Keywords
Theophylline; Kidney dysfunction; Neonatal asphyxia; GFR
Introduction
Perinatal asphyxia is one of the most common neonatal problems which significantly lead to neonatal morbidity and mortality [1]. It is the second most important cause of neonatal mortality after sepsis, it accounts about 30% of neonatal mortality worldwide [2]. The consequences of hypoxic ischemic insult usually tends to develop organ dysfunction after asphyxia [3]. One of the most common complications of birth asphyxia is renal dysfunction and it is of poor prognosis, which result in permanent renal damage in about 40% of survivors [4]. Acute kidney injury (AKI) occurs commonly in critically ill neonates and is associated with adverse outcomes. Studies showing that renal adenosine acts as a vasoconstrictor metabolite in kidney after hypoxemia, causing pre glomerular vasoconstriction and post glomerular vasodilatation and result in falling in Glomerular Filtration Rate (GFR), mainly within first 5-days of life. This pre glomerular vasoconstriction can be suppressed by nonspecific adenosine receptor antagonists like theophylline [5]. A lot of previous trials across the globe have reported that theophylline is effective in improving kidney dysfunction in asphyxial renal injury. Despite the heterogeneity in study populations and the diagnostic criteria used to define asphyxia and renal failure in neonates, results of several studies involving asphyxiated new born consistently demonstrated a common occurrence of renal dysfunction ranging between 17% to 61%. Furthermore, Kidney Disease Improving Global Outcomes 2012 (KDIGO) guidelines suggest that a single dose of theophylline can be given in new-borns with severe perinatal asphyxia, who are at high risk of acute kidney injury [6]. Despite the tremendous advancements in neonatal care in recent years, treatment of acute renal failure has remained essentially supportive and relies on maintaining fluid balance and homeostasis, correction of electrolyte disturbances and management of associated hypotension, acidosis and hypoxemia to reduce renal vasoconstriction and improve renal perfusion. Nevertheless, several experimental studies have been conducted to examine various pharmacological agents to prevent or ameliorate kidney damage post asphyxia. Theophylline has been demonstrated in several animal and human studies to result in improvement of kidney function in conditions associated with increased renal adenosine content such as asphyxia. Moreover, the appropriate dose of theophylline therapy requires further investigation [1]. Thus, the aim of the study is to assess the efficacy of theophylline in the treatment of kidney dysfunction in neonates after asphyxia.
Patients and Methods
This study was conducted in Neonatal Intensive Care Unit at Zagazig University Hospital and Al-Ahrar Teaching Hospital, over a period of 6 months from October, 2017 to March, 2018. Written informed consent was obtained from a parent or guardian, and the study was approved by the Institutional Review Board, Faculty of Medicine, Zagazig University. A total of 82 patients met the inclusion criteria of perinatal asphyxia: 41 neonates were enrolled in theophylline group and 41 were enrolled in the placebo group. Infants eligible to be included in the study were of term gestation with a weight more than 2500 g. All neonates were undergone full general examination for severe birth asphyxia manifested by any two of the following:
(a) An Apgar score of up to three at 1 minute or up to five at 5 minutes [7].
(b) Thompson score of more than 15 [8].
Infants were excluded if they were; preterms, congenital anomalies, history of maternal drugs causing neonatal depression and if the babies required a mechanical mode of ventilation or had culture positive sepsis.
All of 82 infants who were enrolled in the study were divided into two groups:
1. Theophylline-treated group which receive a single dose of intravenous theophylline (5 mg/kg, 0.25 ml/kg) [4],
2. Placebo which receive an equal volume of saline over a five minute period within the first hour of birth.
Fluid intake and urine output were recorded every 24 hr at days 1, 3 and 5 of neonatal life to assess the following investigations: Creatinine levels in serum and urine, Sodium excretion and glomerular filtration rate (GFR) was also estimated on these alternate days using Schwartz’s formula: GFR (mL/minute/1.73 m2)=0.45 × length (cm)/plasma creatinine (mg/100 mL) [9]. In addition, follow up of possible side effects of theophylline which are; Vomiting, Tachycardia, Convulsions, Hypokalemia and Hyperglycemia [10].
Statistical analysis
Data analysis was performed using the software SPSS (Statistical Package for the Social Sciences) version 20. Quantitative variables were described using their means and standard deviations. Categorical variables were described using their absolute frequencies and to compare the proportion of categorical data, chi square test was used. Kolmogorov-Smirnov (distribution-type) and Levene (homogeneity of variances) tests were used to verify assumptions for use in parametric tests. To compare means of two groups, independent sample t test was used. Nonparametric test (Mann Whitney) was used to compare means when data was not normally distributed and to compare medians in categorical data. To assess change in means over time, repeated measure ANOVA was used. The level statistical significance was set at 5% (p<0.05). Highly significant difference was present if p ≤ 0.001.
Results
The demographic and clinical characteristics of the studied asphyxiated neonates were presented in Table 1, the obtained results showed that There is statistically non-significant difference between theophylline group and placebo group regarding clinical data using: ∞ independent sample t test§Mann Whitney test ¥ Chi square test.
| Characteristic |
Theophylline group (41) |
Placebo group (41) |
test |
p |
| Gestational age |
| Mean ± SD |
37.68 ± 1.23 |
38.07 ± 1.35 |
-1.367∞ |
0.175 |
| Range |
36-40 |
36-41 |
| Weight |
| Mean ± SD |
2.86 ± 0.42 |
2.87 ± 0.37 |
-0.209∞ |
0.835 |
| Range |
2.5-4.5 |
2.5-4.5 |
| Length |
| Mean ± SD |
48.8 ± 1.21 |
48.66 ± 0.88 |
0.626∞ |
0.533 |
| Range |
46-52 |
47-51 |
| Thompson score |
| Median |
16 |
16 |
0.198§ |
0.843 |
| Range |
15-19 |
16-20 |
| Mean Blood pressure |
| Mean ± SD |
49.07 ± 3.62 |
48.34 ± 2.59 |
1.053∞ |
0.296 |
| Range |
42-61 |
42-54 |
| UOP |
| Mean ± SD |
1.66 ± 0.5 |
1.52 ± 0.58 |
1.147∞ |
0.225 |
| Range |
0-3 |
0-3 |
| Time of TH/placebo inf. |
| Mean min ± SD |
37.71 ± 11.18 |
37.24 ± 11.7 |
0.183∞ |
0.463 |
| Range |
20-58 |
15-55 |
| HIE |
| Stage 2 |
34 (82.9) |
32 (78) |
0.311¥ |
0.577 |
| Stage 3 |
7 (17.1) |
9 (22) |
Table 1: The demographic and clinical characteristics of the studied groups.
The represented results of serum creatinine levels during first five days of life in two study groups of asphyxiated newborns showed non-significant difference between both groups at baseline readings. Later on, highly statistically significant differences (p ≤ 0.001) were found between both groups in the values of serum creatinine on 3rd and 5th days (Table 2).
| Serum creatinine |
Theophylline group |
Placebo group |
t |
p |
| mg/dl |
Mean ± SD |
Mean ± SD |
-- |
-- |
| First day |
0.92 ± 0.16 |
0.86 ± 0.14 |
1.761 |
0.082 |
| Third day |
0.73 ± 0.19 |
1.03 ± 0.2 |
-7.180 |
<0.001** |
| Fifth day |
0.58 ± 0.18 |
0.75 ± 0.11 |
-5.378 |
<0.001** |
| p∞ |
<0.001** |
<0.001** |
-- |
-- |
| **p ≤ 0.001 is highly statistically significant difference. (∞) repeated measurement. |
Table 2: Serum creatinine during first five days of life in studied groups of asphyxiated new-born.
The estimated urine creatinine during first five days of life showing a significant difference between both groups on 1st, 3rd and 5th days. On pairwise comparison, the difference was noticed only between readings in 3rd and both 1st and 5th days in theophylline-treated group while it was between first readings and those on 3rd and 5th days in placebo group. No difference between 3rd and 5th days in the group and no difference between 3rd and 5th days in placebo group (Figure 1).
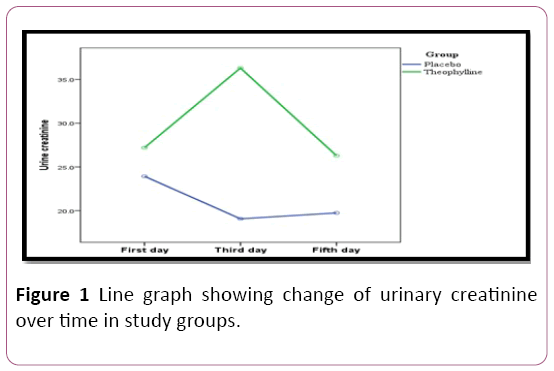
Figure 1: Line graph showing change of urinary creatinine over time in study groups.
In addition, the level of urinary sodium showing highly statistically significant differences were found between both groups on 1st and 5th days on measuring the urinary sodium along five days. There are highly statistical significant differences within each group between values of urinary sodium over time (Figure 2).
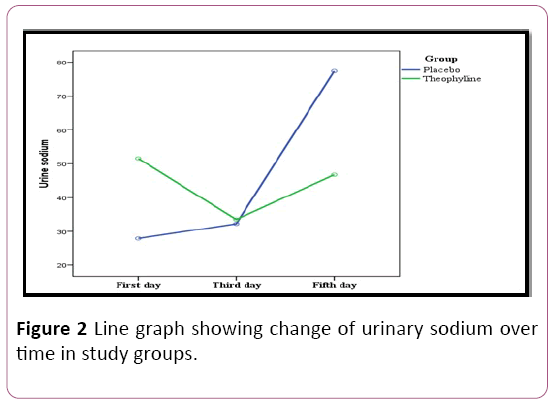
Figure 2: Line graph showing change of urinary sodium over time in study groups.
The obtained data of GFR which calculated using Schwartz formula revealed a non-significant difference statistically between both groups at baseline readings, later on highly statistically significant differences (p ≤ 0.001) were found between theophylline treated group and placebo (31.8 ± 7.67 Vs. 21.87 ± 4.11 and 41.09 ± 10.5 Vs. 29.64 ± 4.58 ml/min/1.73 m2 on 3rd and 5th days, respectively. There are highly statistical significant differences within each group between values of GFR over time (Table 3).
| GFR |
Theophylline group |
Placebo group |
t |
p |
| ml/min/1.73m2 |
Mean ± SD |
Mean ± SD |
-- |
-- |
| First day |
24.55 ± 4.79 |
26.25 ± 4.46 |
-1.665 |
0.100 |
| Third day |
31.8 ± 7.67 |
21.87 ± 4.11 |
7.304 |
<0.001** |
| Fifth day |
41.09 ± 10.5 |
29.64 ± 4.58 |
6.398 |
<0.001** |
| p∞ |
<0.001** |
<0.001** |
-- |
-- |
| **p ≤ 0.001 is highly statistically significant difference. (∞) repeated measurement. |
Table 3: Estimated GFR during first five days of life in the studied groups of asphyxiated new-born.
The occurrence of the complications among the two groups was recorded in numbers and percentage (Figure 3). The complications includes: Acute kidney injuries, encephalopathy, multi organ failure and death. There is no significant difference between occurrences of complications among studied groups, but it is less in theophylline treated group compared to placebo [11].
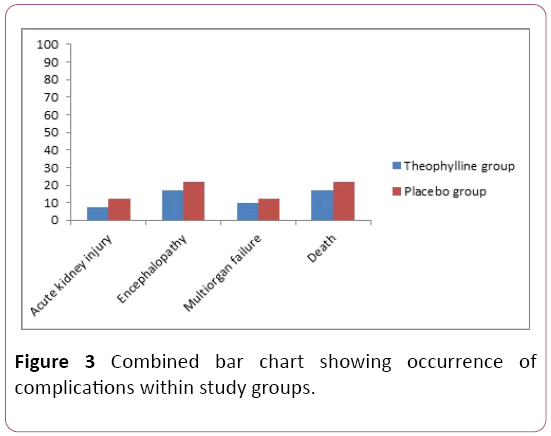
Figure 3: Combined bar chart showing occurrence of complications within study groups.
Discussion
The obtained results indicate that treating term neonates with severe perinatal asphyxia with a single dose of theophylline (5 mg/kg) in the first postnatal hour was beneficial. The presented data of theophylline treated group showed a significant decrease in the serum creatinine levels, with a significant increase in the creatinine clearance and urinary creatinine excretion compared to non-treated group (placebo). Similar results of Eslami et al. [12] was stated that, Serum creatinine levels were not significantly different between the theophylline and control groups on the 1st day as in our study in which p=0.082; however, these levels significantly decreased in the infants of the theophylline group and significantly increased in the controls on the 3rd day (p<0.001) which is similar to our findings(p<0.001). Prophylactic prescription of theophylline as a single dose of 8 mg/kg could decrease serum creatinine and urinary β2- microglubolin and increase creatinine clearance [13,14]. In contrast, there were no significant difference in urine sodium excretion was reported in the study of Bhat et al. [5], but in our study there were high statistically significant difference in urinary sodium excretion especially in the first day due to natriuretic effect of theophylline. Due to the natriuresis effect of theophylline, urinary sodium excretion on the 1st day was significantly higher in the theophylline group than in the control group (p=0.02) as same as our findings (p<0.001) [12]. The estimated GFR was significantly decreased in the control group from 26.25 ± 4.46 at the first day to 21.78 ± 4.11 at the third day compared to theophylline treated group. Bakr [13] and Raina et al. [4] were reported the same results that, a decrease in GFR from 31 ± 13.03 at day one to 20.87 ± 8.5 at day three. Jenik et al. [14] reported that the severity of asphyxia and multi-organ involvement were similar between the two studied groups, while our study the development of complications such as multiorgan failure and encephalopathy were more less in theophylline group compared to placebo.
Conclusion
We concluded that therapeutic use of theophylline has the ability to overcome renal dysfunction associated with neonatal asphyxia.
Conflict of Interest
The authors declare no conflict of interests.
23246
References
- Al-Wassia H, Alshaikh B, Sauve R (2013) Prophylactic theophylline for the prevention of severe renal dysfunction in term and post term neonates with perinatal asphyxia: A systematic review and meta-analysis of randomized controlled trials. J Perinatol 33: 271-277.
- Sharma D, Pandita A, Kumar C (2014) Therapeutic hypothermia in asphyxiated newborn: Its applicability, feasibility and accessibility in developing country India: A long way to go. J Neonatal Biol 3: 150-111.
- Lapointe A, Barrington J (2011) Pulmonary hypertension and the asphyxiated newborn. J Pediatr 158(2): 19-24.
- Raina A, Pandita A, Harish R, Yachha M, Jamwal A (2016) Treating perinatal asphyxia with theophylline at birth helps to reduce the severity of renal dysfunction in term neonates. Acta Paediatrica 194: 208-285.
- Bhat MA, Shah ZA, Makhdoomi MS (2006) Theophylline for renal function in term neonates with perinatal asphyxia: A randomized, placebo-controlled trial. J Pediatr 149: 180-184.
- Apgar V (1953) A proposal for a new method of evaluation of the new-born infant. Curr Res Anesth Analg 32(4): 260-267.
- Thompson CM, Puterman AS, Linley LL, Hann FM, Van Der Elst CW, et al. (1997) The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr 86: 757-761.
- Schwartz GJ, Feld LG, Langford DJ (1984) A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr 104(6): 849-854.
- Carl C, Alice I, Baker J (1985) Clinical pharmacodynamics of theophylline. J Allergy Clin Immuno 76: 292-297.
- Eslami Z, Shajari A, Kheirandish M, Heidary A (2009) Theophylline for prevention of kidney dysfunction in neonates with severe asphyxia. Iran J Kidney Dis 3: 222-226.
- Bakr AF (2005) Prophylactic theophylline to prevent renal dysfunction in newborns exposed to perinatal asphyxia study in a developing country. Pediatr Nephrol 20(9): 1249-1252.
- Jenik AG, Ceriani Cernadas JM, Gorenstein A, Ramirez JA, Vain N, et al. (2000) A randomized, double blind, placebo controlled trial of the effects of prophylactic theophylline on renal function in term neonates with perinatal asphyxia. Pediatrics 105(4): 45.









