Keywords
Right ventricular high septal pacing, Right ventricular apical pacing, Pacing induced dyssynchrony, Cardiac resynchronization therapy, Selected site pacing
Introduction
Independent of permanent pacing indication, a ventricular pacing lead is currently included in 99.5% of all pacemaker implantations [1]. Published data from the DANPACE trial [2] and technical advancements in device algorithms designed to prevent unnecessary ventricular pacing, are likely the major reasons for this. The right ventricular (RV) apex remains the preferred implant site for endocardial transvenous ventricular leads due to ease of placement, electrical stability, reliability, and lead design. It has been documented however that RV apical pacing can induce non physiologic ventricular activation patterns [3] resulting in dyssynchrony. Prolonged dyssynchrony has a negative impact on systolic and diastolic function, resulting in a higher risk for heart failure hospitalization and a negative impact on long term outcome [3,4]. In patients requiring ventricular pacing, alternative RV pacing sites (e.g. RV outflow tract [5], ventricular septum [6] and HIS-Bundle [7], biventricular (BiV) pacing modes [8,9] and isolated left ventricular (LV) pacing [10,11] have been investigated in an attempt to reduce pacing-induced dyssynchrony. High septal ventricular pacing appears to be the most promising configuration of these alternative pacing sites [11]. This study was designed to investigate the procedural burden, implant feasibility and acute and chronic electrical performance of high septal pacing when compared to standard RV apical and BiV pacing.
Methods
Patient cohorts
The study was a single center clinical study designed to evaluate patient outcome over two years of follow up (including procedure, hospital discharge, 6-, 12- and 24 month follow ups). It investigated the procedural burden (defined as average implant procedure and fluoroscopy times) and feasibility (defined as implant success) of three different ventricular lead pacing sites- RV apical pacing (RV apical), biventricular pacing (BiV) and high septal pacing (RV septal). Eligible patients were those with a permanent pacemaker indication anticipated to require a high rate of RV pacing, but without an indication for CRT. The study was approved by the Institutional Ethics Committee and patients were enrolled to the study after written informed consent has been provided.
RV apical and BiV patients were prospectively randomized to their pacing modes via their participation in the BIOPACE trial [8]. Following the completion of the BIOPACE trial, a third, consecutive patient cohort meeting the same inclusion criteria and following the same follow-up schedule, was enrolled and included as the RV septal group. Patients were included over a period of 5.5 years.
Implant procedure
In patients with long lasting persistent atrial fibrillation a pacemaker without an atrial lead was implanted (bradyarrhythmia, Table 1). For all other patients a dual chamber or CRT pacemaker was implanted depending on randomization. The Identity ADx; Victory, Frontier I and II pacemaker models were implanted (St. Jude Medical, St. Paul, Minnesota, USA) with either an active fixation RV pacing lead in the RV septal group (Tendril, St Jude Medical), a passive fixation lead in the RV apical and BiV group (Isoflex, St Jude Medical) and a coronary sinus (CS) lead for the BiV group (Quicksite, St Jude Medical).
If needed, a steerable stylet (Locator, St. Jude Medical) was utilized to reach the parahisian ventricular septum in selected cases. Correct high septal lead placement in the RV septal group was confirmed by fluoroscopy in an angulated projection (left anterior oblique, Figure 1) in combination with standard electrocardiogram (ECG) configuration testing [12]. A similar method was used to ensure a posterolateral CS lead position in the BiV group.
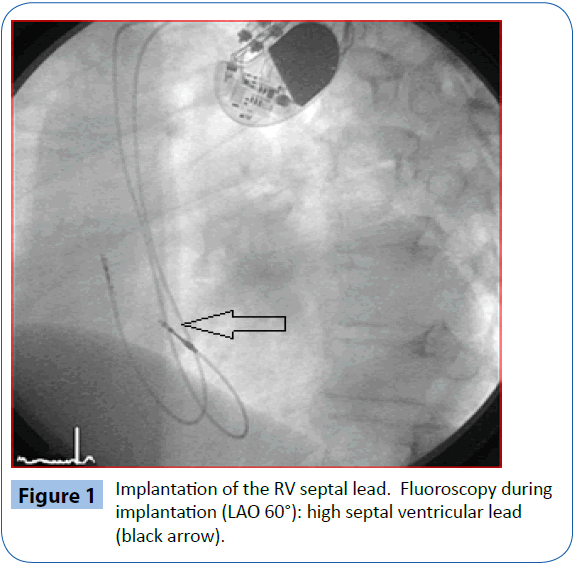
Figure 1: Implantation of the RV septal lead. Fluoroscopy during implantation (LAO 60°): high septal ventricular lead (black arrow).
Study endpoints
The primary objective of this study was to evaluate the procedural burden associated with ventricular lead implantation for the three groups. Burden was defined as average implant duration (including implantation of all system-required leads and the pacemaker) and total fluoroscopy time required for successful implantation. The secondary endpoints were feasibility (defined as implant success) and acute and chronic electrical performance of the RV leads (average pacing and sensing thresholds and pacing impedance).
Statistics
Categorical variables are presented as absolute numbers and percentages and are compared by Chi Square test. Continuous variables are expressed as means ± standard deviation or medians with interquartile ranges and are compared using a Kruskal-Wallis test or Wilcoxon test. In the case of multiple pair comparisons, Bonferroni correction was performed.
Results
Baseline characteristics
During a period of 5.5 years, 164 patients with a preserved ejection fraction (mean 56 ± 13%) and a permanent pacemaker but without CRT indication were included in the study (Table 1). 68.9% of the patients were male (n=113) with a mean age of 74 ± 9.3 years. Randomization procedure assigned 62 patients to the RV apical group (38%) and 60 to the BiV group (37%). In addition 42 consecutive patients were subsequently enrolled to the RV septal group (26%). Baseline characteristics were similar between groups and there were no significant differences in comorbidities (Table 1).
| |
RVapical group |
BiV group |
RVseptal group |
| Pacing Indication |
| Bradyarrhythmia |
n=9 (15%) |
n=11 (18%) |
n=0 (0%) |
| Binodal disease |
n=20 (32%) |
n=10 (17%) |
n=5 (12%) |
| AV-Block II° |
n=11 (18%) |
n=14 (23%) |
n=29 (69%) |
| AV-Block III° |
n=22 (35%) |
n=25 (42%) |
n=8 (19%) |
| Comorbidities |
| Coronary artery disease |
n=10 (16%) |
n=18 (30%) |
n=12 (29%) |
| Dilated cardiomyopathy |
n=7 (11%) |
n=5 (8%) |
n=2 (5%) |
| Valvulopathy |
n=3 (5%) |
n=3 (5%) |
n=2 (5%) |
| Hypertension |
n=57 (92%) |
n=55 (92%) |
n=38 (91%) |
| Renal Disease |
n=11 (18%) |
n=10 (17%) |
n=5 (12%) |
| Diabetes Mellitus |
n=18 (29%) |
n=14 (23%) |
n=11 (26%) |
| Stroke |
n=0 (0%) |
n=2 (3%) |
n=3 (7%) |
Table 1: Pacing indications and comorbidities.
Acute procedural implant data
All enrolled patients were successfully implanted with the appropriate pacing system according to allocated groups leading to 100% implant success (feasibility).
Mean implant duration for the BiV group was significantly longer than for both the RV apical group and RV septal group (p<0.001). The mean implantation duration for the BiV pacing system was 65 ± 30 minutes [(min), median 50 min.] compared to 40 ± 19 min (median 38 min) for the conventional pacemaker system implantation in the RV apical group and 36 ± 10 min (median 35 min) for the RV septal pacemaker implant.
Similar to the implant duration results, the average total fluoroscopy exposure during implantation was significantly shorter in the RV apical group (4.3 ± 5.5 min; median 2.5 min) and the RV septal group (4.0 ± 3.6 min; median 3.4 min) than in the BiV group (13.8 ± 10.9 min; median 10.2 min, p=0.05). Importantly, the overall procedure burden (implantation duration and fluoroscopy exposure) was not different for the placement of an RV septal lead vs an RV apical lead (p=ns).
Acute electrical performance
At implantation, the acute electrical performance of sensing, pacing and impedance parameters were acceptable for all three RV leads (Table 2) however, there were some minor differences documented between groups. In general, the RV septal leads had significantly lower ventricular sensing than both the RV apical and BiV leads (7.8 ± 3.4 mV vs 17.5 ± 20.8 mV and 14.2 ± 7.1 mV respectively, p<0.05) and higher pacing thresholds (0.7 ± 0.3 V/0.5 ms vs 0.5 ± 0.2 V/0.5 ms and 0.4 ± 0.1V/0.5 ms respectively p<0.05). In addition, average pacing impedance was lower (555 ± 73 Ω vs 774 ± 169 Ω and 821 ± 178 Ω p<0.05).
Chronic electrical performance
Electrical performance was tested every 6-months following implantation over a total follow up period of two years (24 ± 1 months). Table 2 and Figures 2-4 documents the values recorded at each follow-up over this time for RV leads of each group. In general, electrical performance remained stable for RV apical or RV septal lead location. However, the significant differences in performance of RV leads documented acutely between the RV septal group and the RV apical and BiV groups, persisted.
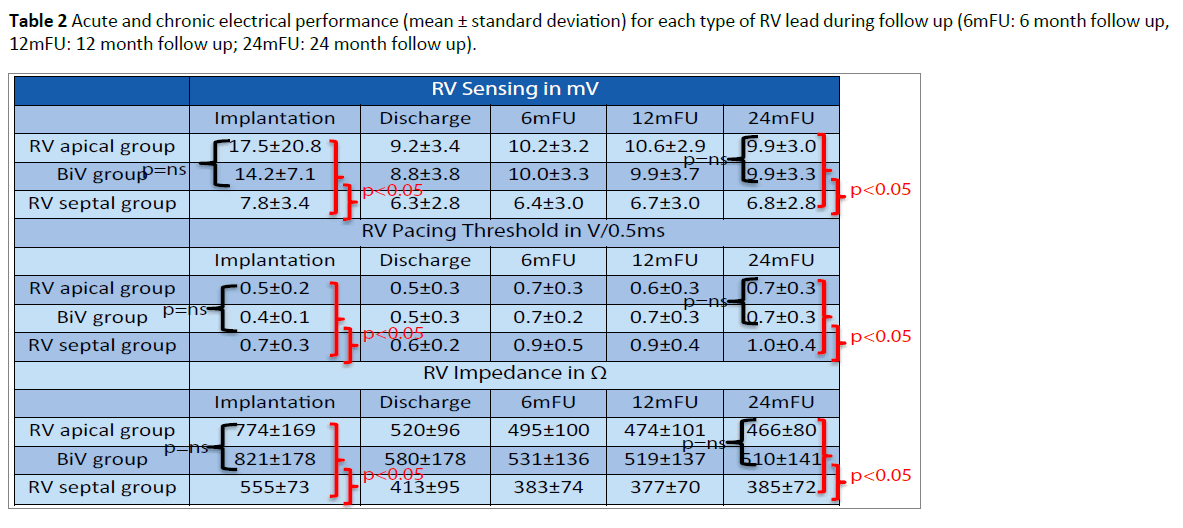
Table 2: Acute and chronic electrical performance (mean ± standard deviation) for each type of RV lead during follow up (6mFU: 6 month follow up, 12mFU: 12 month follow up; 24mFU: 24 month follow up).
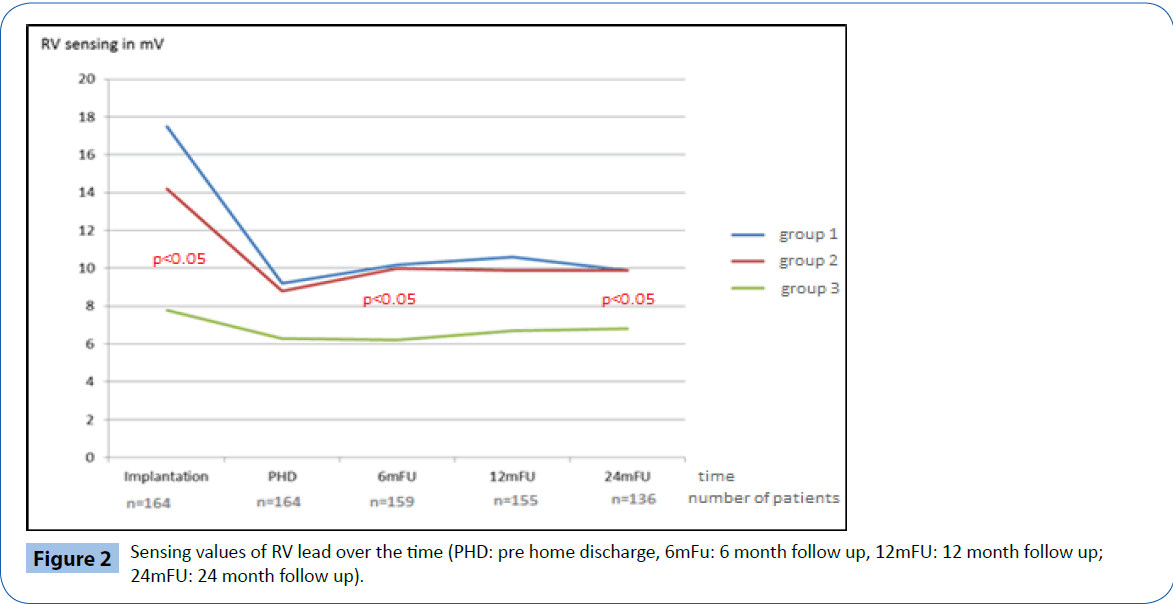
Figure 2: Sensing values of RV lead over the time (PHD: pre home discharge, 6mFu: 6 month follow up, 12mFU: 12 month follow up; 24mFU: 24 month follow up).
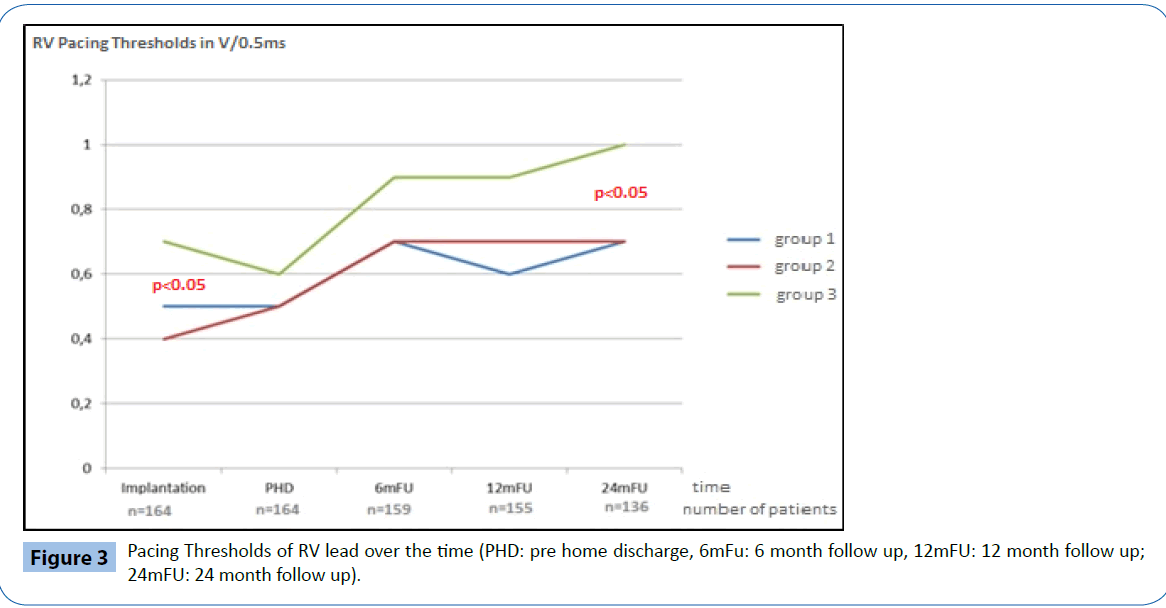
Figure 3: Pacing Thresholds of RV lead over the time (PHD: pre home discharge, 6mFu: 6 month follow up, 12mFU: 12 month follow up; 24mFU: 24 month follow up).
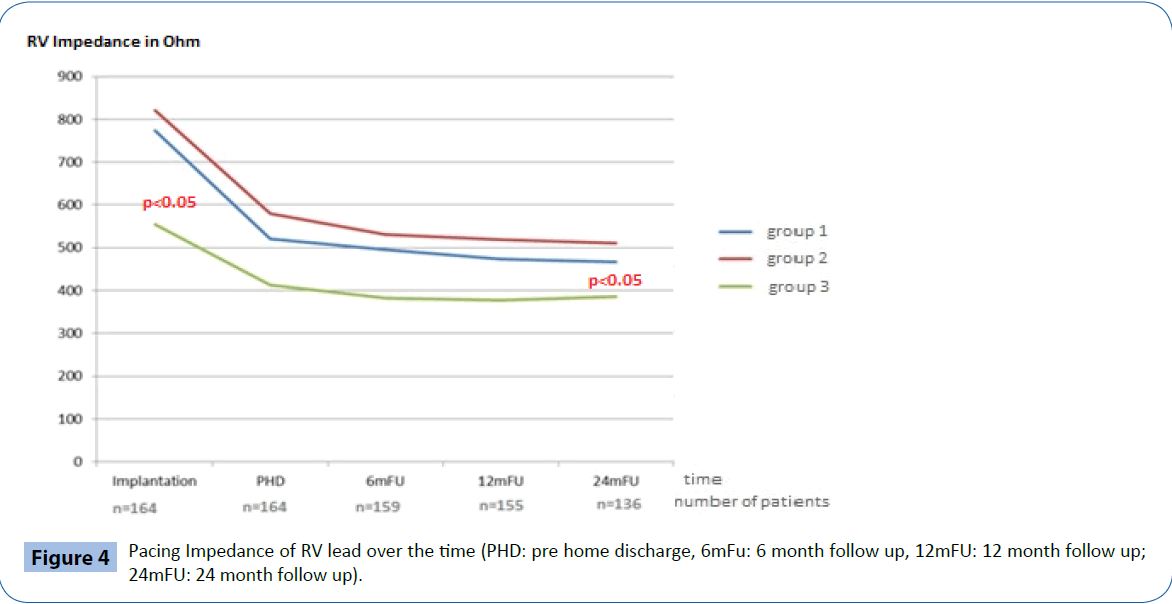
Figure 4: Pacing Impedance of RV lead over the time (PHD: pre home discharge, 6mFu: 6 month follow up, 12mFU: 12 month follow up; 24mFU: 24 month follow up).
Clinical outcome
Figure 5 documents the major clinical complications recorded throughout the duration of the trial. A total of 29 major clinical events were documented from 29 individual patients (17.7%). Of these, ten events were device or procedure-related events (6.4%); two in the RV apical group (3.2%); five in the BiV group (8.3%) and three in the RV septal group (7.1%). Only one RV lead related event was observed: Exit block with lead revision in one patient of the septal group.
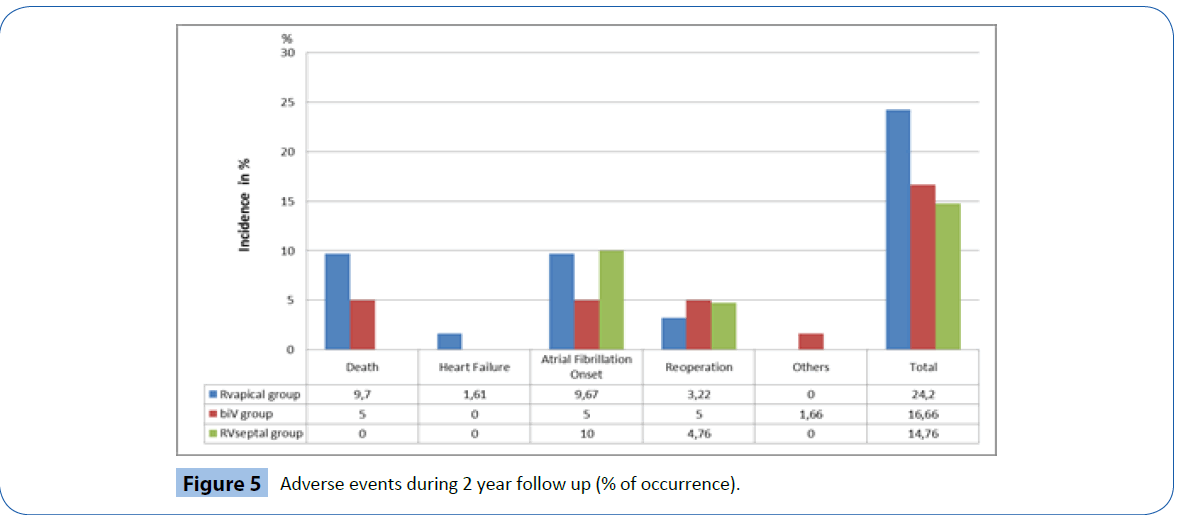
Figure 5: Adverse events during 2 year follow up (% of occurrence).
The majority of other major clinical events were related to atrial fibrillation (n=13) either new onset or chronic, including two patients who required isthmus ablations (one in the BiV group and one in the RV septal group). Two patients were hospitalized for heart failure in the RV apical group (one requiring upgrade to BiV ICD) while one patient was hospitalized for COPD in the BiV group. One patient in the RV septal group required a valve replacement during follow-up.
A total of nine deaths occurred during follow-up (5.5%) none of which were deemed procedure or device-related. Of the six patients that died in the RV apical group (9.8%) two deaths were attributed to heart failure and four to non-cardiac causes. The remaining three deaths were in the BiV group (5%), one death was related to worsening of chronic heart failure and two to noncardiac causes.
Discussion
RV apical pacing is known to cause desynchronization of ventricular contraction through the creation of a left bundle branch block-like morphology due to the change in activation of both ventricles. This may lead to impairment of LV function, induction of heart failure [12] and can also induce histological changes [13]. In contrast, CRT therapy with BiV pacing has become the gold standard in patients with heart failure, reduced LV function and left bundle branch block and data suggests that the wider the bundle branch block the better the response may be to CRT [14]. In patients with atrial fibrillation, it has also been shown that CRT can be beneficial as long as BiV stimulation is delivered sufficiently [14].
The protective effect of CRT on hemodynamic changes remains controversial, especially in patients with preserved LV ejection fraction. Some studies have not demonstrated any benefit of CRT in the prevention of the development of heart failure (PREVENT HF trial; 9) during short term follow up. The superiority of CRT over RV apical pacing in the prevention of the onset of heart failure after two years follow-up has been shown in the Block HFtrial [15]. However, the trial also demonstrated the complexity associated with implantation of an additional CS lead. In total more than 6% of patients in Block HF could not be successfully implanted with a CS lead, mainly because of difficulties related to CS access. In addition to the implant failure rate, 10.3% of successfully implanted patients had significant problems with the CRT system during follow-up resulting in the need for subsequent system revisions. Over two years of follow up we have also observed that the incidence of system related issues with CRT devices is approximately 15% and even in the BIOPACE trial [8], an implant failure rate of 14.8% was observed for the BiV group. In addition to the high failure to implant rate, for those patients who have a successful implantation, there often is a significantly longer implant duration and higher exposure to fluoroscopy, increasing risks for both patients and implanters. Finally, in addition to overall increased burden associated with implantation of a CRT system, the costs are substantially higher when compared to conventional systems.
As long as the advantage of CRT in patients with preserved LV function requiring ventricular pacing is not clearly shown, alternatives right ventricular sites should be considered. For these reasons, the current study was designed to evaluate whether RV septal pacing was a feasible alternative to RV apical pacing.
The data demonstrate that high RV septal pacing is feasible and comparable to RV apical pacing with respect to implantation burden when evaluated by average implantation time and fluoroscopy exposure. These similarities also translate into a comparable cost since you are using the same devices and leads and the same implantation effort is needed. While there was a statistically significant difference in both acute and chronic parameters of electrical performance for the RV septal leads when compared to a traditional apical position, it should be remembered that all measured values were in the normal and acceptable range, providing safe and effective ventricular stimulation from a clinical point of view. This is comparable with data using leads with passive fixation mechanism [16]. In addition, the incidence of lead related events was not significantly higher in the RV septal group vs RV apical.
Our findings are generally in line with the Protect Pace study [6], which shows similar results over long term follow up. In contrast to our observation of no difference in implantation burden, the Protect Pace authors did report a significant difference in implant duration and fluoroscopy time between septal and apical RV pacing. In the Protect Pace study, the location of RV septal lead is discussed extensively which likely resulted in the additional implant time. It is our belief that minor variations in septal lead location are not really critical, due to programming the ventricular output twice of the pacing threshold parahisian stimulation will result [7]. Pacing in the RV outflow tract can be avoided through ECG identification and LAO angulated fluoroscopy.
Some studies with longer observation have shown a continuous improvement in LV function [17,18] in RV septal paced patients.
In addition, the processes of remodeling and reverse remodeling are very dynamic and could be observed echocardiographically. RV septal pacing has been shown to induce a change in activation time (strain analysis) but without the appearance of dyssynchrony in contrast to RV apical pacing, where tissue velocity imaging may also document dyssynchrony. An echocardiographic sub-analysis (LV ejection fraction) of the Protect Pace patients demonstrated a worsening in all patients without any overall group effect, the strain analysis showed greater dyssynchrony in the RV apical group [19,20].
However, the time period of two years in our study might be too short to show hemodynamic benefits.
Limitations
One limitation of the study is that the RV septal pacing group was not enrolled at the same time as the RV apical and BiV groups which were included in the BIOPACE study. This could lead to potential differences in patient population however that was limited by using the same inclusion criteria and follow-up protocol. The absence of clinical and echocardiographic data is also a limitation since it could provide additional support to the hypothesis that LV function can be preserved by high septal RV stimulation rather than RV apical stimulation. Due to a short observational time period of two years hemodynamic outcome of this patients could not be measured.
Conclusion
There appears to be no additional implantation burden associated with high septal placement of RV leads and acute and chronic performance is acceptable. Since the disadvantages of RV apical pacing and the risks associated with BiV pacing are well known, we believe that it may be considered to placing the RV leads into the high RV septum in pacemaker dependent patients. Further hemodynamic data is needed to support this statement, particularly in patients with reduced ejection fraction and narrow native QRS complex.
Acknowledgements
NK, DP and MK received consulting fees from St. Jude Medical and Biotronik.
8205
References
- Akerstrom F, Pachon M, Puchol A, Jimenez-Lopez J, Segovia D et al. (2014) Chronic right ventricular apical pacing: Adverse effects and current therapeutic strategies to minimize them. International Journal of Cardiology 173:351-360.
- Auricchio A, DelnoyPP, Regoli F, Seifert M, Markou T et al. (2013) Collaborative study group. First-in-man implantation of leadless ultrasoundbased cardiac stimulation pacing system: novel endocardial left ventricular resynchronization therapy in heart failure patients. Europace 15: 1191-1197.
- Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G et al. (2013) ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 34:2281-329.
- Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I et al. (2013) Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial Investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 368: 1585-1593.
- de Cock CC,Giudici MC, TwiskJW (2003) Comparison of the haemodynamic effects of right ventricular outflow-tract pacing with right ventricular apex pacing: a quantitative review. Europace 5: 275-278.
- Deshmukh P,Casavant DA, Romanyshyn M, Anderson K (2000) Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation 101: 869-877.
- Domenichini G,Sunthorn H, Fleury E, Foulkes H, Stettler C, et al. (2012) Pacing of the interventricular septum versus the right ventricular apex: a prospective, randomized study. Eur J Intern Med 23: 621-627.
- Funck RC, Mueller HH, Lunati M, Piorkowski C, De Roy L et al. (2014) BioPace study group. Characteristics of a large sample of candidates for permanent ventricular pacing included in the Biventricular Pacing for Atrio-ventricular Block to Prevent Cardiac Desynchronization Study (BioPace). Europace 16:354-362.
- Janousek J, van Geldorp IE, Krupickova S, Rosenthal E, Nugent K et al. (2013) Working Group for Cardiac Dysrhythmias and Electrophysiology of the Association for European Pediatric Cardiology. Permanent Cardiac Pacing in Children: Choosing the Optimal Pacing Site A Multicenter Study.Circulation 27:613-623.
- KarpawichPP, Justice CD, Cavitt DL, Chang CH (1990) Developmental sequelae of fixed-rate ventricular pacing in the immature canine heart: an electrophysiologic, hemodynamic, and histopathologic evaluation. Am Heart J 119:1077-1083.
- Kaye GC, Linker NJ, Marwick TH, Pollock L, Graham L et al. (2015) Protect-Pace trial investigators. Effect of right ventricular pacing lead site on left ventricular function in patients with high-grade atrioventricular block: results of the Protect-Pace study. Eur Heart J 36:856-862.
- Markewitz A (2014)Jahresbericht des DeutschenHerzschrittmacher- und Defibrillatorregisters.
- Molina L, Sutton R, Gandoy W, Reyes N, Lara S, et al. (2014) Medium-term effects of septal and apical pacing in pacemaker-dependent patients: a double-blind prospective randomized study. Pacing ClinElectrophysiol 37: 207-214.
- Nielsen JC, Thomsen PE, Højberg S, Møller M, Vesterlund T, et al. (2011) A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J 32: 686-696.
- Pastore G, Zanon F, Baracca E, Rigatelli G, Corbucci G et al. (2013) How can we identify the optimal pacing site in the right ventricular septum? A simplified method applicable during the standard implanting procedure. Am J Cardiovasc Dis 3:264-272.
- Saito M, Kaye G,Negishi K, Linker N,Gammage M, et al. (2015) Dyssynchrony, contraction efficiency and regional function with apical and non-apical RV pacing. Heart 101: 600-608.
- Schwaab B,Kindermann M, Fröhlig G, Berg M, Kusch O, et al. (2001) Septal lead implantation for the reduction of paced QRS duration using passive-fixation leads. Pacing ClinElectrophysiol 24: 28-33.
- Stockburger M, Gomez-DoblasJJ, Lamas G, Alzueta J, Fernandez-Lozano I et al. (2011) Preventing ventricular dysfunction in pacemaker patients without advanced heart failure: results from a multicentre international randomized trial (PREVENT-HF). European Journal of Heart Failure 13: 633-641.
- Sweeney MO,Prinzen FW (2006) A new paradigm for physiologic ventricular pacing. J Am CollCardiol 47: 282-288.
- Tops LF,SchalijMJ, BaxJJ (2009) The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J Am CollCardiol 54: 764-776.












