Keywords
|
| Biomarker, Breast cancer, Clinical laboratory, Early detection, BRCA 1 and 2 |
Introduction
|
| Breast disease is one of the main sources of tumor-related passings in ladies inside financially created districts of the world. Breast disease viewed as a multifactorial issue brought on by both non-hereditary and hereditary elements. Breast disease is the most widely recognized danger in ladies and is very treatable if analyzed at an early stage. Conventional prognostic elements incorporate the age, axillary lymph hub status, tumor size, tumor evaluation, and hormone receptor status [1]. |
| Among ladies in the United States, bosom malignancy is the most widely recognized disease determined women to have pretty nearly 200,000 new cases reported every year. The second driving reason for growth related passings in ladies, as indicated by the American Cancer Society. Diagnosing the bosom disease as ahead of schedule as could be expected under the circumstances enhances the probability of effective treatment and can spare numerous lives. In any case, utilizing mammography as a present strategy to distinguish bosom tumor has characteristic impediments. Along these lines, early indicative biomarkers are essential for location, conclusion, and checking illness movement in bosom disease. |
| Several prognostic models for breast cancer molecular features have used in biomarker products [1-3]. Which proven to be of value to medical decision making, such as predicting whether an early-stage patient will benefit from adjuvant chemotherapy. |
| The determination of bosom malignancy depends on a coordinated methodology utilizing clinical and physical examinations, imaging mammography and ultrasound, and histopathology. Disregarding the way that serum biomarkers have not yet expected a critical part in chest danger demonstrative or prognostic practice [4,5]. Powerful biomarker board in an open organic liquid would be an important and insignificantly intrusive assistant to other clinical and obsessive methodologies (Elizabeth et al., 2006). As entire blood gives a dynamic representation of physiological and neurotic status, serum or plasma speaks to the most widely examined organic network for tumor biomarkers [6]. Consequently, examination of the serum or plasma proteome may be a vital stride to accomplish exact finding or anticipation. |
| For bosom tumor biomarker revelation, proteins and peptides have been recognized in bosom growth cell lines [7,8]. Areola suction liquid [9,10]. Ordinary, kindhearted, premalignant, and dangerous bosom tissue [11-14]. Notwithstanding serum and plasma [4,6]. |
|
Breast Cancer
|
| The bosom tumor is a malignancy of the bosom glandular tissue. Around the world, a bosom tumor is the fifth most regular reason for malignancy passing (after lung growth, stomach disease, liver disease, and colon growth). Among ladies around the world, bosom malignancy is the most well-known reason for tumor passing (WHO, 2006). There is a worldwide/topographical variety in the rate of Breast Cancer. Occurrence rates are higher in the created nations than in the creating nations. Rate rates are additionally higher in urban zones than in the provincial regions [15]. |
| Ideal approaches to analyzing disease or foresees helpful reaction are to utilize serum or tissue biomarkers. Biochemical markers in oncology are atoms that can distinguish in higher or lower than ordinary sums in the blood, pee, or body tissues of a few individuals with specific sorts of growth. A tumor marker may be delivered the tumor itself, by the encompassing ordinary tissue in light of the vicinity of a tumor or by the tissue of metastases [16]. |
| The boundless use of screening mammography has brought about an increment in the identification of ahead of schedule stage infection, especially in situ (stage 0) and early (stage 1) malignancies. Albeit screening mammography is an extremely touchy bosom disease discovery instrument that has gotten to be standard for ladies at high hazard, it needs adequate specificity and expense viability for utilization as a general screen. In forceful diseases, particularly in ladies less than 50 years old years, presentation in front of an audience 2 or 3 is more regular. Mammography screening is less high [17]. The evaluated number of bosom disease passings in the US in 2011 is ascertained by fitting the quantities of growth passings from 1969 through 2007 to a factual anticipating model. Information on the number of passings gotten from the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention [18]. |
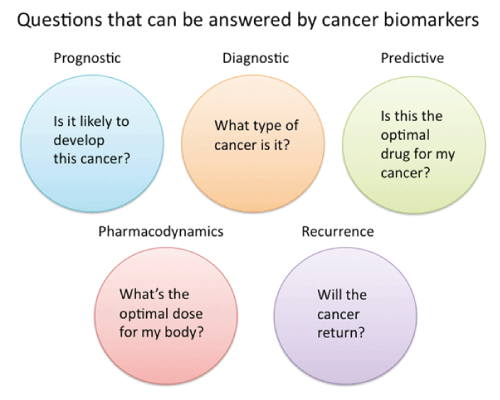
| A biomarker is characterized as a trademark that is unbiasedly measured and assessed as a marker of ordinary biologic procedures, pathogenic procedures or pharmacological reactions to a predetermined remedial intercession [19]. These pointers could incorporate an expansive scope of biochemical elements, for example, nucleic acids, proteins, sugars, lipids, and little metabolites, and additionally entire cells or biophysical attributes of tissues. Recognition of biomarkers, either exclusively or as bigger sets or examples, can be the expert by a wide mixed bag of routines. Running from a biochemical examination of blood or tissue tests for biomedical maging [20,21]. Be that as it may, additionally more intrusive strategies obliging tumor tissue for immunohistochemistry and DNA and RNA examinations are broadly utilized. A prognostic biomarker gives data about the tolerant's general tumor result, paying little heed to treatment. The vicinity or the nonattendance of a prognostic marker can be valuable for the determination of patients for a sure treatment, however not foresee the reaction to this treatment. Prognostic biomarkers can isolate into two gatherings. Biomarkers that give data on repeat in patients who get corrective treatment and biomarkers that connect with the length of time of (movement free) survival in patients with the metastatic illness. A biomarker with prescient worth gives data on the impact of a remedial in a patient. A perceptive biomarker can likewise be objective for treatment [19]. |
|
Biomarkers of risk
|
| Biomarkers of risk can help identify individuals who are at increased risk of developing cancer, before the biological onset of the disease. These biomarkers are based mainly on inherited or somatically acquired susceptibilities, in the form of altered genes such as BRCA1 and BRCA2 mutated genes that predispose to breast cancer [22]. In these cases, there is an inherent familial predisposition to the development of some cancer, although many individuals inheriting mutated genes will not develop cancer. Suggests the involvement of other factors, such as the environment, which could interact with specific genes to initiate cancer. However, risk markers are important in the monitoring of individuals and allow early intervention in those who do develop cancer. Markers of inherited risk include genetic polymorphisms that might affect the metabolism of carcinogens. Mutations in genes such as P53, which implicated in a wide variety of cancers can also serve as risk markers [21]. |
| Biomarkers for early detection |
| Biomarkers can detect the outcomes of the interaction between genetic susceptibility and the environment and are therefore extremely important for early detection. Theoretically, they could provide the opportunity to intervene during the natural progression of cancer, to cause inhibition, regression, or even elimination of the disease. After biological onset, the disease progresses through a preclinical phase before symptoms develop, changes in biomarkers during this phase could be very helpful in early detection [23]. |
|
Genetic and molecular signatures
|
| Genetic and molecular changes are the initial events in carcinogenesis and could be useful if detected before the onset of symptoms and morphological changes [21]. |
|
Genomics
|
| Genomics can be broadly defined as the measurement of gene expression from available sequence information. The expression profile represents the function and phenotype of a cell and is called a transcriptome [21]. |
|
Proteomics
|
| Proteomics methods detect the functioning units of expressed genes, through biochemical analysis of cellular proteins, to provide a protein fingerprint [24]. The protein reflects both the intrinsic genetic program of the cell and the impact of its immediate environment and is, therefore, valuable in biomarker discovery. Particular changes that happen at the protein level amid the change of an ordinary cell into a neoplastic cell incorporate adjusted expression. Differential protein alteration, changes in a particular action, and unseemly limitation, all of which may influence cell capacity [21]. |
Biomarkers Can Separate into the Accompanying Classes
|
|
Early recognition
|
| If utilized for screening patients to discover malignancy right on time as estrogen, progesterone receptors, and C35. |
|
Diagnostic
|
| If used to evaluate the vicinity or nonappearance of disease as epidermal development component receptor 2, p53 and thymidine phosphorylase. |
|
Prognostic
|
| If used to survey the survival probabilities of patients or to identify the forceful phenotype and decide how the malignancy will act as mammaglobin. An extracellular grid protein and cathepsin D |
|
Predictive
|
| If used to foresee whether the medication and different treatments will be successful, or to screen the viability of treatment as topoisomerase II and CA 15-3. 5) Target: if used to recognize the atomic focuses of novel treatments and which subatomic markers. Expressions influenced by treatment as HER2 and WWOX. Much of the time, diverse biomarkers will likely be critical for distinctive undertakings [16]. The tumor's order markers may anticipate on concoction structure (e.g., protein, DNA, polyamine), capacity (e.g., chemical, signal particle). System for identification (e.g., antigenic property-immunologic examine, compound movement test) or anatomic source (e.g., placenta, salivary). In one order plan, tumor markers are arranged in the accompanying gatherings: |
| 1. Compounds and isoenzymes |
| 2. Hormones, neurotransmitters and their metabolites |
| 3. Receptors (estrogen, progesterone, androgen, and corticosteroid) |
| 4. Proteins (immunoglobulin, glycoproteins, carcinoembryonic protein or oncofetal antigens) |
| 5. Hereditary markers (oncogenes and silencer qualities) |
| 6. Different markers (sialic corrosive conjugates, polyamines, and amino acids) [25]. |
Biomarkers
|
|
Estrogen receptor
|
| The estrogen receptor (ER) is an individual from the atomic hormone group of intracellular receptors, which is initiated by the hormone 17β-estradiol [26]. The most seasoned and most profitable prognostic and prescient marker in bosom disease is ER [27]. Estrogen is the principle stimulant in the advancement and development of the bosom disease. Its activities are intervened by two receptors: ER-alpha and - beta. Over 50% of all bosom tumors overexpress ER-alpha, and around 70% of them react to tamoxifen treatment (Hormonal treatment). The vicinity of elevated amounts of ER-alpha in kindhearted bosom epithelium connotes an expanded danger of bosom malignancy. Recommending a part for ER-alpha in bosom disease start, advancement and movement [28]. The estrogen receptor is an atomic interpretation figure that ties responsive estrogen components in the genome and enlisted people various cofactors that encourage quality translation. ER+ bosom disease. Depend on this process for their growth and are thus treated with anti-estrogens. Normal breast tissue mainly expresses the neuropeptide Y (NPY) Y2 receptor, whereas primary human breast carcinomas express the Y1 receptor (Y1R) subtype. Estrogen plays critical roles in the upregulation of Y1R, which in turn regulates estrogen-induced cell proliferation in breast cancer cells [29]. Estrogen receptors are overexpressed in around 70% of bosom malignancy cases, alluded to as "ER positive". Two theories have been proposed to clarify why this reasons tumorigenesis, and the accessible proof recommends that both instruments contribute. Firstly, tying of estrogen to the ER fortifies multiplication of mammary cells, with the subsequent increment in cell division and DNA replication prompting changes. Also, estrogen digestion system produces genotoxic waste. The consequence of both procedures is a disturbance of cell cycle, apoptosis and DNA repair and subsequently tumor development. ERα undoubtedly connected with separated tumors. While proving that ERβ is included disputable [30]. ER is the most capable individual predictive factor examined in breast cancers. ER is critical in the cancer-causing procedure, and its restraint, through endocrine focusing on, either straightforwardly utilizing feeble estrogen agonists (specific estrogen receptor modulators) (SERM). The implication by obstructing the transformation of androgens to estrogen (e.g. aromatase inhibitors) frames the pillar of adjuvant and metastatic bosom growth treatment. Bosom tumors which are ER+ and ⁄ or PR+ give a lower mortality danger contrasted and ER-and ⁄ or PR-tumors. And are to a great extent free of other clinical tumor qualities [31]. A particular estrogen receptor modulator, raloxifene, has been utilized as a protection chemotherapy for ladies judged to have a high danger of creating bosom growth. Another chemotherapeutic against estrogen, Casodex that goes about as a complete rival likewise advances corruption of the estrogen receptor [12]. The most utilized test as a part of the clinical setting for assessing ER protein expression is immunohistochemistry (IHC). Since IHC, dissimilar to compound measures, not require the demolition of tissue examples. It demonstrates the tissue conveyance of ER; it has turned into the favored technique for deciding the ER in bosom malignancy cases. On the other hand, the concentrated and tedious elucidations and the variability among research centers in techniques for tissue acquisition, protection, antigen recovery. All the more critically, the meanings of energy are significant negative components for IHC [17]. In spite of these apparent issues, IHC is broadly accessible, promptly robotized and appropriate for patient administration [32]. |
|
Progesterone receptor
|
| Progesterone is an ovarian steroid hormone is key for ordinary bosom improvement amid adolescence and in planning for lactation. The activities of progesterone intervened by its high liking receptors, including the traditional progesterone receptor (PR). An and - B isoforms, situated in the cerebrum where progesterone controls regenerative conduct, bosom, and conceptive organs [2]. Progesterone incitement of its receptor needed for bosom improvement and impacts the danger of bosom disease. The physiologic consequences of progesterone totally intercede through the progesterone receptors, PR-An, and PR-B, which are results of the same quality through option transcriptional start. Articulation of PR-An and PR-B controlled in a tissue-particular way, and the parity of PR isoform expression has viewed as an imperative variable. Adding to the danger of threat PR-B enacts translation more powerfully than PR-A [33]. Distinguishing proof of both isoforms (PR-An and using reverse transcriptase- polymerase chain reaction (RT-PCR), but only the PR- IHC identified an isoform. The positive mRNA expression could reflect the level of the PR- B isoform [32]. The progesterone receptor quality is powerfully communicated in epithelial and stromal bosom tissue amid sexual advancement and pregnancy. In spite of the fact that bosom advancement and the reaction of mammary tissue to progesterone not modified by the hereditary erasure of murine PR-A. The cancelation of PR-B especially diminished pregnancy-related ductal and alveolar epithelial cell expansion [34]. Since PR-B is essential for mammary cell development, hereditary instruments that impact PR-B expression may add to the bosom danger. Despite the fact that progesterone receptor quality feeling is receptive to estrogen, neither estrogen nor its receptors are needed for PR expression in hormonally responsive tissues [35]. PR is an estrogen-directed quality, help with anticipating reaction to hormonal treatment. Tumors active for PR will probably react to tamoxifen (Hormonal treatment), both in patients with metastatic infection and in the adjuvant setting. PR may be distinguished in cases that show up ER, which may be because of a false-negative ER examine. Low- Level ER or to variation ERs not perceived by the counteracting agent but rather still equipped for invigorating PR expression. PR is a broadly utilized marker, despite the fact that its quality is less entrenched [31]. |
|
Human epidermal development variable receptor 2
|
| Human epidermal development variable receptor 2 (HER2, otherwise called c-erbB-2 or HER2/neu or ERBB2 oncogene). The locale of 17q12 harboring the HER2 quality is the most considered amplicon in bosom growth. HER2 is an individual from the epidermal development variable (EGF) receptor group of receptor tyrosine kinases that intervenes cell development, separation, and survival [36]. Overexpression of HER2 connected with the expanded dangers of cutting-edge stage bosom tumor and poor visualization. HER2 quality intensification or protein overexpression has found in 20 to 30% of human breast disease and have connected with high-review tumors and unfavorable forecast. Clinically, HER2 is an essential biomarker and focus of treatment of bosom malignancy [13]. HER2 is a cell-surface protein included in cell advancement. In typical cells, HER2 controls parts of cell development and division. At the point when enacted in growth cells, HER2 quickens tumor development (Rusiecki et al., 2005). There are a few conceivable employments of HER2 status. HER2 inspiration connected with an awful anticipation (higher rate of repeat and mortality) in patients with recently analyzed bosom growth who don't get any adjuvant systemic treatment. Hence, the HER2 status may be consolidated into a clinical choice, alongside other prognostic elements, in regards to whether to give any adjuvant systemic treatment. HER2 testing ought to be routinely performed in patients with another conclusion of intrusive bosom malignancy [37]. HER 2 positive bosom malignancy tumors have connected with poor anticipation and poor reaction to systemic medications (chemotherapy and endocrine treatment). |
|
BRCA1 deletion
|
| Breast cancer susceptibility gene-1 (BRCA1) is a tumor suppressor gene that encodes a large protein of 1863 amino acids on chromosome 17q21. BRCA1 regulates transcriptional activation, DNA repair, apoptosis, cell-cycle checkpoint control and chromosomal remodeling [38]. BRCA1 germ line mutations have been shown to predispose to both breast cancer and ovarian cancers. Mechanisms of BRCA1 gene inactivation including BRCA1 gene promoter methylation and transcriptional down regulation [39]. In the familial breast cancer, BRCA1 gene deletion (loss of the wild-type allele due to its physical deletion). Leading to BRCA1 inactivation is known to be the loss of heterozygosity (LOH) in the BRCA1 locus. Although LOH often results from physical deletion of one of the alleles, in genetically unstable tumors (such as tumors arising in BRCA1 germ line mutation carriers or high-grade breast cancer). LOH frequently represents mitotic recombination, gene conversion. Or deletion followed by endore duplication events (i.e., one of the alleles lost, but the number of copies of the gene is maintained) [40]. |
|
BRCA2 deletion
|
| Breast cancer susceptibility gene-1 (BRCA2) is a tumor suppressor gene encoding a larger protein of 3418 amino acids and maps to 13q12-q13. BRCA2 involved in DNA repair, transcription, and cell growth [41]. BRCA2 gene deletion lead to BRCA2 inactivation in familial BRCA2 tumors, including BRCA2 physical deletion. Also a combination of deletion of the wild-type allele followed by duplication of the mutant allele, gene conversion, mitotic recombination, and nondisjunction chromosomal loss with or without duplication [40]. |
|
p53
|
| The expert tumor silencer protein p53. The p53 quality limited on the short arm of chromosome 17 encoding a 53-kD atomic phosphoprotein and encodes a 393-amino corrosive translation variable. It has a primary part in controlling the cell cycle. DNA harm builds the level of p53, bringing about cell cycle capture, DNA repair or apoptosis [42]. In typical cells, p53 is normally inert, bound to the protein MDM2 (likewise called HDM2 in people). Its activity and advances its corruption by going about as a ubiquitin ligase [43]. P53 enacted in light of unpleasant boosts (after the impacts of different disease bringing about operators, for example, some DNA-harming medications, hypoxia, UV radiation, and oncogene actuation. P53 is regularly alluded to as the "genome's Watchman" because it assumes a vital part in deciding a cell's destiny taking after DNA harm. At the point when DNA damaged, p53 can trigger cell cycle capture, senescence (lasting capture) or apoptosis to take out the harmed cell. Cell cycle capture gives time to repair the damage in this manner permitting the cell to recoup and survive. The components are administering whether a phone will catch or kick the bucket surely known. But rather these capacities are essential to shield a life form from the impacts of abnormal cell divisions, and deformities can prompt growth advancement and movement. P53 is flawed in > half of tumors backings the affirmation that p53 is a vital player in the a version of tumor improvement. The essential part of p53 in controlling the choice of a cell to live or bite the dust makes it an appealing focus for malignancy therapeutics. Reintroduction or enactment of p53 would actuate apoptosis in the tumor. Cells with deficient p53 are more delicate to specific medications and blends [44]. The p53 quality variations from the norm are much of the time connected with the pathogenesis of neoplasias, especially active tumors, similar to the bosom, lung, and colon cancer. The part of the p53 quality in a mixed bag of cell procedures, including interpretation, DNA repair, cell cycle control and apoptosis. Makes it a potential marker for the location of patients at higher danger of creating growth. A large portion of the transformations in the p53 quality bunch inside of the district coding the DNAtying space weakening the DNA-tying or transactivation elements of the protein. In this manner repressing its key part in cell cycle control [45]. P53 change status once in a while got for a routine examination, notwithstanding collecting confirmation of its prognostic worth. Transformations in the p53 quality have been accounted for to be available in more than a large portion of every single tumor can be that as it. May the recurrence demonstrates variety between sorts/subtypes of the disease. In bosom disease, the recurrence of p53 quality changes is pretty nearly 20%to 30%. Acquiring a p53 transformation has been proposed to be an early occasion in bosom malignancy advancement, and it identified with poor anticipation and chemoresistance. Allelic lopsidedness or loss of heterozygosity at chromosome area 17p13, where the p53 quality found has been accounted for in more than a large portion of bosom carcinomas [46]. The characteristic history of bosom disease can be affected by a few elements affected by p53 hereditary polymorphisms. Incessant presentation to larger amounts of a few endogenous (e.g. estrogens). And exogenous bosom cancer-causing agents bringing about higher aggregation of DNA harm amid a singular's lifetime may change the age at onset of the ailment, Also. It has been proposed that p53 polymorphisms connected with a familial bosom disease by the age of 50 years. Moreover, p53 polymorphisms are a prescient element of a vicinity of lymph hub metastases [47]. |
WWOX tumor silencer quality
|
| The WW space containing oxidoreductase (WWOX) is a malignancy quality, situated on chromosome 16q23.3-24.1. Crossing a genomic district of more than 1 million nucleotide bp, a zone additionally perceived as the typical, delicate site FRA16D. Genomic and expression abnormalities influencing this quality and locus are standard in different neoplasias including bosom disease [48]. Diminished WWOX expression in instances of bosom growth connected with markers of awful prognosis. It found that WWOX may be included in steroid (estrogens) digestion system and flagging pathways. WWOX can be considered as another focus for quality treatment advancement because of the relationship of high WWOX expression with enhanced illness free survival. The WWOX quality at FRA16D habitually quieted in tumors and had as of late been appeared to smother the development of disease got cells from lung and different malignancies. Notwithstanding erasures and translocations, the delicate WWOX quality is often hushed by hypermethylation of its CpG dinucleotide rich promoter districts. Since the substantial part of bosom and lung malignancies promoter hypermethylation is in charge of quieting WWOX in a. Pharmacologic treatment to restore articulation of WWOX. And other tumor silencers, for anticipation or treatment others a possibly viable methodology [49]. |
|
FHIT tumor silencer quality
|
| The delicate histidine triad (FHIT) quality situated on chromosome 3p14.2. It encodes a cytoplasmic protein that has adenosine triphosphate action. The most well-known sensitive site of the genome, FRA3B maps inside of the FHIT quality. This delicate site makes FHIT helpless to revisions incited by a mixture of cancer-causing agents. It is a tumor silencer quality included in the carcinogenesis of bosom disease and different growths [50]. Tumors that have genomic FHIT adjustments or changed FHIT transcripts, for the most part, don't express or show diminished levels of FHIT proteins. Loss of heterozygosity inside of the FHIT quality is one of the changes. Cancelations of the FHIT quality have additionally been seen in preneoplastic sores, proposing that FHIT erasures could be an early occasion in bosom carcinogenesis. Adjusted translation is every now and again because of inside cancelations inside FHIT, and point changes are fairly uncommon. One of the instruments by which loss of expression in bosom growth can happen is because of hypermethylation of FHIT [51]. Albeit changed FHIT transcripts had accounted for in 20% to 38% of essential bosom carcinomas, a decrease or nonattendance of FHIT protein can see in up to 72% of bosom carcinoma. Loss of FHIT protein expression connected with different markers of poor visualization. That misfortune FHIT expression is additionally kept u p in the metastatic axillary lymph hub in patients with bosom tumor [52]. |
|
Mammaglobin A
|
| Mammaglobin is an individual from the uteroglobin quality family, located on chromosome 11q12.2 and gives off an impression of being communicated just in bosom tissue [53]. Mammaglobin A will be an exceedingly particular marker for the bosom tissue. Its demeanor confined to the grown-up mammary organ and mammary tumor cell lines. It is overexpressed in essential human breast tumors when contrasted with ordinary bosom tissue [54]. Because of its particular bosom expression, mammaglobin An is of high enthusiasm as an applicant demonstrative marker for bosom tumor and considered as the most encouraging sub-atomic marker for bosom disease [55]. Assessment of mammaglobin An as a sub-atomic marker for lymph hub metastasis through the identification of mammaglobin. A-mRNA in lymph hubs showed the utility of mammaglobin-particular RT-PCR for the recognition of dispersed tumor cells [56]. The expanded discovery rate of RT-PCR for mammaglobin about the histological examination of axillary lymph hubs may recognize patients at higher danger of backsliding as contrasted and patients with negative RT-PCR results. Bone marrow micrometastases recognized by RT-PCR for mammaglobin was appeared to be a valuable prescient marker for ahead of schedule far off the repeat of a bosom tumor [57]. |
| Mammaglobin A will be an exceedingly particular atomic marker for the discovery of circling tumor cells (CTCs) in bosom growth with imperative prognostic applications. The recognition of CTCs in the fringe blood, bone marrow, and lymph hubs is valuable for deciding anticipation. And checking of the ailment for ahead of schedule intercession in the treatment of bosom growth [54]. |
|
10-C35 [C17orf37]
|
| The C35 quality situated on chromosome 17q12, 505 nucleotides from the 3' end of the HER2 oncogene. The chromosomal game plan of the classes encoding C35 and HER2 is a tail to tail [58]. C35 can be a critical novel biomarker for bosom tumor, incorporating early discovery particularly in invading lobular carcinoma (ILC). Discovery of ILC, the second most standard type of bosom malignancy is especially risky because these tumors may not recognize by palpitation or mammography. Likewise, the vicinity of lymph hub contribution in ILC can be missed amid histologic examination because of flat, uniform appearance of the harmful cells and low mitotic rate [59]. ILC regularly displays a less forceful phenotype (hormone receptor energy and low frequencies of p53 variation from the norm and HER2 expression). However, general survival rates of ILC are more strong penetrating ductal carcinoma (IDC) the most widely recognized sort of bosom malignancy. Which may come about because of poor discovery because of the morphology of ILC and a backward connection of C35 expression with a period of ILC patients. ILC in more youthful patients has a tendency to take a forceful course [58]. |
| The plenitude and commonness of C35 overexpression in tumors and the nonattendance of expression in ordinary human tissues settle on it. An appealing decision as a biomarker for the analysis of right on time stage and late-arrange malignancy and, also, a potential focus for restorative mediation. Articulation of C35 is dictated by utilizing a mixed bag of tests, including PCR, immunohistochemistry immunofluorescence and Western blotches [58]. Despite the fact that declaration of C35 plainly connected with HER2 enhancement sometimes, C35 presents points of interest as a novel biomarker in an equivalent number of IDC that don't overexpress HER2. Especially in ahead of schedule stage and ILC that normally don't overexpress HER2. Regulation and articulation of C35 in ductal carcinoma in situ (DCIS), LCIS, IDC, ILC, and metastases could recommend a part for this quality. In procedure and support of threatening phenotype [58]. Besides, C35 is frequently communicated without HER2 overexpression and is overexpressed in a higher extent of patients. This broad populace likewise incorporates patients with less forceful tumors, for example, grade 1 IDC, ILC, who could with at before phases of sickness. And who might, in this way, be more receptive to novel disease particular treatments. In patients who have advanced to late-organize infection, viable treatment must target metastases. It is essential, in this way, that large amounts of C35 expression recognized in far off metastases from the bosom [60]. |
|
Ataxia telangiectasia changed [ATM] quality
|
| ATM is a quality situated on chromosome 11q22, which encodes a protein kinase that initiates cell-cycle checkpoints after DNA harm, and manages the TP53 protein [61]. Transformations in ATM expand bosom growth hazard and add to acquired bosom malignancy. Changes in the ATM quality are the reason for an uncommon autosomal latent neurologic disorder, ataxiatelangiectasia (AT) [62]. Patients with AT described by ahead of schedule onset dynamic cerebellar ataxia, skin and visual telangiectasia, immunodeficiency, chromosomal shakiness expanded the danger of malignancies. With an inclination towards stable tumors particular bosom disease in ladies [63]. ATM quality assumes a discriminating part in the cell-cycle capture, apoptosis, and DNA repair. ATM quality expression levels assessed in tumor and neighboring ordinary tissue from patients determined to have essential bosom growth utilizing quantitative ongoing opposite interpretation polymerase chain response (RTPCR). ATM expression in tumor tissues diminished by roughly half contrasted and contiguous typical tissues from the same patients. The ATM quality expression was down-managed in bosom growth tissues and a high ATM quality expression level in bosom malignancy tissue was connected with an ideal visualization [64]. |
|
Quality enhancement MYC
|
| MYC is a protooncogene that maps to 8q24.1 and encodes an atomic phosphoprotein that assumes pleiotropic parts in cell cycle movement and apoptosis [65]. Myc is an interpretation component that invigorates the translation of elements that encourage movement through basic cell cycle checkpoints and stifles understanding of variables that smother cell cycle movement [18]. A standout amongst the most widely recognized imperfections in human malignancy cells deregulated articulation of the Myc oncogene. Hoisted Myc expression has reported in a broad mixture of the human tumor. The regulation of Myc expression is mind boggling and is controlled at the level of interpretation, analysis and adjustment [66]. MYC quality enhancement and protein overexpression have reported in all dangerous tumor sorts. In bosom malignancy, MYC quality intensification is found in 1.1% to 94.4% of cases (9). MYC enhancement as characterized by chromogenic in situ hybridization (CISH) or Fluorescent in situ hybridization (FISH) connected with a high histologic evaluation. High expansion rates, an absence of hormone (progesterone) receptors and an unfortunate result. The vicinity of MYC quality enhancement in high-review in situ injuries. For example, pleomorphic lobular carcinoma in situ and high-review DCIS would be vital in the move from in situ to obtrusive ductal carcinoma [36]. |
|
Cyclin D1
|
| Cyclin D1 is one of the as often as possible overexpressed proteins and one of the regularly opened up qualities in the bosom tumor. Cyclin D1 is the result of the CCND1 (PRAD1) quality situated on chromosome 11q13 and enhanced in approximately 15% of bosom malignancies. Then again, cyclin D1 is overexpressed at the mRNA and protein level in more than half of the bosom malignancies in the vicinity or nonappearance of quality intensification [67]. CCND1 enhancement corresponds with the protein's overexpression. High articulation of cyclin D1 is not optional to quality intensification inferring that different systems add to keeping up cyclin D1 overexpression. In bosom, incorporate malignancy estrogen and p53 different variables that could add to protein overexpression [68]. Cyclin D1 assumes a crucial part in estrogen-prompted bosom tumor with estrogen activity interceded through the transcriptional initiation of cyclin D1 and c-myc [69]. This confirmation proposes basic part of cyclin D1 in human bosom tumor cell-cycle control. Given this part, overexpression of cyclin D1 may give a development point of interest to the tumor cells and may additionally contribute towards imperviousness to endocrine treatment [70]. The incitement of development captured cells in light of different oncogenes, for example, myc and mitogenic development components. For instance, individuals from epidermal development variable (EGF) result in the D's affectation sort cyclins. These cyclins join the extracellular signs to the cell-cycle apparatus and of the three D-sort cyclins, it is cyclin D1 that transcendently connected with human tumorigenesis [71]. Cyclin D1 atomic force on immunohistochemistry has compared to the level of enhancement of CCND1. CCND1 opened up can be recognized by CISH strategy [72]. The flow information proposes that cyclin D1 merits further examinations as a marker of illness reaction and visualization and offers future helpful, open doors treatment [70]. |
| 8p11.2-p12 |
| The 8p12-p11.2 region amplified in 7% to 15% of breast cancers. This amplicon often displays complex rearrangement patterns of the chromosomal break, losses, unbalanced translocation, and amplification and is derived from a breakage-fusion-bridge process, often involving chromosome 11. The unbalanced translocations on 8p11-p12 are accompanied by additional complex events, including inversions and multiple independent duplications or amplifications [36]. Using microarray-based comparative genomic hybridization (CGH) revealed that 8p12 DNA amplification encompassed at least four distinct amplification cores (A1-A4) that may be co-amplified or amplified separately and have specific gene targets (amplicon drivers). |
| There are translocations taking place in this genomic region, with breakpoints around NGR1 and between the UNCD5 and FGFR1 regions. The breakpoint region between UNCD5 and FGFR1 revealed that loss was always accompanied by amplification, whereas amplification not necessarily associated with the presence of a breakpoint. Identified FGFR1 as a putative driver of the core A2 of this amplicon by using a combination of array CGH, FISH and CISH [72]. FGFR1 amplification has linked with a shorter disease-free and overall survival in node-negative and estrogen receptor–positive breast cancer. 8p11.2-p12 amplifications associated with 11q13 amplifications and both would be more frequently found in estrogen receptor–positive breast cancers [65]. |
|
20q
|
| Amplification on the long arm of chromosome 20 (20q13~21.3 megabases) has identified in 5% to 83% of breast cancers and breast cancer cell lines. This region of amplification was further mapped using 14 loci on 20q, which highlighted that the amplicon composed of 3 small regions: [1] AIB3 region on 20q11.22 [18] 20q13.2 (ZNF217 gene) and [73] PTPN1 region on 20q13.13 (71). There were two types of amplification patterns: a discrete region of amplification around ZNF217 (type I) and co-amplification of 2 or 3 regions (type II) [69]. Type I showed a strong correlation with 12p13 Enhancement, a relationship with p53 energy, and an absence of estrogen receptor and progesterone receptor expression. Sort II intensification every now and again found in conjunction with 11q13 enhancement and was connected with high review and axillary lymph hub metastasis [65]. |
|
13-Caveolin 1
|
| The Caveolin 1 (CAV1) quality maps to 7q31.1 and encodes a 21- to 24-kDa essential film protein. This protein is the fundamental segment of caveolar movies, which are extraordinary invaginated microdomains of the plasma layer found in the larger part of mammalian cells. CAV1 communicated in a few sorts of human cells, including adipocytes, endothelial cells, fibroblasts, a few types of epithelial cells, and myoepithelial cells (MEC). Attributable to its subcellular limitation and omnipresent appropriation, CAV1 has been accounted for to assume a noteworthy part in lipid transport, layer trafficking, quality regulation, and sign transduction [74]. Both tumor suppressive and oncogenic roles have been proposed for this protein [36]. Clashing results on the part of CAV1 in human tumors have accounted [75]. In light of the high recurrence of erasures of 7q31 (a delicate site known as FRA7G) in human malignancies, the questionable vicinity of CAV1 quality. Promoter methylation and inactivating quality transformations, and the evident lessening of CAV1 expression in bosom carcinomas, it has been proposed that CAV1 is a tumor silencer quality [36]. Interestingly, enhancement of FRA7G site in disease cell lines and additions of genomic material on 7q are as often as possible found in high-review bosom growth [76]. By immunohistochemical study, articulation of CAV1 was fundamentally found in 33% of intrusive bosom carcinomas, though neither one of the normals bosom tissue. Nor considerate bosom sickness, nor ductal carcinoma in-situ indicated pertinent CAV1 expression [77]. |
|
14-Parafibromin
|
| Parafibromin is a novel protein result of HRPT2 oncosuppressor quality, as of late recognized tumor silencer quality. Transformations of the HRPT2 quality are regular in parathyroid carcinomas [78]. The dispersion of parafibromin was resolved in bosom disease tissues and associated its look with traditional neurotic parameters. Bigger tumors were less inclined to express parafibromin than littler ones. Recoloring force corresponded contrarily with tumor size and obsessive stage. Parafibromin in bosom malignancy, as in parathyroid tumors, seems to have tumor silencer capacities, with the loss of protein expression connected with antagonistic obsessive parameters. These discoveries may demonstrate a potential part of parafibromin as a prognostic marker in bosom disease [79]. |
|
15-Bcl-2 expression
|
| Bcl-2 is an inhibitor of apoptosis and overexpressed in more than a large portion of every single human malignancy. Overexpression of Bcl-2 happens in 40-80% of human bosom tumors [73]. BCL-2 was the first antideath quality found. Since its revelation over two decades back, various individuals from the human Bcl-2 group of apoptosis-managing proteins have been distinguished. Counting six antiapoptotic proteins, three fundamentally comparative proapoptotic proteins and a few assorted proapoptotic cooperating proteins that work as upstream agonists or foes. Bcl-2–family proteins direct every single real kind of cell demise, including apoptosis, corruption and autophagy, [80]. Bcl-2 quality encodes for a mitochondrial protein thought to counteract apoptosis in typical cells. Dysregulation of this quality can add to tumor movement and expanded medication resistance [81]. The Bcl-2 quality at first distinguished in human B-cell lymphoma in light of its action as an inhibitor of apoptosis in growth cells. Different individuals from the Bcl-2 family, for example, box, additionally advance apoptosis [82]. As a result, the outflow of bcl- 2 in malignancy cells is thought to restrain apoptosis and, in this manner, identify with a more regrettable result. Notwithstanding, the declaration of Bcl-2 in bosom disease has been observed to connected with ideal prognostic components, for example, Littler tumor size, ER energy, and low atomic evaluation. Bcl-2 likewise predicts an excellent result in metastatic malady and also in right on time bosom malignancy patients who got heterogeneous adjuvant chemo and hormonal treatments [83]. The significance of apoptotic marker Bcl-2 family protein is in anticipating the reaction to neoadjuvant chemotherapy in carcinoma of the bosom. Diminish in Bcl-2 expression after chemotherapy, about the phrase from the pretreatment test, corresponds with sickness response [73]. |
|
16-Serum Glycomics
|
| Since the glycosylation of proteins is known not in tumor cells amid the advancement of bosom growth, a glycomics methodology is utilized to discover significant biomarkers of bosom disease. These glycosylation changes are identified not with expanding tumor weight and poor guess. This glycomics way to deal with find glycan biomarkers of the bosom disease. Breast malignancy tumor cell lines are glycosylated proteins discharged by artificially cutting oligosaccharides (glycans) [84]. Glycosylation of proteins is known not in the breast and different sorts of growth. Adjustments in glycosylation impact development, separation, change, bond, metastasis, and resistant observation of the tumor. In bosom growth, the vicinity of expanding groupings of very glycosylated proteins (mucins) and different changes in glycosylation correspond with developing tumor weight and poor anticipation, [85]. O-Linked Glycosylation of the mammary organ is likewise known not adjusted amid threat in huge part because of the adjustments in mucin glycosylation [86]. Glycosylation of proteins changes in an extensive and sensational style in disease cells. Glycans get to be shorter and all the more contrarily charged, and center structures change. These sorts of glycans not regularly created in solid people [87]. Mucin 1 (MUC1) known as the polymorphic epithelial mucin, MUC1 is a huge, polymorphic and vigorously glycosylated mucin. The part of mucins is fundamentally one of the hydrating and greasing up epithelial linings. However, these proteins have additionally involved in regulating both development element flagging and cell attachment. It has proposed that MUC1 expression at the surface of tumor cells could diminish cell attachment and favored spread. Then again, MUC1 could assume a part in the introductory connection of bosom tumor cells to tissue at removed locales, encouraging the foundation of metastatic destinations. Despite the fact that MUC1 communicated in a greater part of bosom tumors, its overexpression has been connected with a lower evaluation and a higher ER-positive phenotype [88]. MUC1 has long been viewed as an objective for immunotherapeutic and immunodiagnostic measures, since it is very overexpressed in many adenocarcinomas, including bosom, ovarian, and pancreatic malignancies [89]. |
|
17-CA 15-3 and CA 27.29
|
| There are two general sorts of examines m 15-3 (CA15-3) and measures for malignancy antigen 27.29 (CA 27.29). CA15-3 is one of the first circling prognostic components for bosom malignancy. Preoperative focuses along these lines may joined with existing prognostic variables for the foreseeing result in patients with the recently analyzed bosom tumor. An essential clinical utilization of CA 15-3 is in observing treatment in patients with cutting edge bosom disease that is not assessable by existing clinical or radiologic strategies. CA 15-3 is the most broadly utilized serum marker as a part of the bosom disease, [85]. An increment in CA 15-3 or CA 27.29 after essential and adjuvant treatment can foresee a normal of 5 to 6 months. Before other measuring MUC1 quality inferred glycoprotein: means for growth antigen manifestations or tests [90]. For observing patients with metastatic malady amid dynamic treatment, CA 15-3 or CA 27.29 can utilize as a part of conjunction with analytic imaging, history, and physical examination. Be that as it may, without promptly quantifiable sickness, an expanding CA 15-3 or CA 27.29 may be utilized to show treatment disappointment. Alert ought to be used when deciphering a rising CA 15-3 or CA 27.29 level amid the initial 4 to 6. Weeks of another treatment, given that spurious early ascents may happen [91]. |
|
18-Carcinoembryonic antigen
|
| Carcinoembryonic antigen (CEA) is a glycoprotein included in cell grip. It typically delivered amid fetal advancement. However, the generation of CEA stops before conception. Accordingly, it is not displayed in the blood of sound grown-ups. Bosom carcinomas had more elevated amounts of CEA than solid people. For checking patients with the metastatic sickness amid dynamic treatment, CEA can be utilized as a part of conjunction with demonstrative imaging, history, and physical examination. Present information is lacking to prescribe the utilization of CEA alone to monitor reaction to treatment. Then again, without promptly quantifiable sickness, an expanding CEA may be utilized to demonstrate treatment disappointment. Alert ought to be employed when deciphering a rising CEA level amid the initial 4 to 6 weeks of another treatment, given that spurious early ascents may happen [91]. Carcinoembryonic antigen has been the favored marker for recognition of small scale metastases in lymph hubs in all carcinomas [53]. |
|
19-Urokinase Plasminogen Activator and Plasminogen Activator Inhibitor 1
|
| The urokinase plasminogen activator (uPA) framework has included in disease cell attack and metastasis. uPA action is controlled by its essential inhibitor, the plasminogen activator inhibitor sort 1 (PAI-1) (Julia et al., 2008). uPA is one of the serine proteases that catalyzes the transformation of plasminogen to plasmin, a dynamic catalyst that can corrupt a mixed bag of extracellular grid proteins. It trusted that uPA starts a proteinase course at the cell surface and advances tumor attack and angiogenesis. uPA repressed by PAI-1, which is an individual from the superfamily of serine protease inhibitors, [92]. PAI-1, the essential inhibitor of the plasminogen initiation framework, inactivates tissue sort plasminogen activator (tPA) and uPA, additionally assumes a vital part in sign transduction, cell adherence, and in cell relocation. In a few studies, increments in both uPA and PAI-1 have been either connected to forceful tumor attributes or a weak anticipation in a few diseases, including bosom malignancy [93]. A few creators discovered uPA immunostaining in stromal cells additionally in tumor cells of bosom malignancy tissue [94]. While different producers found that uPA is solely or principally limited in disease as well as in stromal cells [95]. PAI-1 protein has identified in tumor cells, myoepithelial cells, endothelial cells, fibroblast and macrophages in bosom growth tissue. A quantitative continuous RT-PCR technique to evaluate PAI-1 and uPA mRNAs in bosom malignancy. The limitation of the uPA, PAI-1 proteins and mRNAs in bosom malignancy tissue by immunohistochemistry and in situ hybridization [96]. |
|
20-Osteopontin
|
| Osteopontin is an emitted phosphorylated glycoprotein that was initially separated from bone and controls biomineralization, osteoclast separation, and bone resorption [97]. Osteopontin emitted by initiated macrophages, leukocytes, and enacted T lymphocytes, and is available in extracellular liquids, at destinations of irritation, and in the extracellular framework of mineralized tissues. A few physiologic parts have credited to osteopontin, i.e., in irritation and safe capacity, in mineralized tissues, in the vascular fabric, in the kidney. Osteopontin collaborates with a mixture of cell surface receptors, including a few integrins and CD44. Tying of osteopontin to these phone surface receptors fortifies cell bond, relocation, and particular flagging capacities. Overexpression of osteopontin has found in a mixed bag of tumors, including bosom growth, lung disease, colorectal malignancy, stomach malignancy, ovarian growth, and melanoma. Besides, osteopontin is available in hoisted levels in the blood and plasma of a few patients with metastatic growths. Osteopontin is measured in plasma, utilizing ELISA examine [98]. It has investigated as a potential non-intrusive biomarker for the analysis or movement of malignancy. High osteopontin levels are connected with poor guess in the bosom tumor [97]. The metastasis quality osteopontin is liable to options grafting, which yields three messages, osteopontin-an, osteopontin-b and osteopontin-c Osteopontin-c specifically communicated inobtrusive. However not in noninvasive, bosom tumor cell lines, osteopontin-c present in 78% of growths, 36% of encompassing tissues and 0% of the ordinary fabrics. Moreover, osteopontin-c recognizes a higher division of bosom tumors than estrogen receptor, progesterone receptor or HER2. In conjunction, osteopontin-c, estrogen receptor, and HER2 dependably foresee grade 2-3 bosom disease. Henceforth, osteopontin-c is an indicative and prognostic marker that may have esteem in an analytic board together with ordinary bosom growth markers [99]. |
|
21-Cell cycle checkpoint kinase 2
|
| Cell cycle checkpoint kinase 2 (CHEK2) is a serine-threonine kinase that is actuated by ataxia telangiectasia changed (ATM) protein because of DNA twofold strand breaks. CHEK2 not just directs the capacity of BRCA1 protein in DNA repair additionally applies some basic parts in cell cycle control and apoptosis [100]. Introduction to ionizing radiation can bring about a mixed bag of sorts of harm to the DNA, of which the most genuine are twofold strand breaks. At the point when unrepaired, DNA dual strand breaks can bring about the loss of hereditary material while mistakenly repaired twofold strand breaks can cause harm extending from restricted transformations at the first's site injury to vast scale genomic revisions. The results of qualities for which recognized variations or changes build hazard for bosom growth act prevalently inside of a typical cell pathway utilized by human cells to sense, flag, and repair such harm from DNA twofold strand breaks [101]. Incitement of this pathway by presentation to ionizing radiation or other DNA twofold strand break instigating specialists initiates the ATM protein, a serinethreonine kinase, which phosphorylates a full cluster of substrates including BRCA1 and CHEK2. ATM and CHEK2 assume key parts in the essential flagging pathway that controls cell reactions to twofold strand breaks and both straightforwardly manage the elements of BRCA1 [102]. ATM - interceded phosphorylation and actuated HEK2 kinase phosphorylates a few substrates, e.g. p53 and BRCA1, bringing about cell-cycle capture and apoptosis [103]. Phosphorylation of proteins assumes a critical part in the regulation of numerous flagging systems that control cell development, separation, senescence and customized cell demise (apoptosis). Protein phosphorylation happens mainly on serine, threonine and tyrosine buildups, and >500 kinases that catalyze the phosphorylation of proteins have distinguished in the human genome. Protein phosphatases catalyze protein dephosphorylation, and in people there are three noteworthy sorts of protein phosphatases: tyrosine phosphatases (PTPases), serine/threonine phosphatases (PPases) and "double specificity" phosphatases (DSPases). Activities of a few protein kinases have been appeared to advance or encourage the improvement of human diseases. Medications change the activities of these kinases (i.e. trastuzumab, imatinib, bevacizumab, gefitinib. And cetuximab) have created by treatment choices for the medicinal administration of human growths [104]. Serine/threonine protein phosphatases have ensnared in the regulation of numerous sign transduction falls that control development. Serine/threonine phosphatase sort 5 (PP5) has developed as a possibly critical controller of cell gr wth. PP5 found in a relationship with a few proteins that impact intracellular flagging falls started by hormones. (i.e. glucocorticoids) or cell stress (i.e. hypoxia, oxidative anxiety, and DNA-harm) [105]. |
|
22-Nectin-4
|
| Nectins are cell bond atoms included in the regulation of epithelial physiology. Nectins are individuals from the immunoglobulin superfamily and are parts of E-cadherin-based adherence intersections in epithelial cells [106]. Nectins and E-cadherin connected to F-actin through AF-6/Afadin and Catenins. The Nectin/Afadin and E-cadherin/Catenins frameworks connect with one another through Afadin and α-catenin. Nectins and Afadin have included in tumor science. All Nectins aside from Nectin-4 communicated in epithelial, endothelial, hematopoietic and neuronal cells in grown-up tissues. Nectin-4, for the most part, expressed amid embryogenesis yet is not distinguished in typical adult clothes nor serum [107]. Nectin-4 expression in bosom growth broke down by IHC investigation; expression distinguished in 62% of the ductal sort and 6% of lobular sort carcinoma. Nectin-4 communicated in non-intrusive and obtrusive tumors [106]. Circling types of cell grip particles reported in diverse maladies, particularly in malignancy. The vicinity of a Nectin-4 flowing structure in the sera of patients with bosom carcinoma examination performed by protein connected immunosorbent test (ELISA). Nectin-4 is serum marker for patients with metastatic bosom carcinoma and can enhance patient preliminary [108]. Nectin-4 speaks to a profitable marker for assessing infection movement and the metastatic status of patients. Nectin-4 can likewise use to quantify restorative effectiveness after treatment of the metastatic ailment [106]. |
|
23-Cytokines
|
| Cytokines, flagging atoms that intervene and control safety, aggravation, and hematopoiesis, are a vital part of the natural milieu connected with the bosom tumor. Cytokines have utilized as biomarkers for visualization and have been combined with side effects and antagonistic results in different conditions, including bosom malignancy [109]. Despite the fact that there are a few characterization frameworks for cytokines, a typical classification marks cytokines that advance irritation as proinflammatory cytokines, though cytokines that stifle the action of proinflammatory cytokines and diminish aggravation are called mitigating cytokines. Most research has concentrated on measures of proinflammatory cytokines as biomarkers, [110]. |
| In persons with disease, the insusceptible reaction to tumor cells adds to an unfavorable proinflammatory state. High interleukin (IL)- 6 levels have been connected reliably to poorer results in the all inclusive community and malignancy populaces. IL-6 is an autocrine and paracrine development variable for a few diseases, including bosom growth, [111]. IL-6 may animate malignancy cell development and add to repeat and metastasis in bosom disease. Raised levels of circling proinflammatory cytokines, including IL-1, IL-6, IL-8 and IL-18, may be connected with abbreviated survival in persons with malignancy. IL-6 and IL-18 have corresponded with infection stage and movement in the bosom tumor [112]. Alongside proinflammatory cytokines, hematopoietic cytokines have imperative parts in people with growth. Province animating elements are hematopoietic development figures that fortify the advancement of neutrophils and macrophages. Hoisted level of granulocyte-monocyte settlement animating variable (GM-CSF) was noted in patients with bosom malignancy [113]. Notwithstanding antagonistically influencing sickness movement in persons with disease, lifted levels of proinflammatory cytokines have connected with upsetting side effects in people with bosom growth. Cytokines may assume a part in the pathophysiology of neuropsychiatric side effects through associations among segments of the insusceptible and neuroendocrine frameworks. Proinflammatory cytokines have been connected with misery in persons with growth amid treatment and in weariness in survivors of bosom disease [114]. |
|
24-Small bosom epithelial mucin
|
| Little breast epithelial mucin (SBEM) is a quality item that shows guarantee as another bosom biomarker. The SBEM quality, otherwise called BS106 and B511S, was initially distinguished by a putative bosom particular condition. SBEM encodes a discharged 90 amino acids glycoprotein, which comprises of an emission signal peptide. Three tandemly rehashed octapeptide themes (TTAAXTTA) and displays attributes of individuals from the mucin family, [115]. SBEM quality expression, as evaluated by converse transcriptase-polymerase chain response (RT-PCR), has been seen in > 90% of essential or metastatic bosom tumors. The joined utilization of SBEM, cytokeratin (CK) 19, trefoil element 3 (p1B) and epithelial glycoprotein-2 (EGP-2). Expression permits the distinguishing proof of micrometastases in sentinel lymph hub biopsy of bosom diseases, missed by standard histological assessment [116]. SBEM expression saw in 22% of ERα− and 13% of ERα+ bosom diseases. In ER+ bosom growths, SBEM may be connected with more regrettable survival and shorter time to the movement. In ER− growths, SBEM protein expression was exceedingly combined with a few markers of poor visualization as HER-2 [117]. |
|
25-cyclooxygenase-2
|
| Cyclooxygenase-2 (COX-2) protein is the inducible type of cyclooxygenase, the rate-restricting chemical for prostaglandin blend [118]. There is the stable biologic method of reasoning fundamental COX-2 as an applicable biomarker in bosom carcinogenesis. Cyclooxygenase compounds catalyze the combination of bioactive prostaglandins from arachidonic corrosive, which got from layer phospholipids. COX-2 prompted because of different incendiary and mitogenic boosts, has been appeared to assume a tumorigenic star part in preclinical models of a few tumor frameworks. Including upgraded expansion, improved angiogenesis, imperviousness to apoptotic cell demise, immunosuppression, advancement of intrusion, and metastasis. It overexpressed in a few human malignancies and their precancerous sores, [119]. COX-2 communicated by immunohistochemistry in intrusive bosom disease examples. In ductal carcinoma in situ, the recurrence of COX-2 overexpression gives off an impression of being higher. Overexpression is connected with forceful histologic and clinical elements [120]. Overexpression of COX-2 in ductal carcinoma in situ injuries is an in number danger variable for neighborhood repeat in the preserved bosom [118]. Atypical hyperplasia in bosom tissue, albeit generous, is connected with a high danger of bosom tumor. Lifted COX-2 expression was more typical in atypical lobular hyperplasia than atypical ductal hyperplasia. COX-2 overexpression in atypical hyperplasia is a risk component for the movement of the bosom tumor [120]. |
Part of Biomarkers in Disease
|
| A harm biomarker insinuates a substance or system that is reasonable for the region of infection in the body. A biomarker may be a molecule released by a tumor or a specific response of the body to the area of development. Inherited, epigenetic, proteomic, glycomic, and imaging biomarkers can use for danger discovering, representation, and the investigation of infection transmission. Ideally, such biomarkers can be tried in non-prominently accumulated biofluids like blood or serum [121]. Tumor biomarkers, particular those joined with innate changes or epigenetic changes, routinely offer a quantitative way to deal with the center when individuals slanted to specific sorts of malignancies. Striking instances of possibly insightful development biomarkers join changes on qualities KRAS, p53, EGFR, erbB2 for colorectal. Esophageal, liver, and pancreatic harm; changes of characteristics BRCA1 and BRCA2 for chest and ovarian malady. Irregular methylation of tumor silencer qualities p16, CDKN2B, and p14ARF for brain threat; hypermethylation of MYOD1, CDH1, and CDH13 for cervical development; and hypermethylation of p16, p14, and RB1, for oral disease [122]. |
Determination
|
| Development biomarkers can in like manner be useful in setting up a particular investigation. Particularly the circumstance when there is a need to make sense of if tumors are of the crucial or metastatic source. To make this capability, researchers can screen the chromosomal alterations found on cells arranged in the key tumor site against those found in the helper site. In case the progressions arrange, the helper tumor can be perceived as metastatic. However in the event that the alterations shift, the discretionary tumor can be recognized as a particular fundamental tumor [123]. |
Perception and Treatment Expectations
|
| Another usage of biomarkers in development remedy is for ailment representation, which happen after an individual has been resolved to have the tumor. Here biomarkers can be important in choosing the forcefulness of a recognized harm and, additionally, its likelihood of responding to a given treatment. To some degree, this is because tumors indicating particular biomarkers may be responsive to prescriptions altering to that biomarker's appearance or region. Delineations of such prognostic biomarkers join raised levels of metallopeptidase inhibitor 1 (TIMP1), a marker associated with more compelling sorts of distinctive myeloma, [121]. Raised estrogen receptor (ER) and progesterone receptor (PR) expression. Markers associated with better broad survival in patients with chest growth [9,124]. HER2/neu quality increase, a marker exhibiting a chest illness will likely respond to trastuzumab treatment [125]. An adjustment in exon 11 of the proto-oncogene c-KIT, a marker demonstrating a gastrointestinal stromal tumor (GIST) will likely respond to imatinib treatment [3,126]. Changes in the tyrosine kinase range of EGFR1, a marker exhibiting a tenacious. Non-little cell lung carcinoma (NSCLC) will presumably respond to gefitinib or erlotinib treatment [127,128]. |
Pharmacodynamics and Pharmacokinetics
|
| Development biomarkers can moreover be used to center the best treatment organization for a particular singular's disease, [129]. Given complexities in each's innate beautifying agents, a couple of people metabolize or change the creation structure of drugs in a surprising way. From time to time, decreased absorption arrangement of particular solutions can make hazardous conditions in which unusual measures of the drug accumulate in the body. In that limit, prescription screening so as to dose decisions particularly tumor pharmaceuticals can benefit for such biomarkers. A case is a quality encoding the compound thiopurine methyltransferase (TPMPT) [130]. People with changes in the TPMT quality are not ready to metabolize a great deal of the leukemia drug, mercaptopurine, which possibly causes a dangerous drop in white blood mean such patients. Patients with TPMT changes are in this way endorsed to be given a lower estimations of mercaptopurine for security contemplations [131]. |
Checking Treatment Reaction
|
| Tumor biomarkers have also demonstrated utility in checking how well a treatment is working after some time. Much research is going into this particular reach, taking after productive biomarkers have the ability to give paramount cost diminishment inpatient thought. The rhythmic movement picture based tests, for instance, CT and MRI for checking tumor status are outstandingly expensive [14]. One remarkable biomarker gaining discriminating thought is the protein biomarker S100- beta in monitoring the response of undermining melanoma. Melanomas, melanocytes, the cells that make shade in our skin, convey the protein S100-beta in high obsessions subject to some development cells. Response to treatment is in this way joined with decreased levels of S100-beta in the blood of such people [132,133]. Likewise, additional lab examination has exhibited that tumor cells encountering apoptosis can release cell parts, for instance, cytochrome c, nucleosomes, cut cytokeratin-18, and E-cadherin. Studies have found that these macromolecules and others can be discovered accessible for utilization in the midst of development treatment, giving a potential wellspring of clinical estimations for checking treatment [14]. |
Repeat
|
| Development biomarkers can in like manner offer worth in envisioning or watching sickness rehash. Chest development looks used to predict the likelihood of chest illness rehash. This test expected for women with the privilege of time (Stage I or II), center point negative, estrogen receptor-positive (ER+) the noisy chest tumor that will treat with hormone treatment. Oncotype DX looks at a leading group of 21 qualities in cells taken in the midst of tumor biopsy. The test's outcomes are given as a rehash score that demonstrates the likelihood of rehash at ten years [134,135]. |
Making Pharmaceutical Targets
|
| Despite their usage in development pharmaceutical, biomarkers are frequently used all through the tumor drug exposure process. For example, in the 1960s, researchers discovered the lion's offer of patients with unending myelogenous leukemia had a particular inherited abnormality of chromosomes 9 and 22 named the Philadelphia chromosome. Right when these two chromosomes unite, they make an infection bringing on quality known as BCRABL. In such patients, this quality goes about as the rule starting point in most of the physiological indications of leukemia. For quite a while, the BCR-ABL was used as a biomarker to stratify a certain subtype of leukemia. Tranquilize creators were over the long haul prepared to make imatinib, a dangerous prescription that effectively frustrated this protein and lessened era of cells containing the Philadelphia chromosome [136]. |
Conclusion
|
| We see a couple biomarkers especially premier for the determination and representation upgrading the adjustment of slipping into sin and expanding persistent survival while decreasing patient. The way that mid-section risk is not a uniform advancement substance yet rather incorporates several distinct subtypes with arranged sub-atomic profiles, appropriate conduct, and danger profiles identify with a test for the clinical association. Prognostic and farsighted segments constitute fundamental gadgets for the individualization of mid-section tumor treatment to give effective treatment and to additional patients with real distinct profiles from undesirable reactions of overtreatment. The information showed that tumor biomarkers can additionally be helpful in building up an accurate determination. The predominant piece of the people was mindful so as to test biomarker the medical crises in mid-section advancement and the consideration was higher among major powers. Meanwhile, there is more need to learn as these crises can actuate setbacks. Like this, it ought to be compulsory however well-being couldn't be thoughtless authorities astoundingly mid-section tumor ailment to know about differing customs for dealing with the accommodating crises. Especially the condition when there is a need to comprehend if tumors are of the key or metastatic root. This refinement, powers can screen the chromosomal changes found on cells organized in the particular tumor site against those found in the alternative site. The chest malady is a common sex hormone neoplasm and estrogen are acknowledged to expect an essential part in the pathogenesis and necessary extension of this tumor. Estrogen binds to the estrogen receptor (ER) achieving an ordered compound that goes about as an interpretation segment through binds to the goal qualities. Estrogen advancement can be ruined by antagonistic to estrogen, which follows binds to the estrogen receptor. The evaluation of ER expression in the chest tissue has accomplished discernible progressions in the treatment of chest development, especially in the area of endocrine treatment. Chest tumor common subtypes tend to offer to rise to first far away metastases at particular body destinations. A couple of key tumor proteins associated with homing of danger chest cells. Chest development is a real fundamental issue with basic general wellbeing and general results and is the essential wellspring of Cancer destruction in women. Chest carcinoma is the most understood undermining neoplasm, with more than a million cases happening annually. Several threat variables for the headway of chest danger have developed, and been propose that the common element for by far most of these segments drawn out estrogen affectation taking a shot at an innately small establishment. Estrogen and progesterone receptors and HER-2/ neu protein expression showed significant concordance amidst vital and metastatic chest carcinoma to the relating lymph centers. Hormone receptor status is commonly chosen just for the reason of receptor substance in the primary tumors as it acknowledged that hormone receptor material of the metastases is similar to that of vital carcinomas. Our study favors recent thought because there is no particular qualification of hormone receptor expression amidst key and metastatic tumors. Of course metastatic stores in the lymph centers can in like manner be used to overview hormone receptor when tissue from the crucial tumor is not available for receptor examination. In any case, there is a mean rate of ER and PR positive patients in which metastatic stores don't express hormone receptors. In this way, the patients who made unavailable metastasis despite the endocrine treatment may encounter radiologically helped biopsies and full hormone measures remembering the deciding objective to know the alert status of the hormone receptor expression. In a summary, the finding that ER/PgR-positive DCIS exists together in a minority of patients with ER/PgR-negative chest development raises the issue of chemoprevention in this partner of patients. Clinically, hormonal treatment is not used as a piece of the treatment of ER/PgR-negative great chest development and chemoprevention has not had displayed to be off point of preference in ER/PgR-negative. Prominent chest danger or ER/PgR-negative DCIS. In any case, chemoprevention routinely used as a piece of ER/PgR-positive DCIS. The finding of ER/PgR-positive DCIS in a couple of patients with ER/PgR-negative chest sickness may be clinically germane and may require a more cautious examination of the tissue for this setting. The assertion of our data by individual establishments, by fused immunohistochemistry and converse interpretation PCR (RT-PCR) is legitimized. Screening for considerable genomic conformities in both BRCA1 and BRCA2 is unequivocally maintained by this concentrate, particularly for various circumstance chest/ovarian families with an energetic time of onset (BRCA1) and families containing no under one example of male chest danger (BRCA2). BRCA1 methylation to join with AI at the BRCA1 locus. All BRCA1 methylated tumors with truant/remarkably diminished BRCA1 expression (8/9) demonstrated BRCA1 cancelation. BRCA1 methylated and familial BRCA1 tumors, in light of BRCA1 eradication, TP53 changes, ER status, energetic age at determination and tumor grade. The plasma urokinase-sort plasminogen activator (uPA), plasminogen activator inhibitor-1 (PAI-1), and urokinase-sort plasminogen activator receptor (uPAR) levels were measured in sound volunteers and chest tumor patients. In pre-menopause sound females, blood was assessed after quite a while in the midst of one ladylike cycle and FSH/LH levels controlled month to month cycle stages (follicular, ovulatory, luteal). uPA, PAI-1, and uPAR levels were at the nadir in the midst of ovulatory stage. uPA level was most lifted at the follicular stage while the PAI-1 level was most significant in a luteal phase. In chest infection patients, uPA, PAI-1, and uPAR positive rates were low when we use the menopause-stateunmatched cut-off core interests. As we adjusted the cut-off centers by menopause communicates, the PAI-1 vitality extended in post-menopause ailment patients. These disclosures suggest that there is a minor, however, possible subsequent change of these in the midst of ladylike cycle which may cloud the psychotic motivation in pre-menopause malady patients. The shaky ascent of PAI-1 all around distinguished in post-menopause illness patients, yet this tallness did not unite with tumor weight, for instance, the number of metastatic destinations or metastatic territory. Considering modification of physiological changes of uPA, PAI-1, and uPAR required in choosing over the top tallness of the plasma levels in tumor patients, especially in females. Both u-PA and PAI-1 suggests a section for these proteins in rudeness and angiogenesis in glioblastoma multiforme. Estimation of u-PA in tumor tissue may be useful to choose representation and survey ampleness of treatment in patients with undermining. Osteopontin (OPN) is a released, integrin-tying phosphoprotein that has ensnared in both typical and obsessive procedures; subjective increments in OPN blood levels have been account for in a little number of patients with metastatic tumors of different sorts. This study shows a factually significant rise in plasma OPN in the lion's share ( roughly 70%) of a physical arrangement of patients with metastatic bosom growth when thought about (95th percentile) to real ladies or patients who had finished adjuvant treatment for right on time stage bosom malignancy. Moreover, this is the first study to exhibit that higher OPN levels in patients with the metastatic bosom disease may connect with an expanded number of included destinations and diminished survival. The pilot study reported here recommends a potential utility for plasma OPN determination in patients with metastatic carcinoma of the bosom, both for the estimation of tumor weight and as a possible marker of reaction to treatment. Plasma OPN could be a clinically useful parameter in checking the adequacy of treatment and, possibly, the choice to change treatment. In the dominant part of patients with metastatic bosom tumor, who don't have a quantifiable ailment, successive plasma OPN determinations could in this way give a tremendously required device to direct clinical administration. Our outcomes emphatically bolster the requirement for a next actual trial to address the utility of measuring plasma OPN levels in ladies with the bosom tumor. We reason that bosom disease common subtypes connected with the first site of inaccessible repeat, and that numerous ordinarily surveyed proteins are compared with homing of bosom tumor at far off locales. Bosom malignancies that first offer ascent to lung metastases all the time express either EGFR or HER2, which proposes that such growths may be a potential target bunch for dual HER1 and HER2 inhibitors, for example, lapatinib or erlotinib. |
Acknowledgement
|
| The Authors are appreciative to Dr. Nawaf Aljhany general administrator Personnel of Alfarabi Dentistry and Nursing for giving the essential workplaces to the papers arrange |
Figures at a glance
|
 |
| Figure 1 |
|
| |
| |






