Commentary - (2025) Volume 0, Issue 0
Revolutionizing Liver Diagnostics: Velacur™ as a Non-Invasive Tool for MASLD and MASH – A Commentary on Recent Advances
Muhammad Y. Sheikh1,
Muhammad Nameer Hasan1 and
Caitlin Schneider2*
1Fresno Clinical Research Center, Fresno, California, USA
2Sonic Incytes Medical Corp, Vancouver, Canada
*Correspondence:
Caitlin Schneider,
caitlin@sonicincytes.com,
Canada,
Email:
Received: 15-Apr-2025, Manuscript No. IPACLR-25-15663;
Editor assigned: 17-Apr-2025, Pre QC No. IPACLR-25-15663 (PQ);
Reviewed: 28-Apr-2025, QC No. IPACLR-25-15663 ;
Revised: 05-May-2025, Manuscript No. IPACLR-25-15663 (R);
Published:
14-May-2025
Keywords
Velacur, MASLD, MASH, Non-invasive
diagnostics, Liver fibrosis, Ultrasound-based technology,
Steatosis, Biopsy alternatives
Introduction
The increasing prevalence of MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease) and MASH (Metabolic Dysfunction-Associated Steatohepatitis) poses a growing challenge in the field of hepatology. Traditional diagnostic methods, particularly liver biopsy, remain the gold standard but come with several limitations, including invasiveness, high cost, post-procedural pain/complications, and sampling variability [2]. Non-invasive imaging techniques, such as Velacur™, an advanced ultrasound-based technology, are emerging as potential game-changers, providing an opportunity to assess liver stiffness and fat content with high accuracy and without needing biopsy [3].
Sheikh et al.'s recent study (2025) on Velacur highlighted its promising diagnostic accuracy compared to liver biopsy results in patients with moderate-to-advance MASLD and MASH. This commentary will analyze the findings from their work and review recent advances and updates related to the development and application of Velacur in liver diagnostics.
Velacur™: The Technology and Its Role in Liver Disease Diagnostics
Velacur™ uses quantitative ultrasound to measure liver stiffness (a marker for fibrosis) and fat fraction (a marker for steatosis) [3], [6]. These two features are critical in clinically diagnosing MASLD and MASH. Excessive fat accumulation and liver fibrosis development, if left untreated, can lead to cirrhosis and hepatocellular carcinoma [4].
Recently, Sheikh et al. compared Velacur's evaluation with a liver biopsy, which remains the gold standard for the histological staging of liver disease. In this study, Velacur demonstrated high accuracy in diagnosing advanced liver fibrosis and steatosis, making it an excellent candidate for replacing or supplementing liver biopsy in routine outpatient practice [1]. This study confirmed previous findings, in which Velacur™ showed excellent correlation and discriminatory ability when using MRE and MRI-PDFF as the gold standard [3].
As new medications are becoming available for patients with MASLD and MASH, it is becoming ever more important to correctly assess and monitor these patients at the point of care in a more cost-effective and less invasive manner. Seen in Figure 1, Velacur shows little overlap in defining patients in the F1 vs. F2 range, which is critical to determining which patients will be eligible for currently available treatments [10].
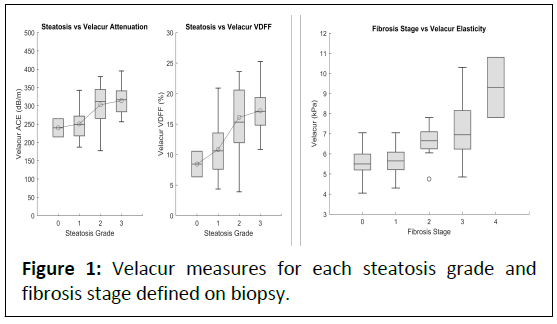
Figure 1: Velacur measures for each steatosis grade and
fibrosis stage defined on biopsy.
Recent Advances and Innovations
Recent updates in Velacur’s development further support its growing role in liver diagnostics in clinical practice. Improved software algorithms and machine learning have improved the device’s accuracy and allowed for real-time, automatic analysis of liver images. These improvements increase the device’s precision and broadly impact its applications in various clinical settings, including primary care and low-resource environments [5].
Key updates include
Improved AI-Based Image Analysis: Recent developments have introduced AI-driven analysis into Velacur’s imaging system, enabling further accurate segmentation of liver tissue and improving the detection of shear waves within the liver. This development reduces operator dependency and enhances the reproducibility of results across different healthcare settings [5].
Velacur Determined Fat Fraction (VDFF): Recently, interest in creating a clinic-based tool that can match MRI-PDFF led to the development of VDFF [7]. The VDFF is a measure designed to estimate MRI-PDFF by using a combination of ultrasound imaging parameters. It was shown to be more accurate than CAP when compared to MRI-PDFF [8] and biopsy [1].
Wider Clinical Applications: While the initial study focused primarily on clinical diagnosis of MASLD/MASH, recently published results have expanded Velacur™’s application use for assessing and monitoring patients on newly developed medications, such as Rezdiffra [8]. This versatility has increased its potential as a universal tool for non-invasive liver disease diagnostics.
Challenges and Limitations
Despite its promise, Velacur™ faces several challenges that
need to be addressed for wider clinical adoption:
Population Diversity: Most early studies have focused on specific populations with metabolic risk factors, such as obesity and diabetes. More extensive, multi-center studies involving diverse patient populations must fully validate Velacur™’s performance across various ethnic groups and disease profiles.
Comparative Efficacy: While Velacur™ has shown excellent results compared to liver biopsy [1], MRE and MRI-PDFF [3], further validation trials with larger cohorts of patients comparing these technologies will be crucial to defining its roles in clinical practice.
Cost and Accessibility: While Velacur™ promises to reduce the need for invasive procedures, cost is always a consideration, particularly in low-income regions. Cost-effectiveness studies are needed to determine the device’s long-term value in resource-constrained settings.
Future Directions and Conclusion
As non-invasive technologies continue to evolve, Velacur™ stands at the forefront of liver disease diagnostics, with its potential to significantly alter the clinical management of MASLD and MASH. Future directions for research include:
• Expanding multi-center, randomized controlled trials to confirm the device’s utility across diverse populations.
• Integrating AI-based diagnostic tools to enhance Velacur™’s interpretative capabilities.
• Investigating the long-term predictive value of Velacur™ in monitoring liver disease progression and treatment outcomes.
In conclusion, Velacur™ represents a promising advance in providing safe, cost-effective, and accurate diagnostics for liver diseases. As research progresses, its widespread use could help address the growing global burden of MASLD, MASH and other liver conditions.
Conflict of Interest
MYS: Research grants from Akero, Allergan, Altimmune, Boston Pharmaceutical, Bausch Health, Conatus, Eli Lily, Genentech, Gilead, Hanmi, Intercept, Madrigal Pharmaceuticals, NGM Biopharmaceuticals, Northsea, Poxel, Salix Pharmaceuticals, Shire, Sonic Incytes, Terns Pharmaceuticals and Viking Therapeutics. Speaking engagements: AbbVie, Salix Pharmaceutical, Intercept, Gilead, Madrigal, Phathom Pharmaceuticals.
MNH: None
CS: Caitlin Schneider is an employee of Sonic Incytes Medical Corp.
References
- Sheikh MY, Hasan N, Almozuaghi M, Akhtar NM, Grewal Y, et al. (2025) Accuracy of Velacur in Assessing MASLD and MASH Patients Using Biopsy as the Gold Standard. Diagnostics. 15:615.
[Crossref] [Google Scholar] [Pubmed]
- Harrison SA, Dubourg J (2024) Liver biopsy evaluation in MASH drug development: Think thrice, act wise. J Hepatol.
[Crossref] [Google Scholar] [Pubmed]
- Loomba R, Ramji A, Hassanein T, Yoshida EM, Pang E, et al. (2024) Velacur ACE outperforms FibroScan CAP for diagnosis of MASLD. Hepatol Commun. 8:e0402.
[Crossref] [Google Scholar] [Pubmed]
- Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, et al. (2023) AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatol. 77:1797-1835.
[Crossref] [Google Scholar] [Pubmed]
- Honarvar M, Lobo J, Schneider C, Wolfe N, Gawrieh S, et al. (2024) Deep learning based shear wave detection and segmentation tool for use in point-of-care for chronic liver disease assessments. Ultrasound Med Biol. 50:1812-20.
[Crossref] [Google Scholar] [Pubmed]
- Curry MP, Tam E, Schneider C, Abdelgelil N, Hassanien T, et al. (2024) The Use of Noninvasive Velacur® for Discriminating between Volunteers and Patients with Chronic Liver Disease: A Feasibility Study. Int J Hepatol 2024:8877130.
[Crossref] [Google Scholar] [Pubmed]
- Honarvar M, Lobo J, Schneider C, Klein S, Smith GI, et al. Methods and validation of velacur determined fat fraction in patients with MASLD. WFUMB Ultrasound Open. 2:100061.
[Crossref] [Google Scholar]
- Schneider, C., Hennan, j. et al. (2025) Velacur's VDFF outperforms FibroScan CAP in assessment of liver steatosis in a large cohort of MASLD and MASH patients. MashTag proceedings.
- Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, et al. (2024) A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. NEJM. 390:497-509.
[Crossref] [Google Scholar] [Pubmed]
- Noureddin M, Charlton MR, Harrison SA, Bansal MB, Alkhouri N, et al. (2024) Expert panel recommendations: practical clinical applications for initiating and monitoring resmetirom in patients with MASH/NASH and moderate to noncirrhotic advanced fibrosis. Clin Gastroenterol Hepatol.
[Crossref] [Google Scholar] [Pubmed]
Citation: Kim H, Hur M, d Onofrio, Zini G (2025) Commentary on “Real-World Application of Digital Morphology Analyzers: Practical Issues and
Challenges in Clinical Laboratories’’. Ann Clin Lab Vol.13 No.S5: 001
Copyright: © 2025 Kim H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which
permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.







