Keywords
Merkel cell carcinoma; Neuroendocrine tumors; No skin involvement; Retroperitoneal tumor; Unknown primary
Background
Merkel cell carcinoma was described by Toker in 1972. It is a rare neuroendocrine tumor that represents a small percentage of skin tumors, with an incidence ranging from 0.18 to 0.41/100,000 patients and a survival rate at 5 years of 20% to 80%. Ultraviolet (UV) radiation, other tumors and polyomavirus have been considered risk factors. Studies also show high prevalence in immunosuppressed patients, including HIV infected patients.
Merkel cells are located in the basal layer of the dermis and follicles of the epidermis, being associated to the mechanoreceptors of the papillary dermis known as Merkel cells. It has been proposed that skin neuroendocrine carcinoma arises from these cells, however there are several hypotheses about its origin [1].
Of the total cases reported, 78% of patients are diagnosed over 59 years old, being more frequent in men according to recent demographic studies about MCC [2]. According to largest published series, only 2% to 5% had no skin involvement [3,4]. Among regions involved, sun exposed ones are the most frequent, being head and neck the most affected with 50.8% of cases or 33.7% in limbs and lymph nodes. It usually presents as a firm and painless cutaneous nodule, and a debut as skin ulceration is not common [1].
Case Presentation
72 years old male and a history of type 2 diabetes mellitus and dyslipidemia, who came to the emergency room because of a recent mass in the right inguinal region. In addition, he referred pain in right back during the last days that followed an inflammatory pattern and irradiated to gluteal and thigh ipsilateral level.
Physical examination only highlighted a 3 cm adenopathy in the right inguinal region and stony consistency, so we performed fine needle aspiration (FNA) of the lesion. The analysis performed showed lack of leukocytosis or parameters suggestive of infectious/inflammatory reaction. FNA results were consistent with Merkel cell carcinoma: atypical small cells with very small cytoplasm, hiperchromatic nucleous without nucleolus and frequent mitosis. Immunohistochemistry was also compatible with MCC, with positiveness for cytokeratine 20 (CK-20), with characteristical perinuclear dot-like staining pattern, neurofilaments (NFP), CD56 and synaptophisine (SYP) and negativity for cytokeratine 7 (CK-7) and thyroid transcrption factor 1 (TTF-1).
In light of these findings the patient was assessed in Dermatology Department, but given the fact there was no skin involvement and the pain was out of control, he was finally derived to Internal Medicine Department to complete the study and receive treatment.
Once entered, PET-CT was performed with 18- Fluorodeoxyglucose as a marker, observing a large retroperitoneal mass of 13.5 × 14.5 × 15 cm in size with a SULpeak 10.9 (normal range from 2.5 to 3), infiltrating psoas muscle and contacting several lumbar vertebrae, without clear infiltration of them, and invasion of the back muscles of the posterior abdominal wall (Figures 1 and 2). Right inguinal lymphadenopathy of 37 mm and 21 mm were also objectified with SULpeak 6.2 and 7.9 respectively.
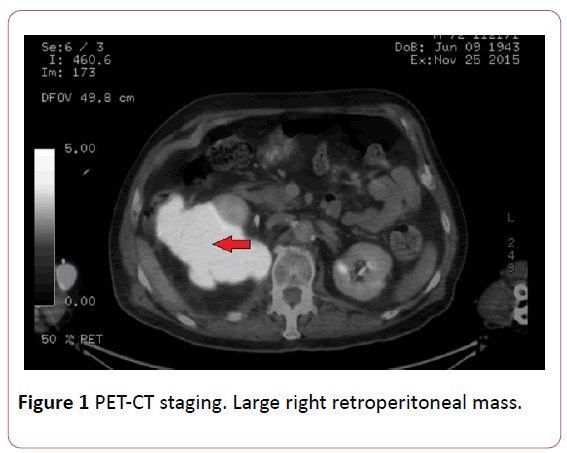
Figure 1: PET-CT staging. Large right retroperitoneal mass.
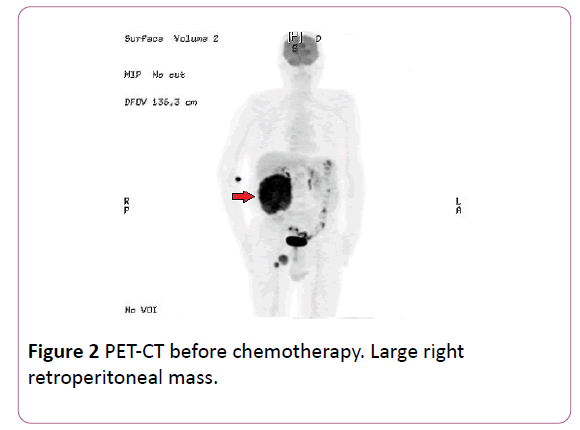
Figure 2: PET-CT before chemotherapy. Large right retroperitoneal mass.
Given the infrequency of Merkel cell tumors without primary skin lesion, retroperitoneal core needle biopsy was performed to rule out a tumor of different origin, occurring at the same time than the inguinal one already diagnosed. However, in the immunohistochemical study, the result found was again compatible with Merkel cell carcinoma.
Once the extension study and pathological diagnosis was completed, he started palliative chemotherapy (cisplatin 100 mg/m2 + etoposide 100 mg days 1-3/21 days) given the fact that the tumor was unresectable and had progressed rapidly.
Pain was successfully controlled l with opioids (oxycodone/ naloxone).
The 2nd and 3rd cycle cycles of chemotherapy were administered without dose reduction. PET-CT performed in January 2016, showed almost complete metabolic response of retroperitoneal mass, being unable to rule out viable tumor tissue, and partial response of the right inguinal lymph nodes (Figure 3).
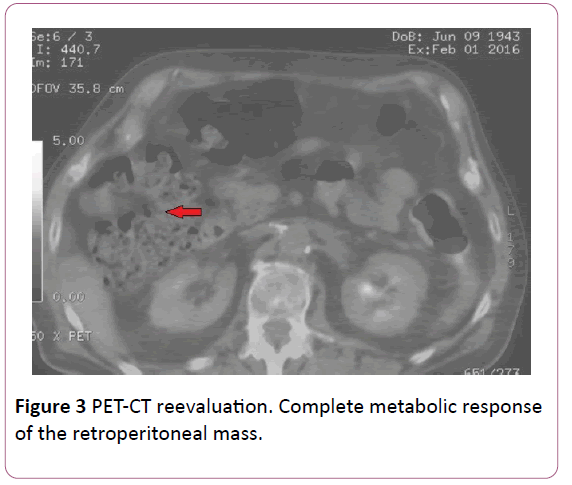
Figure 3: PET-CT reevaluation. Complete metabolic response of the retroperitoneal mass.
After that, he received chemotherapy (5th cycle), with good tolerance and ECOG 1. There were no signs of retroperitoneal disease (Figure 4).
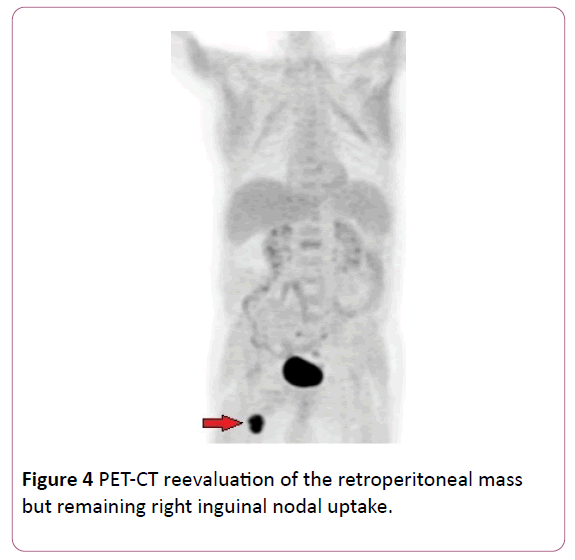
Figure 4: PET-CT reevaluation of the retroperitoneal mass but remaining right inguinal nodal uptake.
Material and Method
We conducted a systematic research in MEDLINE. To do this, the following terminological combinations were included: "Merkel cell carcinoma retroperitoneum" (3 results), "Merkel cell retroperitoneal carcinoma" (11 articles), "Merkel cell pancreatic carcinoma" (28 articles), "Merkel cell carcinoma unknown primary" (71 items). In addition, citations of previously selected for review articles were revised, adding two more publications to the selected publications.
Articles which included references to cases of Merkel cell carcinoma with retroperitoneal involvement and without cutaneous tumor, written in English or Spanish, were selected. We found 16 articles with pancreatic involvement but 14 of them with primary cutaneous tumor, so they were not included. Finally, 13 articles were included in the review; 7
with retroperitoneal nodal involvement, 2 with pancreatic involvement and only 4 cases of Merkel cell carcinoma without known primary cutaneous tumor with a retroperitoneal nonnodal mass. These items were compared with our case.
Literature Review Results
Only 13 cases [5-17] met the condition of having a retroperitoneal Merkel cell involvement without primary skin lesion, they are summarized in Table 1.
| ARTICLES |
Current case |
Quiroz-Sandoval (5) |
Boghossian (6) |
Rossini (7) |
Evoli (8) |
Taran
Tola (9) |
Noto (10) |
Silberstein (11) |
De Cicco (12) |
Abul Kasim (13) |
Noell (14) |
Kontis (15) |
Ghouri (16) |
Yaramada (17) |
| Age |
72 |
58 |
81 |
67 |
52 |
Unknown |
72 |
69 |
54 |
65 |
61 |
60 |
70 |
85 |
| Sex |
Male |
Male |
Male |
Male |
Male |
Unknown |
Female |
Male |
Male |
Male |
Male |
Male |
Male |
Male |
| Location |
Right inguinal adenopathy, Right retroperitoneal mass |
Right flank, left para-aortic and iliac nodes |
Right iliac fossa |
Inguinal |
Retroperitoneal, ureter |
Retroperitoneal, brain, stomach |
Inguinal, mediastine |
Inguinal |
Inguinal, iliac |
Brain, inguinal, iliac, para-aortic |
Retroperitoneal nodal mass obstructing
both ureters |
Left inguinal adenopathy,
Left iliac fossa and infra renal nodes affection |
Right groin mass ? Abdominal wall ? Metastatic disease (brain, chest wall, and abdomen including the pancreas). |
Epigastric mass (invading pancreas and duodenum), left inguinal nodal mass |
| Size of the main mass |
13.5 ÃÂâ€â€Â 14.5 ÃÂâ€â€Â 15 cm |
11 ÃÂâ€â€Â 9 ÃÂâ€â€Â 7 cm |
5 ÃÂâ€â€Â 5 ÃÂâ€â€Â 7.5 cm |
4 and 3 cm |
Unknown |
Unknown |
Unknown |
3 cm |
Unknown |
Unknown |
14 ÃÂâ€â€Â 7 cm |
Unknown |
Unknown |
10.2 cm ÃÂâ€â€Â
6.3 cm |
| Image |
PET-CT |
PET-CT |
PET,octreo-scan |
CT |
CT |
CT |
CT |
CT |
CT, octreoscan |
PET-CT |
CT |
 PET-CT |
PET-CT |
CT |
| Treatment |
Cisplatin+Etoposide |
Carboplatin+ Etoposide Surgery, RT |
Surgery, RT |
Carbo-platin+Etoposide, RT |
Surgery, Carboplatin+Etoposide |
Unknown |
No |
Metotrexate, 5FU, surgery |
Surgery, Carboplatin+ Etoposide, RT |
RT |
RT. Carboplatin and y etoposid
àGemcitabine
àTemozolo-mide |
Surgery+ Cisplatin and Etoposide |
Surgery+ QT (1. Cisplatine+ Etoposide; 2. Vincristine, Adriamycin and Ifosfamide;) |
Surgery+RT (nodal mass) ? QT (Carboplatine+ Etoposide) |
| Relapse |
No |
Lung, liver |
Yes |
No |
Yes (RT, surgery, quimiotherapy) |
Unknown |
Unknown |
Yes (RT, cisplatin+ Etoposide) |
Nodes, Pancreas |
Yes |
Yes, Skin nodules |
No |
Yes (local excision+ intra-arterial chemotherapy with cisplatin) |
No |
| Survival |
Unknown |
Unknown |
Unknown |
Unknown |
Unknown |
Unknown |
Unknown |
2 years |
17 months |
6 months |
9 months |
Unknown |
25 months |
Unknown |
Table 1: Review of Merkel Cell tumor with retroperitoneal involvement without primary skin tumor.
Most of the cases had retroperitoneal nodal affection (7 cases) or pancreatic involvement (2 cases), with only 4 plus our case with a real retroperitoneal mass of unknown origin that could be classified as the main tumor [5-8].
The average age of these patients was 66 years old, being female only one case. They were all diagnosed by computerized tomography (CT) or Positron emission tomography-computed tomography (PET-CT). The size of the mass was unknown in 50% of the cases, <5 cm in one case and >5 cm in the remaining [6].
Selected treatments were chemotherapy, surgery and radiation therapy. If the tumor was initially removable, surgery was the first choice, if not, neoadjuvant chemotherapy was used prior to surgery ± radiotherapy (RT).
Survival rate is unknown in most of them, but it was described a survival rate less than 24 months in four of the reviewed cases and 25 months in one of them.
Discussion
Our article collects cases of retroperitoneal tumors without skin primary lesion, in which the pathologic diagnosis is essential because there is no visual way to identify it nor have characteristic all patterns in imaging tests. It also has a different therapeutic approach to other tumors such as sarcomas that more often occur in this location.
Regarding diagnosis approach for MCC, in the European Guidelines Interdisciplinary Diagnosis and Treatment of Tumor Cells Merkel, a thorough physical examination is the first step, (which could identify a primary skin lesion) and after that, a ultrasonography can be performed to assess a sentinel node biopsy close to the lesions observed by history and physical examination. Then, it is proposed an extension study by CT or PET-CT.
Although is not common that these tumors are cured, we should perform follow-up post-treatment with physical examination and locoregional ultrasound test every 4 months for the first 3 years and every 6 months to reach 5 years, with CT or PET-CT annually during these first 5 years accordingly to European interdisciplinary consensus-based guidelines [18]. National Cancer Comprehensive Network guidelines suggest similar recommendations for the follow-up post-treatment although they don´t mention ultrasonography role neither establish any specific frequency for image studies [19]. About magnetic resonance imaging (MRI), some studies recommend it as a valid method for the extension study image [20], however the use of PET with 18-flurodesoxiglucose seems to be more useful for this purpose in this profile of patients, achieving a sensitivity of 90% and a specificity of 90%, with limitations in small lesions, less metabolic activity or in regions with high uptake as may be the nervous system [21].
Immunohistochemical markers for diagnosis include: positivity for cytokeratin 20 (CK 20) with paranuclear dot-like staining pattern, neuron-specific enolase (NSE), gromogranin A (GrA), neurofilament proteins (NFP), neural adhesion molecule (CD56) and synaptophysin. Negativity for thyroid transcription factor 1 (TTF-1), S-100 protein, vimentin and leukocyte common antigen (CD 45). Cytokeratin 7 (CK 7) is also usually negative (69% to 77% of the cases). CDK20 is the most important marker for differential diagnosis of MCC, because it is not usually expressed in other neuroendocrine carcinomas. CK 20 positivity with perinuclear dot-like pattern has good sensibility and high specificity for MCC, being positive in 97% of cases [22].
Differential diagnosis includes both squamous and basal cell skin carcinomas, Ewing's sarcoma, neuroblastoma, retinoblastoma, pyogenic granuloma and cutaneous melanoma metastases from small cell carcinoma. Immunohistochemistry and electron microscopy are those that help us to confirm the diagnosis because they usually express CK 20 and marked cytoplasmic reactivity for NSE with S-100 protein and CK 7 being negative. There are three characteristic histologic patterns such as the trabecular, intermediate cell type and small cell, being the second one the most common.
Staging is performed according to the Guidelines of the American joint committee on cancer (AJCC) Staging Manual [23]; which describes the stage I as negative nodal involvement with a tumor <2 cm, stage II negative nodal involvement but tumor >2 cm, stage III with nodal involvement and IV distant metastases. In the last edition of the AJCC Manual, changes have been made in the stage III disease (nodal involvement cases), due to recent findings regarding prognosis differences. A difference has been made between nodal involvement detected through clinical examination or image technics and those cases of nodal disease that has been detected on pathological exam (because the last one is considered more accurate, and thus, this affects to the recurrence chance). Also, stage III has a new category for MCC cases with nodal involvement and unknown primary, because of their better prognosis [24]. Although, these changes won´t be implemented until January 1, 2018 [25].
Treatment choice will depend on the tumor stage, including those without known primary tumor. If there is no distant metastasis, surgery with wide margins ± technique of sentinel node is the choice therapy since the prognosis differs depending on having > <2 affected lymph nodes.
Regarding chemotherapy, platinum has been the drug of choice. Pectasides described 9 patients in the last 15 years of advanced or metastatic disease, receiving carboplatin (AUC 5) and etoposide (VP-16) (100 mg/m2 on days 1-3) every 3 weeks. For a second line, the scheme of cisplatin (CDDP) (60 mg/m2 to 100 mg/m2), ifosfamide (IFO) (3 g/m2 to 5 g/m2) and epirubicin (EPI) (30 mg/m2 to 50 mg/m2 is preferred.
Overall response rate of patients receiving chemotherapy was 66.6% (16.6% of them achieving partial response and 50% complete response) and overall response. After the response, two patients received RT. The overall median survival was 56 months (range 15-114 months) [26].
According NCCN guidelines, the most used regimens are cisplatin/carboplatin ± etoposide, cyclophosphamide +doxorubicin+vincristine (although high toxicity) or topotecan for elderly patients.
The Merkel cell carcinoma (MCC) can therefore show high sensitivity to chemotherapy, but it can also progress very quickly with a poor prognosis.
Merkel cell carcinoma may respond well to immunotherapeutic approaches. It has been shown that aberrant PI3K pathway activation may be a potential therapeutic target. Idelalisib is a PI3K inhibitor approved by the FDA for the treatment of B-cell lymphoma. Recent studies suggest that inhibition of this pathway not only influences the signaling pathway of B cells but also affects to the immunological tolerance and deregulate the removal of regulatory T cells [27].
Another possible therapeutic targets, although the mutation in c-kit and PDGFR are rare, would be the inhibition of these targets by drugs such as imatinib or pazopanib with improved survival in stage II studies [28].
And finally, other options that are not chemotherapy, as we have seen reflected in any of the cases reviewed is radiotherapy. The study also explains Ghadjar indications of radiotherapy, based primarily on locally advanced tumors as an improvement is achieved at the time of local recurrence vs. 93% 64% and distant disease 70% to 42%, but however not improve overall survival (56% vs. 46%; p=0.2) [29].
Regarding survival rates, they are comparable to those of melanoma, very often presenting metastasis and recurrence; lymph node involvement being the best predictor of survival. Five-year survival decreased progressively with increasing stage, from 60% to 80% for patients with stage I disease to 20% for those with stage IV disease. The difference in survival between stage I and II are significant, with 66% survival at 5 years versus 51% respectively [23,29].
Risk factors include tumors >2 cm, truncal lesions, male, age >65 years, lymphovascular invasion, locoregional metastatic involvement or input, previous tumors [29,30].
There are some studies that have shown that node-positive patients with an unknown primary tumor have an improved survival outcome compared to node-positive cases who have a visible tumor [9,31,32]. Regarding retroperitoneal cases without known primary tumor, conclusions are limited due to the lack of survival rates provided in most of the cases, with only 5 of 13 being known: less than 24 months in four of the reviewed cases and 25 months in one of them. This could be explained by the fact that most of the cases are single reports that are published before the follow up is completed.
There are two main theories about the origin of Merkell cell carcinomas without primary skin involvement that could explain an apparent retroperitoneal origin for MCC such as our case: the tumor is originated from immature pluripotential stem cells of the lymph nodes that acquire neuroendocrine differentiation upon malignant transformation or is a skin lesion that has spontaneously regressed after producing metastatic involvement of the retroperitoneum [6].
Cutaneous MCC regression has been reported in the literature and Cirillo established an incidence for spontaneous regression of 1.7% to 3% of the cases. This would support the second abovementioned theory for MCC with unknown primary [33].
Conclusion
Merkel cell tumor appears at a frequency of 1 case per million population, behaving a poor prognosis because it usually appears in advanced stages.
Although rare, the absence of skin lesion does not exclude the diagnosis and it has been reported in 2% to 5% of the cases.
As it happens in our case, PET-CT is postulated as a very sensitive and specific for this tumor type technique.
The characteristic form of expression by immunohistochemistry is positivity for CK20 and CD117 +, and negativity for CK7.
Treatment of choice is surgery in localized stages, receiving also a benefit from chemotherapy (cisplatin+etoposide is the most studied) and radiotherapy in advanced stages.
Five-year survival rate decreased progressively with increasing stage, from 60% to 80% for patients with stage I disease to 20% for those with stage IV disease. There are poor prognostic markers such as tumor size >2 cm, lymph node involvement, age >65 years, male sex and previous tumors. Node-positive cases with unknown primary tumors seem to have better prognosis. Conclusions about retroperitoneal cases with unknown primary tumor are limited due to the lack of data in most of the reported cases, but we found a survival rate less than 24 months in four and 25 months in one.
Retroperitoneal location for MCC without primary skin lesion is very rare, and there are only 4 published cases plus ours that could be classified as “primary retroperitoneal MCC”. Nodal origin or metastatic disease after primary cutaneous regression could explain these few reported cases.
18733
References
- Sidhu GS, Chandra P, Cassai ND (2005) Merkel cells normal and neoplastic: An update. Ultrastruct Pathol 29: 287-294.
- Albores-Saavedra J, Batich K, Chable-Montero F (2010) Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol 37: 20-27.
- Tai PT, Yu E, Tonita J (2000) Merkel cell carcinoma of the skin. J Cutan Med Surg 4: 186-195.
- Akhtar S, Oza KK, Wright J (2000) Merkel cell carcinoma: Report of 10 cases and review of the literature. J Am Acad Dermatol 43: 755-767.
- Quiroz-Sandoval OA, Cuellar-Hubbe M, Lino Silva LS (2015) Primary retroperitoneal Merkel cell carcinoma: Case report and literature review. Int J Surg Case Rep 19: 21-24.
- Boghossian V, Owen ID, Nuli B, Xiao PQ (2007) Neuroendocrine (Merkel cell) carcinoma of the retroperitoneum with no identifiable primary site. World J Surg Oncol 5: 117.
- Rossini D, Caponnetto S, Lapadula V (2013) Merkel cell carcinoma of the retroperitoneum with no identifiable primary site. Case Rep Oncol Med.
- Evoli LP, Marino E, Rosati E (2014) Recurrence of retroperitoneal Merkel cell carcinoma: A case report. Ann Ital Chir 85: 189-194.
- Tarantola TI, Vallow LA, Halyard MY (2013) Unknown primary Merkel cell carcinoma: 23 new cases and a review. J Am Acad Dermatol 68: 433-440.
- Noto R, Giaquinta A, Alessandria I (2008) Right leg swelling as primary presentation of metastatic Merkel cell carcinoma. Minerva Med 99: 341-345.
- Silberstein E, Koretz M, Cagnano E (2003) Neuroendocrine (Merkel cell) carcinoma in regional lymph nodes without primary site. Isr Med Assoc J 5: 450-451.
- de Cicco L, Vavassori A, Jereczek-Fossa BA (2008) Lymph node metastases of Merkel cell carcinoma from unknown primary site: Report of three cases. Tumori 94: 758-761.
- Abul-Kasim K, Söderström K, Hallsten L (2011) Central nervous system extensive involvement in Merkel cell carcinoma: A case report and review of the literature. J Med Case Rep 5: 35.
- Noell CM, Rose E, Nesbitt LT (2014) Merkel cell carcinoma presenting as right leg edema in a multiple myeloma patient. J Am Acad Dermatol 71: 50-52.
- Kontis E, Vezakis A, Pantiora E, Stasinopoulou S (2015) Merkel cell carcinoma of unknown primary site: Case presentation and review of the literature. Ann Surg Med 4: 434-437.
- Ghouri YA, Krishna SG, Kundu UR (2015) A case series and literature review of Merkel cell carcinoma metastasizing to pancreas. Dig Dis Sci 60: 1805-1812.
- Yaramada P, Lim BS, Flannery CM (2016) Merkel cell carcinoma of unknown primary with lymph node and mesenteric metastasis involving the pancreas and duodenum. J Gastrointest Oncol 7: 66-70.
- Lebbe C, Becker JC, Grob JJ (2015) Diagnosis and treatment of Merkel Cell Carcinoma. European interdisciplinary consensus-based guideline. Eur J Cancer 51: 2396-2403.
- (2016) National Cancer Comprehensive Network. NCCN Guidelines version 1.2016. Merkel Cell Carcinoma, 2016.
- Peloschek P, Novotny C, Mueller-Mang C (2010) Diagnostic imaging in Merkel cell carcinoma: Lessons to learn from correlation of 16 cases With sonography, CT, MRI and PET. Eur J Radiol 73: 317-323.
- Treglia G, Kakhki VR, Giovanella L (2013) Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in Patients With Merkel cell carcinoma: A systematic review J Clin Dermatol and meta-analysis 14: 437-447.
- Quijano SL (2016) Merkel cell carcinoma. Update. Rev Esp Patol 49: 158-168.
- Merkel Cell Carcinoma. In: AJCC Staging Manual (7thedn), Springer, New York 2010. 315.
- Merkel Cell Carcinoma. In: AJCC Staging Manual (8thedn), Springer, New York 2017.
- Pectasides D, Pectasides M, Psyrri A (2006) Cisplatin based chemotherapy for Merkel cell carcinoma of the skin. Cancer Invest 24: 780-785.
- Shiver MB, Mahmoud F, Gao L (2015) Response to idelalisib in a Patient With Stage IV Merkel-cell carcinoma. N Engl J Med 373: 1580-1582.
- Hughes MP, Hardee ME, Cornelius LA (2014) Merkel cell carcinoma: Epidemiology, target, and therapy. Curr Rep Dermatol 3: 46-53.
- Ghadjar P, Kaanders JH (2011) The essential role of radiotherapy in the treatment of Merkel cell carcinoma: A study from the rare Cancer network. Int J Radiat Oncol Biol Phys 81: 583-591.
- Tai PT, Yu E, Tonita J, Gilchrist J (2000) Merkel cell carcinoma of the skin. J Cutan Med Surg 4: 186.
- Chen KT, Papavasiliou P, Edwards K, Zhu F, Perlis C, et al. (2013) A better prognosis for Merkel cell carcinoma of unknown primary origin. Am J Surg 206: 752-757.
- Foote M, Veness M, Zarate D, Poulsen M (2012) Merkel cell carcinoma: The prognostic implications of an occult primary in stage IIIB (nodal) disease. J Am Acad Dermatol 67: 395-399.
- Cirillo F (2015) Spontaneous regression of primitive Merkel cell carcinoma. Rare Tumors 7: 5961.










