Key words
|
| |
| Amnesia; Piracetam; Scopolamine; Shatavri; Sodium Nitrite |
| |
Introduction
|
| |
| Dementia refers to several pathological states of brain, leading to disruption of multiple higher cortical functions including memory, reasoning, orientation, comprehension, learning capacity and emotional stability [1]. It is a major health problem in normal life as well as in various pathological conditions such as Alzheimer's disease, Pick's disease, cerebrovascular disease, hypoxic and ischemic encephalopathy, Parkinson's disease, alcoholism, drug abuse, brain tumor and infections like HIV and syphilis [2]. Senile dementia is a clinical syndrome affecting the elderly persons with loss of memory and cognition [3, 4]. Cure of cognitive disorders such as amnesia, attention deficit and Alzheimer’s disease is still a nightmare in the field of medicine. Nootropic agents such as piracetam[5] pramiracetam, aniracetam[6] and cholinesterase inhibitors like donepezil [7] are primarily used to improve memory, mood and behavior. However, the resulting adverse effects associated with these agents have limited their use [8, 9, 10 ]. Herbal medicines offer therapeutics for age related disorder like memory loss [11]. Catechin and gallic acid from Sanguisorbae radix, epicatchin and 3, 4 dihydroxy benzoic acid from Smilax rhizoma are Aβ induced neurotoxicity inhibitors [12]. Therefore, it seems worthwhile to explore the utility of traditional medicines for the treatment of various cognitive disorders. |
| |
| Traditionally, Asparagus racemosus (Shatavari) commonly known as “queen of herbs” has been used to treat neurodegenerative disorders such as senile dementia associated with Alzheimer disease [13, 14]. This herbaceous perennial plant (family: Liliaceace) is common at low altitudes in shade and tropical climates throughout India, Australia and Africa [15]. Its roots are finger like and clustered [16]. The plant is a well known medha rasayana [17]. ‘Rasayana’ enhances the functions of the whole body system and has been reported to treat generalized weakness [18]. Rasayana therapy arrests ageing (‘Vayasthapam’), increases life span (‘Ayushkaram’), intelligence (‘Medha’) and strength (‘Bala’) and thereby prevents disease [19]. Rasayana plants prevent ageing, re-establish youth, strengthen life, brain power and prevent diseases [20]. This implies that they increase the resistance of the body against any onslaught. Traditionally, the root extract of Shatavri is used as health tonic [21]. This common Indian home remedy is also used as a rejuvenator, promoter of strength, breast milk and semen [22]. It is reported to increase the formation and release of estrogens in brain and has potent antioxidant, immunostimulant, anti-dyspepsia and antitussive effects. Therefore, the present study was designed to investigate the effect of Asparagus racemosus Wild to prevent the scopolamine and sodium nitrite induced impairment of memory in mice using elevated plus-maze and Morris water maze as exteroceptive behavioral models. |
| |
|
Materials and Methods
|
| |
| Animals Swiss albino male mice (30±2 g) were procured from Indian Institute of Toxicology Research (IITR), Lucknow (Uttar Pradesh) India. They were housed in animal house provided with 12 hours light and dark cycles at 25±2ºC and had free access to water and standard laboratory diet (Ashirwad Industries, Chandigarh, India). The experimental protocol was approved by the Institutional Animal Ethical Committee and experiments were conducted according to the CPCSEA guidelines on the use and care of experimental animals. Experiments were carried out between 09:00 and 17:00 hours. Efforts were made to minimize animal suffering and optimum number of animals was used. |
| |
| Drugs Piracetam, scopolamine and sodium nitrite were procured from Sigma-Aldrich, Poole U. K., Cadila Health Care (Ahmedabad) and Center Drug House (New Delhi) respectively. All other chemicals used in the study were purchased from S. D. Fine Chemicals Ltd. (Boisar, India). All the drug solutions were prepared freshly prior to use. |
| |
| Plant material The roots of Asparagus racemosus Wild were purchased, from Himgiri Traders, Dehradun, in the month of July 2006. The roots were authenticated by Dr. H. B. Singh, Head and Taxonomist, Raw Materials, Herbarium and Museum at National Institute of Science Communication and Information Resources (NISCAIR, CSIR) New Delhi-110067, India. A voucher specimen no: NISCAIRE/RHMD/consult/06/730/47 is deposited in the same herbarium. |
| |
| Extraction Air dried roots (20 gm) of the plant were coarsely powered and extracted with methanol by continuous hot percolation method using Soxhlet apparatus” [23]. This was repeated thrice with fresh solvent each time. The extracts from all the three washes were pooled and concentrated using Rotaevaporator (Perfit) to obtain dark viscous mass. The residue was then dried at room temperature. The % yield of the methanolic root extract was found to be 22.59. The extract was subjected to phytochemical analysis as per protocols[24]. Phytochemical screening of the methanolic extract of the roots (M. Ar.) indicated presence of alkaloids, carbohydrates, glycosides, phenolic compounds, tannins, flavanoids, aminoacids, proteins, steroids, terpene, gum and mucilage. The extract was suspended in normal saline (0.9% w/v sodium chloride). |
| |
Exteroceptive Behavioral Models
|
| |
|
a) Elevated plus maze apparatus
|
| |
| Elevated plus-maze serves as the exteroceptive behavioral model to evaluate acquisition and retention of memory in mice [25]. The elevated plus maze for mice consists of two open arms (16 cm x 5cm) and two covered arms (16 cm x 5cm x12 cm) extended from a central platform (5cm x 5cm) and is elevated to a height 25 cm from the floor. Each mouse was placed at the end of an open arm, facing away from the central platform. Transfer latency (TL) time taken by mice to move from open arm to covered arm with all its four legs in elevated plus maze was noted. The mouse was allowed to explore the maze for another 2 min and then returned to its home cage. After 24 hrs of acquisition trials, TL was again noted as an index of retrieval. |
| |
|
(b) Morris water maze
|
| |
| Morris water maze was employed to evaluate learning and memory [26]. It consists of a circular water tank (diameter 150 cm and height 45 cm), filled with water maintained at 25°C. The water is made opaque with a white colored dye. The tank is divided into four equal quadrants with the help of two threads, fixed at right angle to each other on the rim of the pool. A platform (10 cm2) of 29 cm height is located in the center of one of these four quadrants. The position of platform and clues were kept consistent throughout the training session. In the present study, target quadrant was Q4. |
| |
| Acquisition trials: Each animal was subjected to four consecutive trials on each day with an interval of 5 min, during which mouse was allowed to escape on the hidden platform and was allowed to remain there for 20 sec. In case of the inability of the animal to locate the hidden platform within 90 sec, it was gently guided by hand to the platform and allowed to remain there for 20 sec. Escape latency time (ELT) to locate the hidden platform in water maze was noted as an index of acquisition and learning. In preliminary study, trial was conducted to familiarize the mice with the task and was not counted. Mouse was subjected to acquisition trials for four consecutive days. Starting position on each day to conduct four acquisition trials was changed as follow: |
| |
 |
| |
| Retrieval trial : On the next day, platform was removed and each mouse was allowed to explore the pool for 90 s. Mean time spent of the animal in each of four quadrants was noted. The mean time spent by the mouse in target quadrant (Q4) for searching the hidden platform was noted as an index of retrieval. The experimenter always stood at the same position. Care was taken that relative location of water maze with respect to other objects in the laboratory, serving as prominent visual clues was not disturbed during the total duration of study. |
| |
| Interoceptive Behavioral Models: (a) Scopolamine amnesia (b) Sodium nitrite amnesia. Scopolamine hydrochloride (0.4 mg/kg, i.p.) and sodium nitrite (75 mg/kg, i.p.) were administered interaperitonealy to induce experimental amnesia in male albino mice. |
| |
| Experimental Protocol: The animals were divided into twenty six groups. Each group comprised of six animals. |
| |
| • Group I (Control) normal untreated mice were exposed to EPM for measuring TL on first day and again after 24 hrs. |
| |
| • Group II [Vehicle control (normal saline)] was administered 0.9% sodium chloride (10 ml/kg, i.p.). TL was recorded after 45 min and then after 24 hr (2nd day) using elevated plus maze. |
| |
| • Group III and IV (Scopolamine hydrochloride and sodium nitrite) were injected with scopolamine hydrochloride (0.4 mg/kg, i.p.) and sodium nitrite (75 mg/kg) respectively 45 min before exposure to elevated plus maze on first day. TL was recorded 45 min after the injection and again after 24 hrs i.e. on 2nd Day. |
| |
| • Group V (Piracetam) was administered piracetam (400 mg/kg; i.p.) 60 min prior to elevated plus maze exposure on first day. TL was measured on first day and 2nd day. |
| |
| • Groups VI and VII (M. Ar. per se) were injected low and high dose of methanolic root extract (75 and 150 mg/kg, i.p., respectively) for 7 days. TL was noted after 60 min of administration on 7th day and after 24 hrs i.e. 8th day. |
| |
| • Group VIII (Piracetam and scopolamine hydrochloride) was administered piracetam (400 mg/kg, i.p.) and scopolamine hydrochloride (0.4 mg/kg, i.p.), 60 and 45 min respectively before first day exposure on elevated plus maze. TL was recorded on first day and 2nd day. |
| |
| • Group IX (Piracetam and sodium nitrite) was injected piracetam (400 mg/kg, i.p.) and sodium nitrite (75 mg/ kg, i.p.) 60 and 45 min respectively before first day exposure on elevated plus maze. TL was recorded on first day and 2nd day. |
| |
| • Group X and XI (M. Ar. + scopolamine) were administered methanolic root extract (75 and 150 mg/kg) and scopolamine 60 and 45 min respectively, before first day exposure on elevated plus maze. TL was recorded on 1st day and 2nd day. |
| |
| • Group XII and XIII (M. Ar. + sodium nitrite) were administered methanolic root extract (75 and 150 mg/kg) and sodium nitrite 60 and 45 min respectively, before first day exposure on elevated plus maze. TL was recorded on 1st day and 2nd day. |
| |
| • Group XIV (Control) normal untreated mice were subjected to MWM for measuring ELT (from day 1 to day 4) and time spent in target quadrant (TSTQ) on day 5. |
| |
| • Group XV [Vehicle control (normal saline)] was administered 0.9% sodium chloride (10 ml/kg, i.p.), 45 min before acquisition trials conducted on four consecutive days (day1 to day 4) and 45 min before retrieval trial conducted on day 5. |
| |
| • Group XVI (Piracetam per se): was injected with Piracetam (400 mg/kg) 60 min before acquisition trials conducted on four consecutive days (day1 to day 4) and saline was administered 45 min before retrieval trial conducted on day 5. |
| |
| • Group XVII and XVIII (M. Ar. per se) were injected with methanolic root extract (75 and 150 mg/kg) 60 min before acquisition trials conducted on four consecutive days (day1 to day 4) and saline was administered 45 min before retrieval trial conducted on day 5. |
| |
| • Group XIX (Scopolamine hydrochloride) was injected with scopolamine hydrochloride (0.4 mg/kg, i.p.) 45 min before acquisition trials conducted on four consecutive days (day1 to day 4) and saline was administered 45 min before retrieval trial conducted on day 5. |
| |
| • Group XX (Sodium nitrite) was injected with sodium nitrite (75 mg/kg) 45 min before acquisition trials conducted on four consecutive days (day1 to day 4) and saline was administered 45 min before retrieval trial conducted on day 5. |
| |
| • Group XXI (Piracetam + scopolamine hydrochloride) was administered piracetam (400 mg/kg, i.p.) and scopolamine (0.4 mg/kg, i.p.) 60 min and 45 min respectively before acquisition trials conducted on four consecutive days (day1 to day 4). On day 5, these mice were administered saline 45 min before retrieval trial. |
| |
| • Group XXII (Piracetam + sodium nitrite) was administered piracetam (400 mg/kg, i.p.) and sodium nitrite (75 mg/kg, i.p.) 60 min and 45 min respectively before acquisition trials conducted on four consecutive days (day1 to day 4). On day 5, these mice were administered saline 45 min before retrieval trial. |
| |
| • Group XXIII and XXIV (M. Ar. + scopolamine) were administered methanolic root extract (75 and 150 mg/kg) and scopolamine (0.4 mg/kg, i.p.) 60 min and 45 min respectively before acquisition trials conducted on four consecutive days (day1 to day 4). On day 5, these mice were administered saline 45 min before retrieval trial. |
| |
| • Group XXV and XXVI (M. Ar. + sodium nitrite) were administered methanolic root extract (75 and 150 mg/kg) and sodium nitrite (75 mg/kg) 60 min and 45 min respectively before acquisition trials conducted on four consecutive days (day 1 to day 4). On day 5, these mice were administered saline 45 min before retrieval trial. |
| |
| Rota-rod test: Each mouse was used only once and total of 6 mice were used for each treatment. Motor co-ordination was considered to be impaired if the animal fell-off from the rotating-rod within 9 sec. In control or drug treated groups, assessment of motor coordination was made before and after administration of vehicle or drugs during acquisition and retrieval trial. |
| |
| Statistical analysis: Results are expressed as means + standard error of the mean (SEM). The data was analyzed with Graph Pad Prism statistical analysis using two-way analysis of variance followed by Bonferroni post-hoc test except in retrieval trial of Morris water maze for which analysis was done by one way analysis of variance followed by Tukey’s test. p value of less than 0.05 was considered to be statistically significant. |
| |
Results
|
| |
|
Effect of scopolamine and sodium nitrite on TL of mice using elevated plus-maze
|
| |
| Transfer latency (TL) of first day reflected learning behavior of animals. Whereas, TL of next day reflected retention of learning behavior. TL of control group animals decreased significantly on 2nd day i.e. after 24 hr of training on elevated plus-maze. Scopolamine (0.4 mg/kg, i.p.) and sodium nitrite (75 mg/kg, i.p.) administered in respective groups III and IV before elevated plus maze exposure on 1st day, significantly increased (p<0.05) 1st and 2nd day TL, when compared to respective 1st and 2nd day TL in control group (Table 1). |
| |
|
Effect of Piracetam and methanolic root extract (MAr) on scopolamine and sodium nitrite induced amnesia using elevated plusmaze
|
| |
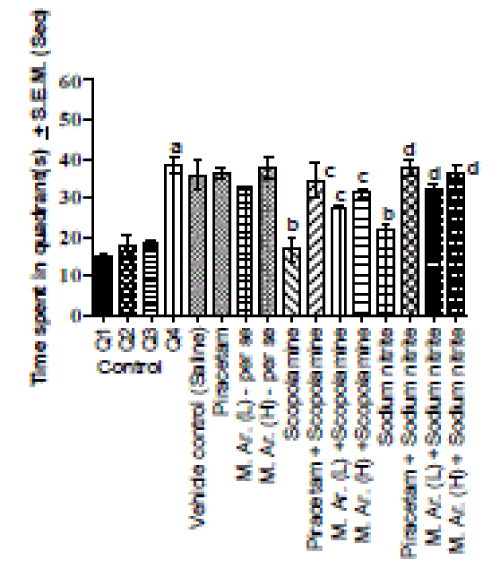
| Pretreatment for seven days with piracetam (group V) per se at the dose of 400 mg/kg, i.p. did not produce any significant effect on 2nd day transfer latency time of mice as compared to transfer latency time of control group on day 1. Pretreatment for seven days with M. Ar. (group VI and VII) per se at the doses of 75 and 150 mg/kg, i.p. significantly (p<0.05) decreased the transfer latency in mice as compared to transfer latency of control group on day 2 (Table 1). The extract per se at the dose of 150 mg/kg, i.p. exhibited improvement in normal short term memory by 85% (Fig. 1). Pretreatment for seven days with piracetam (400 mg/kg, i.p.), the standard drug and MAr (150 mg/kg, i.p.) have significant increase in percent retention by 74% and 64%; 92% and 91% respectively in scopolamine and sodium nitrite treated mice with respective control indicating the reversal of scopolamine and sodium nitrite induced amnesia. |
| |
|
Effect of Piracetam and M. Ar. on ELT and TSTQ during retrieval trial of memory using Morris water maze
|
| |
| In control groups, mice administered with saline (10 ml/kg, i.p.) 30 min before acquisition trials demonstrated significant decrease in ELT as compared to day 1 (Table 2). Learning is improving at the same time. Moreover, mice administered with saline (10 ml/kg, i.p.) 30 min before retrieval trial conducted on day 5 spent significantly more time in target quadrant (Q4) in search of missing platform as compared to time spent in other quadrants (Q1, Q2 and Q3) during retrieval trial (Fig. 1). Control group shows normal retrieval of memory. Piracetam (400 mg/kg, i.p.), M. Ar. (150 mg/kg, i.p.) administered 60 min and saline (10 ml/kg, i.p.), vehicle used for plant drug (M. Ar.) administered 30 min before acquisition trials did not produce any significant per se effect on decrease in ELT and an increase in time spent in Q4 target quadrant noted in control group during acquisition and retrieval trials conducted on day 1 to day 4 and day 5 respectively (Table 2 and Fig. 1). The above mentioned treatments did not alter normal retrieval of memory. |
| |
|
Effect of scopolamine and sodium nitrite on acquisition and retrieval of memory using Morris water maze
|
| |
| Scopolamine (0.4 mg/kg, i.p.) and sodium nitrite (75 mg/kg, i.p.) significantly attenuated the decrease in ELT during successive acquisition trials conducted on day 1 to day 4 (Table 2) and also reduced the increase in time spent in target quadrant (Q4) markedly in search of missing platform during retrieval trial conducted on day 5 (Fig. 1). Scopolamine and sodium nitrite both produced amnesia. |
| |
|
Effect of Piracetam and M. Ar. on scopolamine and sodium nitrite induced amnesia using Morris water maze
|
| |
| Pretreatment of mice for seven days with piracetam (400 mg/kg) and M. Ar. (150 mg/kg, i.p.) 60 min before training significantly decreased EL ?FScopolamine (3,40) =169.1; FScopolamine (3,40) =50.12?in scopolamine and sodium nitrite treated mice (Table 2) during the acquisition trials conducted on four consecutive days (day 1 to day 4). Like piracetam, the extract significantly increased the time spent in target quadrant (Q4) by mice?FScopolamine(3,20) =7.515; Fsodium nitrite (3,20)=15.38?treated with scopolamine and sodium nitrite during the retrieval trial on day 5 (Fig. 1). a = p>0.05 Vs time spent in other quadrants in control; b = p>0.05 Vs time spent in target quadrant (TSTQ) in control; c = p>0.05 Vs time spent in target quadrant (TSTQ) in scopolamine; d p>0.05 Vs time spent in target quadrant (TSTQ) in sodium nitrite. |
| |
Discussion
|
| |
| A significant decrease in transfer latency time of mice noted on 2nd day as compared to their transfer latency on first day indicated normal memory in elevated plus maze tests. Similarly, a marked decrease in escape latency time (ELT), during subsequent trials as compared to first exposure on MWM, denotes normal learning ability. Whereas, enhancement in the time spent by the animal in the target quadrant (in search of missing platform) reflects successful retention of learned task (or memory). The Morris water maze-test [27] and elevated plus-maze [28] have been used extensively to investigate learning and memory in rodents. Since, both of these different memory models produced uniform results on memory scores, the built in limitation if any present in an individual experiment model was automatically taken care of. Pilot studies indicated that single dose administration of M. Ar. had no acute behavioral effects, hence it was administered interaperitonealy at two dose levels (75 and 150 mg/kg, i.p.). |
| |
| Scopolamine and sodium nitrite treatments produced a significant learning and memory deficits as indicated by decrease in EPM and MWM performance. These findings are in line with earlier reports [29, 30, 31]. Central cholinergic system plays a crucial role in the process of learning and memory. Cholinomimetic drugs have been shown to enhance memory, whereas centrally acting, muscarinic cholinergic receptor blockers like scopolamine [32, 33] are reported to impair memory and therefore have been widely used as animal models to study the antiamnestic potential of new drugs [34, 35]. The root extract of Asparagus racemosus wild is reported to increase the formation and release of estrogens in brain [36] and has potent antioxidant [37], rejuvenative [38] effects and aid in the treatment of neurodegenerative disorders [39, 40]. |
| |
| Sodium nitrite has been reported to induce severe vasodilatation [41] and methemoglobinemia [42] which may be responsible to produce cerebral hypoxia [43] that initiates generation of free radicals and may damage hippocampus. Hypoxia is noted to release adenosine [44] and consequent inhibition of synaptic transmission [45, 46] Hippocampus formation is rich in adenosine A1 receptors [47]. Transient hypoxia or ischemia induced release of adenosine [48, 49] and consequent activation of A1 receptors and opening of K+ channels [50] may contribute to sodium nitrite induced amnesia. Administration of vehicle did not produce any modification in the EPM and MWM performance of control animals. Treatment of piracetam and M. Ar. significantly and dose dependently attenuated memory deficits induced by scopolamine and sodium nitrite, as reflected by improvement in the EPM and MWM performance. |
| |
Conclusion
|
| |
| Administration of methanolic roots extract of Asparagus racemosus prevented scopolamine and sodium nitrite induced experimental amnesia and may be a great potential in memory deficits, nevertheless further studies are required to elucidate memory improving mechanisms of the plant. |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
 |
| Figure 1 |
|
| |








