP2 appear to increase gene transcription by alltrans retinoic acid and increase cell sensitivity to all-trans retinoic acid. CRABP2 transfer all-trans retinoic acid in a direct collision process to RAR. The distribution pattern of cytosolic retinolbinding proteins 1 and CRABP1 and CRABP2 in the adult central nervous system differs from foetus and infant one. In adult brain, the distribution of proteins bound to retinol cytosolic 1 is consistent with retinal dehydrogenase and all-trans retinoic acid with high expression in the meninges, hippocampus, amygdala, and olfactory bulb [26].
This information indicates that retinol active form is produced by proteins that bind to cytosolic retinol-1 in adult brain. Interestingly, CRABP1are expressed in hippocampus and olfactory bulb. The hippocampus and olfactory bulb are where the concentration of all-trans retinoic acid is supposed to be adjusted by facilitating the degradation of all-trans retinoic acid to -4-exo-retinoic acid, thereby reducing the retinoic acid effect of all-trans [27].
The expression of CRABP2 in adult brain is limited to cholinergic neurons at the base of the midbrain and nucleus accumbens. Because retinoid receptor transcripts are not found in these sites, the proteins that bind to retinol cytosolic 2 act as a retinoid reservoir in these neurons [28].
Retinoic Acid Response Elements
To affect transcription, all-trans retinoic acid must bind to RA. RAR receptors are heterodimers with RXR and coupled to retinoic acid response elements, while RXR receptors can form homodimers and bind to x-retinoid response elements and retinoid response elements [29].
The elements of the retinoic acid response are usually formed by the consensus of direct half-site repeats of the AGGTCA sequence separated by five nucleotides. One form of retinoic acid response element that is less common is called retinoic acid response element DR-2, and some genes, such as CRBPS, are found in the promoter site. The arrangement and space between the halves of the elements affect the response to retinoic acid binding and the properties of the receptors. Although direct repetitions are common, half-locations may be active [30].
Furthermore, when the retinoid pathway became over activated, some proteins such AP-1, Fos, and Jun components suppress that. AP-1 controls several genes that incline to upregulate cell proliferation [31]. Adversely, retinoids generally supress cell proliferation and differentiation induction.
Consequently, it is assumed that the collaboration amongst AP-1 and RAR-RXR alters cell phenotype from proliferation to differentiation. AP-1 in retinoid-mediated gene expression suppression effect in the adult brain has not yet been investigated [32].
Retinoids and Co-regulators
In all-trans retinoic acid deficiency, co- suppressor binds to RAR. When all-trans retinoic acid interacts to RAR, co-suppressor is released and replaced with co- activator, thereby inducing gene transcription. These suppressors - activate their action by proteins that modulate histones acetylation around DNA containing retinoic acid-responsive elements [33].
Therefore, RAR co- suppressor proteins use histone deacetylase complex to eliminate acetyl groups commencing histone lysine residues and to suppress gene transcription. Unlikeness, RAR adjuvant activating proteins use histone acetyltransferase to acetylate these lysine residues, open DNA, and associate gene transcription.
Thyroid Hormones Nuclear Receptors as Main Co- suppressors
These receptors do not coupled with ligand-bound RAR, nonetheless binding to ligand-free RAR and use histone deacetylase complex to suppress transcription.
Upon binding of all-trans retinoic acid, suppress this process. Co- activators are affected by RAR also Co -activators can increase basal transcription or use further proteins, such as silent mediators for retinoid receptors and thyroid hormones; Like Phospho CREB binding proteins that has histone acetylation activity and release DNA from histone which led to transcription [34] yet like AP-1, role of retinoid-mediated gene transcriptional co-activators and suppressors in the adult brain.
Retinoid in Neurodegenerative Diseases
Neuroinflammation provide synaptic dysfunction and nerve death. Overproduction of proinflammatory mediators led to Aβ peptide assembly and inflammatory molecules synthesis and hyperphosphorylated cytokines. These inflammatory factors interrupt blood-brain barrier integrity and upsurge Aβ42 oligomers creation (Figure 4) [35].
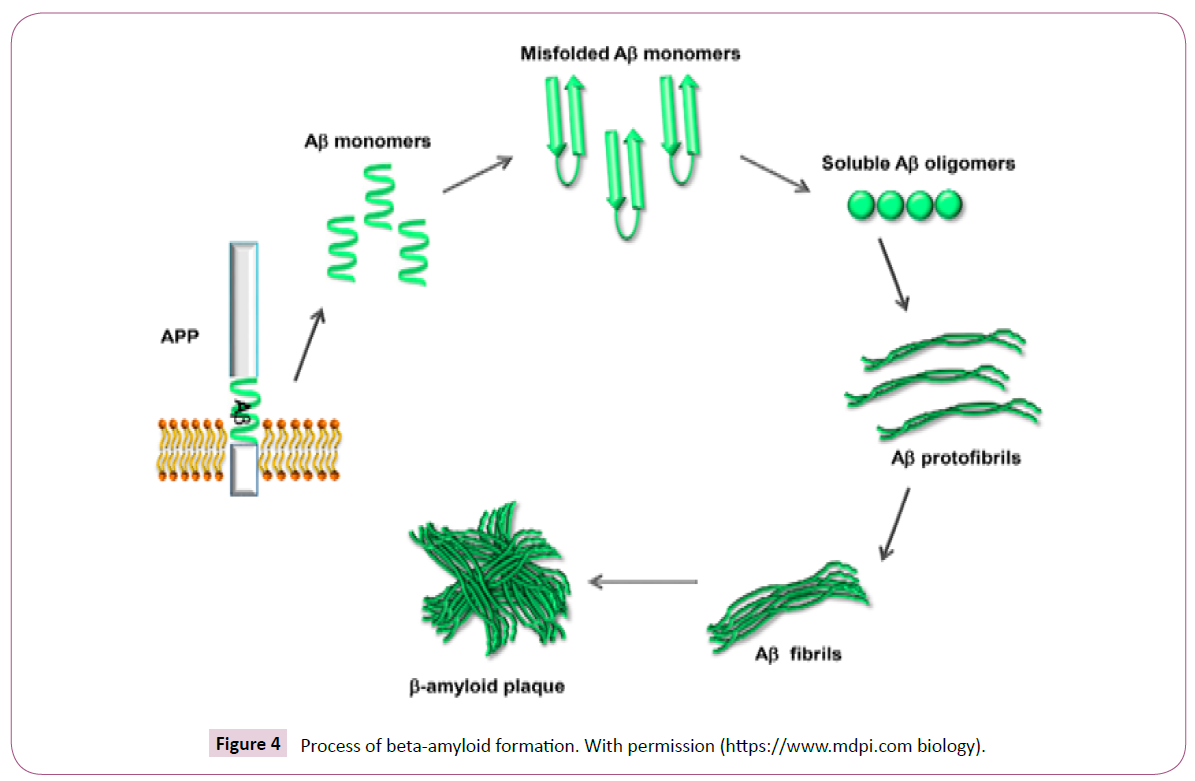
Figure 4: Process of beta-amyloid formation. With permission (https://www.mdpi.com biology).
Retinoids are participate in cellular morphogenesis, proliferation, and differentiation. Retinoids have significant effects in modulating numerous brain functions for instance neuronal evolution and differentiation, release of neurotransmitter, neural tube development, and long-term potentiation [36]. Retinoids performance their effects via nuclear receptors comprising RARα, RARβ, and RARγ and RXRα, RXRβ, and RXRγ which every one have various subtypes. For instance, RARβ have four isoforms: RARβ1, RARβ2, RARβ3, and RARβ4 [37]. RXRs are heterodimer zed with frequent nuclear receptor proteins like peroxisome proliferator-activated receptors (PPARs), liver X receptors (LXRs), and receptors (FXRs) [38]. It is reported that, knockout RXR ligands and RAR agonists can trigger RAR/RXR heterodimers. Nonetheless, in inactivated RAR ligands, RXR agonists cannot stimulate RAR/RXR heterodimers. Nuclear receptors Activation distress numerous functions cell differentiation, proliferation and apoptosis, glucose, and lipid metabolism (Table 1) [39].
| Retinoid name |
Study |
Signaling pathway |
| Xanthophylls |
RBC, in vitro and in vivo studies |
Protective oxidative damage, spatiality phospholipid peroxidation |
| Lutein |
Whole brain, rat |
Suppress Aβ synthesis |
| NEP(neprilysin protease) |
Whole brain, rat |
Anti-neurodegenerative effects |
| Brain of rats |
Vitamin A |
Deficiency can interrupt normal retinoid signaling cascade causes Aβ accretion in cortex and cerebral blood vessels |
| Lycopene |
Caenorhabditis elegans strain GMC101 |
Inhibit APP formation with no alteration in apoptosis |
| Lycopene |
Rat cortical neurons |
Suppress ROS synthesis and apoptosis via regulating proapoptotic/antiapoptotic protein levels |
| Lycopene |
Whole brain, rat |
Suggestively decreased Aβ1-42-mediated mitochondrial malfunction, inflammatory cytokine metabolites involved NF-κB, IL-1β, TNF-α, and TGF-β, |
| Astaxanthin |
HT22 cells |
Triggers antioxidant metabolites e.g. HO-1 and nuclear Nrf2 synthesis. |
| NEP (neprilysin protease) |
Microglia cells |
Caused Aβ peptide proteolytic lyses and increased neurons expression via RARα process. |
| Bexarotene |
Hippocampal neurons |
Inhibit Aβ25-35-, via coupling with the insulin signal transduction |
| Astaxanthin |
PC12 Cells |
Suppress of apoptotic pathways, repression of IL-1β and TNF-α action, and adverse kappaB and ROS nuclear translocation |
| Vitamin A and β-carotene |
In vitro Study |
Prevent Aβ40 and Aβ42 interaction dose-dependent |
| Β-carotene |
Animal Study |
An AD prevention metabolit which can be administrated as significant therapeutic agent concerned in neuroinflammatory treatment |
| Crocin (a natural drug derived from carotenoid) |
In vitro Study |
Prevent Aβ42 amyloid formation |
| Crocetin |
Mouse |
Improve cell recurrence , repress ROS production, and improvement mitochondrial membrane potential. |
| Crocin |
In Vivo models |
Prevent Aβ-induced apoptosis |
| Retinoids |
In vivo studies |
Down-regulation NF-κB signaling cascade |
| Lycopene, lutein, α-carotene, and β-carotene |
Human Studies |
Diverse association among lymphocyte DNA 8-OHdG and plasma lycopene, lutein, alpha-carotene, and beta-carotene, respectively, was observed. |
| Bexarotene |
Mouse model |
Decreases Aβ40 levels and improves cognition ( |
| Retinoic acid |
In vivo studies |
Inhibit early stages of Aβ |
| Retinol and β-carotene |
In vitro studies |
Intensely Downregulate Aβ Intensely esis and destabilize fibril preformation |
| All-trans-retinoic acid (ATRA) |
APP/PS1 transgenic mice |
Reduces tau hyper phosphorylation, amyloid accumulation, and significant memory performance improvement |
| RA isomers |
Mice astrocytes |
Provide apoE secretion through RXR and RAR action |
| All-trans-retinoic acid |
Animal Study |
Provoke PS1 and BACE1 to APP cleavage impairment |
| Retinoids |
Cultured cortical astrocytes |
Provoke prostanoid production |
| Am80 and HX630 |
In AβPP23 mice |
Decrease insoluble Aβ peptide amounts and trigger memory processes |
| (Tamibarotene), a retinoid receptor agonist |
APP23 transgenic mice |
Reduced insoluble Aβ-40 and Aβ-42 levels |
| Am580 |
Animal Study |
As RAR activating agent , upregulation Aβ synthesis |
| ATRA, 9-cis-RA, and 13-cis-RA |
Hippocampal cell cultures |
Protective effect in neurons against cell death induced by Aβ peptide |
| Retinoids |
Rat microglial cells |
Prevent Aβ or LPS-induced TNF-α synthesis along with iNOS expression |
| Retinoids |
Murine astrocyte cultures |
Reduce IL-6 generation |
Table 1. Retinoids neuroprotective effects in different studies.
In mice with vitamin A deficiency revealed severe imperfections in spatial learning and memory indicating its significant effects in the memory functions providing. Retinoid signaling influences development of AD pathology through several process. RAR and RXR activation in APP/PS1 transgenic mice improved the AD symptoms and diminished amyloid accretion and tau hyper phosphorylation [40]. Retinoids also repress pro-inflammatory cytokines and chemokine’s synthesis by astrocytes and microglia.
There’re evidences that neuronal cell lines treated with retinoid agonists unveiled overexpression and activity of choline acetyltransferase (ChAT). Retinoic acid isomers improve, genes expression which linked with cholesterol efflux e.g. apoE, abca- 1 and abcg-1 proteins in astrocytes. Likewise studies reported that retinoids antioxidant properties. These compounds have antioxidants and anti-inflammatory effects. Studies revealed that retinoids have significant effects as endogenous antioxidants.
Retinoids via scavenging of cellular free radical molecules, provide cell destructions. Retinoids in oxidative stress situations for instance metal exposure, ROS synthesis are increased within the cells [41].
Study on genetically engineered mice proves indicate that supplements given to newborn mice with low levels of vitamin A showed decreased brain progression nerve cells development.
This effects mediate via intrusion ROS synthesis, scavenging free radicals directly, up regulation of antioxidant enzymes, and signaling pathways encompassed in immune system for instance Nrf2 signaling modulating . It has been reported that retinoic acid (RA) has a protecting properties on neuron apoptosis and oxidative damage via glutathione reduction.
It similarly reinstates SOD-1 and SOD-2 levels in hippocampal cells. Retinoids have alternative anti-inflammatory mechanisms which prevent of NF-κB transcription factor translocation and suppressing of proinflammatory cytokine synthesis [42].
Furthermore, RA suppress Th1 and Th17 cells affectedly and has main protective effect in neuroinflammation. Inversely, RA can endorse Th2 in T cells exert anti-inflammatory effects. Furthermore, RA improve FoxP3 T cells which have repressive effects on inflammatory response. These evidences revealed that retinoids are anti-inflammatory metabolites and may concern as therapeutic agents in neuroinflammatory situations [43].
All-trans retinoic acid has been suggested in the physiological function of the hippocampus in presence of retinoid signaling pathway components. Retinal dehydrogenase protein 2, one of the enzymes that synthesizes retinoic acid, is trapped in the meninges around the hippocampus of adult mice. Immunoreactivity for binding proteins like cytosolic 1-binding proteins and CRBP2 have been studied in the dendritic layers of hippocampus [44]. Moreover, mRNA transcripts of RXR alpha, beta and gamma have been recognized in the hippocampus [45].
Studies revealed that hippocampus has significant effect in memory and spatial learning, it is not surprising to show that retinoid signaling is effective in this process. Long-term changes in synapses are thought to be the basic cellular mechanism of memory and learning [46].
There are some studies about all-trans retinoic acid in hippocampus processes which has been suggested in retinoid signaling pathway components existence.
All-trans retinoic acid signaling is implicated in long-term amplification modifications and long-term suppression of synapse function in the hippocampus. Long-term amplification disorder and long-term suppression have been observed in mice nockouted beta RAR receptors, or lacking beta RAR and gamma RAR receptors. Although alpha RAR is abundant in adult hippocampus, disruption of alpha RAR in mice causes the death of the newborn. In hippocampus CA1 region of mice in which RAR beta or RAR beta-RXR gamma is mutated, there was no long-term amplification at all.
Interestingly, long-term amplification remained healthy and flawless in mice with only RAR-gamma deficiency, indicating the involvement of beta-RAR receptors, in particular, in the regulation of long-term amplification.
Retinoids on the Dopaminergic System
Dopaminergic neurons are found mainly in midbrain, and basic dopaminergic pathways are developed from the midbrain to the striatum, anterior cortex, and limbic apparatus [47]. Dopaminergic pathway distribution is based on dopamine attribution, comprising of coordination of movement, reward, and emotional process [48]. The major components of retinoid messaging, including retinal dehydrogenase, RAR, and retinoic acid binding proteins, are established in the dopaminergic pathway (Figure 3) [44].
Furthermore, retinoic acid in neurons expresses the activity of tyrosine hydroxylase and dopamine beta-hydroxylase, enzymes that synthesize dopamine (and regulate dopamine D2 receptors) in adult brain [49]. In mice nockouted retinoid receptors, a movement defect is evident, accompanied by a decrease in dopamine D2 receptor expression. In mice with double mutations in RARβ-RXRβ, RARβ-RXR and RXRβ-RXRγ), forward motion decreased [50].
Retinoids in Alzheimer's Treatment
Nerve cells beta-amyloid annihilation has significant effect in Alzheimer's disease improvement. Most of drugs improve neurotransmission but do not alter the disease process [51].
A limited number of drugs are useful in Alzheimer's treatment, most of which are acetylcholinesterase inhibitors such as Donepezil galantamine and rivastigmine, and others memantine, which delay neuronal death. Nonetheless, these drugs have side effects for instance diarrhea, nausea, vomiting and drowsiness [52,53]. Beta-amyloid accumulation is a critical stage in the development of Alzheimer's disease. Vitamin A, which is produced by the central nervous system more than any other cells, regulates genes expression in the central nervous system [54]. Furthermore, vitamin A has significant effect in central nervous system development. Vitamin A, traditionally regarded as an antioxidant compound, has noteworthy effect in maintaining central nervous system function in old age. Alzheimer's patients have been unraveled have low level of plasma and serum vitamin A and beta-carotene concentration. It has been shown that among 442 Alzheimer's patients aged 65-94 years (those with higher plasma beta-carotene concentrations, their memory function also improved [55].
It has been revealed that vitamin A and beta-carotene constrain beta-amyloid fibrils construction from fresh beta-amyloid and cause instability in the fibrillar structure of beta-amyloid in vitro [56]. Retinoic acid, the active metabolite of vitamin A, has been reported to control gene expression dependent on amyloid precursor protein processing. Retinoic acid regulates gene expression in nuclear receptors such as retinoic acid receptors and RXR. Lack of vitamin A causes accumulation of beta-amyloid and reduces the long-term strengthening of the hippocampus in rats. Studies have shown that all-trans retinoic acid induces the expression of the MNSOD2 gene in neuroblast cells, thereby reducing oxidative damage, an important pathological factor in Alzheimer's. Reports indicate that impaired memory and spatial learning, and decreased synaptic flexibility, are due to RXR and RAR mutations [57-60].
Conclusion
Retinoid signaling has been suggested as a potent viable pathway for new therapies for Alzheimer's. With considering retinoic acid has been used to treat Alzheimer's and there are conflicting reports, it is unclear whether Alzheimer's patients have retinoid signaling deficiency. For example, GL Fata and colleagues reported the plasma level of vitamin A in Alzheimer's decreased While Connor and Seidel showed that content of hippocampal retinoids in Alzheimer's is similar to that of controls, A decline in RARα concentration and retinal dehydrogenase 2 in Alzheimer's brain, indicating that retinoid signaling may be impaired in patients, In older mice showed that the frequency of RAR decreased, which may eventually lead to a reduction in retinoic acid-dependent effects. In fact, perceptive deficiencies seen in older mice can be inverted by activating retinoic acid. However, Alzheimer's pathology involves multiple and complex signalling pathways and retinoids may modify these procedures by gene expression regulation along with by antioxidant properties.
In order to treat Alzheimer's with retinoid, it is necessary to consider of vitamin A and its receptors effects in various processes such as in plaque regulating, neuroinflammation and cholinergic system.
Conflict of Interest Disclosures
The authors declare no potential conflicts of interest relevant to this article
40265
References
- Hardeland R, Cardinali DP, Brown GM, Pandi-Perumal SR (2015) Melatonin and brain inflammaging. Progress in neurobiology 127: 46-63.
- Srinivasan V, Cardinali DP, Srinivasan US, Kaur C, Brown GM et al. (2011) Therapeutic potential of melatonin and its analogs in Parkinson’s disease: focus on sleep and neuroprotection. Therap adv neuro dis 4: 297-317.
- Damm K, Heyman RA, Umesono K, Evans RM (1993) Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proceedings of the National Academy of Sciences 90: 2989-2993.
- Ross AC (2010) Vitamin Bioactive compounds and cancer: Springer 335-356.
- Chaudhuri R, Bojanowski K (2014) Bakuchiol: a retinolÃÂâ?ÃÂÃÂlike functional compound revealed by gene expression profiling and clinically proven to have antiÃÂâ?ÃÂÃÂaging effects. Int j cosm sci 36: 221-230.
- Lefebvre P, Martin PJ, Flajollet S, Dedieu S, Billaut X (2005) Transcriptional activities of retinoic acid receptors. Vit horm 70: 199-264.
- Bass NM (1993) Cellular binding proteins for fatty acids and retinoids: similar or specialized functions? Cellular Fatty Acid-Binding Proteins II 191-202.
- Redmond KA, Nguyen T-S, Ryan RO (2007) All-trans-retinoic acid nanodisks. Int J phar 339: 246-250.
- Siddikuzzaman, Guruvayoorappan C, Berlin Grace V (2011) All trans retinoic acid and cancer. Immunopharmacol and immunotoxicol 33: 241-249.
- Tang X-H, Gudas LJ (2011) Retinoids, retinoic acid receptors, and cancer. Annual Review of Pathology: Mechanisms of Disease 6: 345-364.
- Blaner WS (2001) Cellular metabolism and actions of 13-cis-retinoic acid. J Am Acad Dermatol 45: S129-S35.
- Brun PJ, Yang KJZ, Lee SA, Yuen JJ, Blaner WS (2013) Retinoids: Potent regulators of metabolism. Biofactors 39: 151-163.
- von Lintig J (2012) Metabolism of carotenoids and retinoids related to vision. J BiolChemi 287: 1627-1634.
- Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, et al. (2006) International union of pharmacology. LX. Retinoic acid receptors. Pharmacol rev 58: 712-725.
- Mark M, Ghyselinck NB, Chambon P (2009) Function of retinoic acid receptors during embryonic development. Nuclear receptor signal 7: 07002.
- Duong V, Rochette-Egly C (2011) The molecular physiology of nuclear retinoic acid receptors. From health to disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis Dis 1812: 1023-1031.
- Brand N, Petkovich M, Krust A, Chambon P, de Thé H, et al. (1988) Identification of a second human retinoic acid receptor. Nature 332: 850-853.
- Lidén M, Eriksson U (2006) Understanding retinol metabolism: structure and function of retinol dehydrogenases. J Biol Chem 281: 13001-13004.
- Theodosiou M, Laudet V, Schubert M (2010) From carrot to clinic: an overview of the retinoic acid signaling pathway. Cell mol life sci 67: 1423-1445.
- Dollé P (2009) Developmental expression of retinoic acid receptors (RARs). Nuclear receptor signalling 7: 07006.
- Asefy Z, Hoseinnejhad S, Ceferov Z (2021) Nanoparticles approaches in neurodegenerative diseases diagnosis and treatment. Neurol Sci 12: 1-8.
- Noy N (2000) Retinoid-binding proteins: mediators of retinoid action. Biochem J348: 481-495.
- Napoli JL (2017) Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: Effects on retinoid metabolism, function and related diseases. Pharmacol Therap 173: 19-33.
- Napoli JL (2016) Functions of intracellular retinoid binding-proteins. The Biochemistry of Retinoid Signaling II 21-76.
- Newcomer ME (1995) RetinoidÃÂâ?ÃÂÃÂbinding proteins: structural determinants important for function. The FASEB J 9: 229-239.
- Welch ID, Cowan MF, Beier F, Underhill TM (2009) The retinoic acid binding protein CRABP2 is increased in murine models of degenerative joint disease. Arthritis Res Ther 11: 1-9.
- Favorskaya I, Kainov Y, Chemeris G, Komelkov A, Zborovskaya I, et al. (2014) Expression and clinical significance of CRABP1 and CRABP2 in non-small cell lung cancer. Tumor Biol 35: 10295-10300.
- Doldo E, Costanza G, Agostinelli S, Tarquini C, Ferlosio A, et al. (2015) Vitamin A, cancer treatment and prevention: the new role of cellular retinol binding proteins. BioMed Res Int.
- Balmer J, Blomhoff R (2005) A robust characterization of retinoic acid response elements based on a comparison of sites in three species. The Journal of steroid biochemistry and molecular biology 96: 347-354.
- Abbasi-Oshaghi E, Khodadadi I, Mirzaei F, Ahmadi M, Tayebinia H, et al. (2020) Alleviates Sperm Damage by Limiting Oxidative Stress and Insulin Resistance in Diabetic Rats. The Open MedChem J 14.
- Eferl R, Wagner EF (2003) AP-1: A double-edged sword in tumorigenesis. Nat Revi Can 3: 859-868.
- Kirchmeyer M, Koufany M, Sebillaud S, Netter P, Jouzeau J-Y, et al. (2008) All-trans retinoic acid suppresses interleukin-6 expression in interleukin-1-stimulated synovial fibroblasts by inhibition of ERK 1/2 pathway independently of RAR activation. Arthritis Res Ther 10: 1-12.
- Goodarzi MT, Khodadadi I, Tavilani H, Abbasi Oshaghi E (2016) The role of Anethum graveolens L.(Dill) in the management of diabetes. J Trop Med 18: 2016.
- Umesono K, Murakami KK, Thompson CC, Evans RM (1991) Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell 65: 1255-1266.
- Li B, Zhong L, Yang X, Andersson T, Huang M, Tang S-J (2011) WNT5A signaling contributes to Aβ-induced neuroinflammation and neurotoxicity. PloS one 6:e22920.
- Handberg-Thorsager M, Gutierrez-Mazariegos J, Arold ST, Nadendla EK, Bertucci PY, et al. (2018) The ancestral retinoic acid receptor was a low-affinity sensor triggering neuronal differentiation. Science Advances 4: eaao1261.
- Schug TT, Berry DC, Shaw NS, Travis SN, Noy N (2007) Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 129: 723-733.
- le Maire A, Alvarez S, Shankaranarayanan P, R de Lera A, Bourguet W, et al. (2012) Retinoid receptors and therapeutic applications of RAR/RXR modulators. Current topics in Med Chem 12: 505-527.
- Crowe DL, Chandraratna RA (2004) A retinoid X receptor (RXR)-selective retinoid reveals that RXR-α is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands. Breast Cancer Res 6: 1-10.
- Behairi N, Belkhelfa M, Rafa H, Labsi M, Deghbar N, et al. (2016) All-trans retinoic acid (ATRA) prevents lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment in aged rats. J Neuroimmunol 300: 21-29.
- Nunes-Tavares N, Santos LE, Stutz B, Brito-Moreira J, Klein WL, et al. (2012) Inhibition of choline acetyltransferase as a mechanism for cholinergic dysfunction induced by amyloid-β peptide oligomers. J Biol Chem 287: 19377-19385.
- Um H-S, Kang E-B, Koo J-H, Kim H-T, Kim E-J, et al. (2011) Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer's disease. Neurosci Res 69: 161-173.
- Oberstein TJ, Taha L, Spitzer P, Hellstern J, Herrmann M, et al. (2018) Imbalance of circulating Th17 and regulatory T cells in Alzheimer’s disease: a case control study. Frontiers in Immunol 9: 1213.
- Lane MA, Bailey SJ (2005) Role of retinoid signalling in the adult brain. Progress in Neurobiol 75: 275-293.
- Mingaud F, Mormede C, Etchamendy N, Mons N, Niedergang B, et al. (2008) Retinoid hyposignaling contributes to aging-related decline in hippocampal function in short-term/working memory organization and long-term declarative memory encoding in mice. J Neurosci 28: 279-291.
- McCaffery P, Zhang J, Crandall JE (2006) Retinoic acid signaling and function in the adult hippocampus. J Neurobiol 66: 780-791.
- Maden M (2007) Retinoic acid in the development, regeneration and maintenance of the nervous system. Nature Rev Neurosci 8: 755-765.
- Lévesque D, Rouillard C (2007) Nur77 and retinoid X receptors: crucial factors in dopamine-related neuro adaptation. Trends in Neurosci 30: 22-30.
- Wolf G (1998) Vitamin A functions in the regulation of the dopaminergic system in the brain and pituitary gland. Nut Rev 56: 354-355.
- Bremner JD, McCaffery P (2008) The neurobiology of retinoic acid in affective disorders. Progress in Neuro-Psychopharmacol Biol Psy 32: 315-331.
- Shudo K, Fukasawa H, Nakagomi M, Yamagata N (2009) Towards retinoid therapy for Alzheimer's disease. Current Alzheimer Res 6: 302-311.
- Fukasawa H, Nakagomi M, Yamagata N, Katsuki H, Kawahara K, et al. (2012) Tamibarotene: a candidate retinoid drug for Alzheimer’s disease. Biol Pharmaceu Bull 35: 1206-1212.
- Lerner AJ, GustawÃÂâ?ÃÂÃÂRothenberg K, Smyth S, Casadesus G (2012) Retinoids for treatment of Alzheimer's disease. Biofactors 38: 84-89.
- Sodhi RK, Singh N (2014) Retinoids as potential targets for Alzheimer's disease. Pharmacol Biochem and Behav 120: 117-123.
- Honarvar NM, Saedisomeolia A, Abdolahi M, Shayeganrad A, Sangsari GT, et al. (2017) Molecular anti-inflammatory mechanisms of retinoids and carotenoids in Alzheimer’s disease: A review of current evidence. J Mol Neurosci 61: 289-304.
- Fahrenholz F, Tippmann F, Endres K (2010) Retinoids as a perspective in treatment of Alzheimer’s disease. Neurodegenerative Dis 7: 190-192.
- Fata GL, Weber P, Mohajeri MH (2014) Effects of vitamin E on cognitive performance during ageing and in Alzheimer’s disease. Nutrients 6: 5453-5472.
- Connor MJ, Sidell N (1997) Retinoic acid synthesis in normal and Alzheimer diseased brain and human neural cells. Mol Chem Neuropath 30: 239-252.
- Pierzchalski KA (2015) Impact of cellular retinol-binding protein, type I on retinoic acid biosynthesis and homeostasis.
- Etchamendy N, Enderlin V, Marighetto A, Pallet V, Higueret P, et al. (2003) Vitamin A deficiency and relational memory deficit in adult mice: relationships with changes in brain retinoid signalling. Behavioural Brain Res 145: 37-49.










