Keywords
Craniopharyngioma; Inflammation; Immune response; Immunohistochemical; Recurrence; Interleukin 6, 12 and 4; CD68; Fascin; Plasma cells; CD4; CD8
Abbreviations
CPs: Craniopharyngiomas; Ada: Adamantinomatous form; Pa form: Papillary form; SR: Stellate Reticulum; WK: Wet keratin; DC: Dystrophic Calcification; GC: Giant Cells; CC: Cholesterol Clefts; FLI: Finger”-Like Interdigitation; RF: Rosenthal Fibers; IFI16: Interferon factor inducible; NFκB: Nuclear factor κB; TNF-α and TNF- λ: Tumor necrosis factor α and tumor necrosis factor λ; GFAP: Glial Fibrillary Acidic Protein; MIF: Macrophage Migration Inhibiting Factor; CNS: Central Nervous System; BBB: Blood-Brain Barrier; TILs: Tumor Infiltrating Lymphocytes; PSGL-1: P-Selectin Glycoprotein Ligand-1; NK cells: Natural killer cells; HIF-1α: Hypoxia inducible factor-1 alpha; VEGF: Vascular endothelial growth factor; CA9: Carbonic Adhydrase 9; MVD: Microvessel Density; TAMs: Tumorassociated macrophages
Introduction
Craniopharyngiomas (CPs) account for 1.2% to 4% of all brain tumors and 6 to 9% of all childhood tumors [1]. They are the most common non-glial tumors in childhood [2], and originate from the embryonic remains of squamous cells through the hypophyseal-pharyngeal duct [1-3]. The pathology of cyst formation, inflammation and immune response have been always questioned [4]. The cystic portion of the CP is filled with secreted fluid, cholesterol crystals, and epithelial cells. Moreover, its presence is associated with a major risk of recurrence, and thus suggesting a proliferative appliance in its genesis and growth, rupture, and inflammatory and immune response. Indeed, the cyst fluid recurs after multiple aspirations and the epithelium of the cyst is the actively proliferating component due to the presence of secretory squamous cells and of zymogen granules [4]. Due to the secretive properties of this epithelium, most part of PC has a cystic portion containing secreted fluid, cholesterol crystals, and epithelial cells [1]. Chemical meningitis is a rare phenomenon due to rupture of a craniopharyngioma cyst; it develops because of presence of cholesterol crystals in cyst fluid secreted by the squamous epithelium lining of the cyst [5]. Spontaneous rupture of the cyst described mainly in adults may present with or without meningitis, depending upon the cholesterol contents of cystic fluid of tumor [6,7]. The mechanism of rupture of a craniopharyngioma cyst may depend on weakness of the cyst wall caused by cyst expansion inducing degeneration of cyst wall [8,9]. Chemical meningitis in CPs is supposed to be caused by the cholesterol crystals contained in the cystic fluid. Chemical meningitis is therefore strong possibility in operated cases of residual or recurrent CP though the occurrence of meningitis depends on a leak and contents of cholesterol in particular for a cyst of CPs [7,8].
The aim of this work was to evaluate the immune response in the boundary of the craniopharyngioma. A clinical pathological and immunohistochemistry approach.
Material and Method
Clinical cases
Craniopharyngiomas were retrieved from the files of the department of Neuropathology at the National Institute of Neurology and Neurosurgery in Mexico City. The review was conducted during the 2000-2007 period. Clinical information and follow-up data were available for 56 cases. Age, gender, location and size of tumor, time of onset of symptoms, surgical exeresis, recurrence and follow-up were recorded.
All tumours analyzed in the study were obtained from a first surgery. Histological subtypes/grades were classified according to the guidelines given by the World Health Organization (WHO) [1].
Surgically removed specimens were fixed immediately in 10% formalin and subsequently paraffin-embedded and hematoxylineosin- stained. Four pathologists without previous knowledge of the specimen’s source independently carried out the morphological analysis.
Pathology
Surgical and biopsy specimens from 56 patients with CP were reviewed. 35 adaCps and 15 pCPs. All biopsies were fixed overnight in 4% formalin and routinely processed into embedded paraffin. Sections were cut every 4 μm. All tumors analysed in the study were obtained from the first surgical procedure, and were assigned to two categories, according to the presence (group 1) or absence (group 2) of brain invasion.
The criteria to declare brain invasion included the presence of cohesive cells forming nest or compactly arranged as a “finger”- like interdigitation, single cells, nests, cords in the form of wet keratin, ghost cells, and dystrophic calcification in brain parenchyma as well as inflammatory response; macrophages, plasma cells, hemosiderophages. In addition, wet keratin, ghost cells and calcifications on the tumor boundaries were evaluated. Rupture of external epithelium, tumoral cells proliferation, stellate reticulum density, presence of oil machinery fluid, inflammation, granuloma formation, cholesterol cleft macrophages, gliosis, reactive astrocytes, Rosenthal fibers and vessel proliferation were also considered, paying attention to their characteristics for each case. Chronic Inflammation (presence of lymphocytes and plasma cells infiltration) and macrophages were evaluated in surrounding and adjacent brain tissue.
Immunohistochemistry
Immunohistological staining procedures employed the Dual Link Envision+ detection system (BioSB San Ramon CA). Immunohistochemical studies were performed upon 5μ formalinfixed, paraffin-embedded sections. Standard immunostaining techniques were performed with appropriate positive and negative controls included, as described by Hsu et al. with an appropriate positive and negative controls were used. The primary antibodies used are seen in Table 1. The degree of reactivity was denoted subjectively with crosses in images, according to the intensity of expression: negative (-), weak (+), moderate (++) and strong (+++). On the basis of the percentage of positive cells, each tumor was defined within 1 of 4 groups as follows: negative, <30%, 30– 60%, or >60% positive. The expression of the different antibodies used was also evaluated inside (in the outer epithelium stellate reticulum, ghost cells, wet keratin, world likes arrays, dystrophic calcification) and outside the tumor, in the boundary of tumor vessels, reactive gliosis and Rosenthal fibers, as well as the form of brain invasion.
| |
Source |
Dilution |
Clone |
| CD68 |
DAKO |
1:100 |
SPM130 |
| CD4 |
DAKO |
1:100 |
MT310 |
| CD20 |
DAKO |
1:100 |
MHM6 |
| CD56 |
DAKO |
1:100 |
Mog-1 |
| Il-6 |
Santa Cruz |
1:100 |
Poly-A183 |
| MHC-II |
DAKO |
1:100 |
DK22 |
| IFI-16 |
Santa Cruz |
1:100 |
Cs8020 |
| TNF-α, |
Abcam |
1:100 |
ab9635 | |
| TNF- λ, |
AbDSerotec |
1:100 |
CC302 |
| Fascin |
Abcam |
1:100 |
EP5902
(ab126772) | |
| plasma cells |
Santa Cruz |
1:100 |
(LIV11)sc-53416 |
DAKO. Carpintery Ca. Santa Cruz, Dalas Tex. IL-6 .- interleukine 6, IFI-16.- Interferon, gamma-inducible protein. MHC-II.- Major Histocompatibility complex clase II. CD56.-Natural killer (NK) cells, TNFa transforming growth factor, CD4.- T lymphocytes, CD20.- B-lymphocytes, CD68 (macrophages). Fascin. Dendrite cells marker
Table 1: Antibodies used and their characteristic.
Statistical analysis
The analyzed parameter was the relationship between inflammation and clinico-pathologic analysis and positive immunostaining for MHC-II, TNFα, NTFγ, IFI-16, α, CD68, IL-6, CD4, CD20, and CD56, fascin and plasmatic cells. Descriptive statistics (frequency distribution) were determined for continuous and categorical variables. Odds ratios, 95% confidence interval and Phi correlations were performed in relation to the inflammatory response. Survival analysis was made by means of Kaplan- Meier method. A p-value of p <0.05 was considered statistically significant.
Results
Tables 2 and 3 shows the clinical characteristics according to histological type. Fifty-six (56) cases were included in this study. Forty five (80.4%) cases were adamantinomatous craniopharyngiomas (ACF) and eleven (19.6%) were papillary craniopharyngiomas (PCF) (p=0.005). Twenty-nine (51.8%) samples were from female subjects and twenty-eight (48.2%) from male subjects. Age ranged from 23 to 87 years (mean 49.89). Time elapsed from onset of symptoms was 3-24 months (mean 10.89mo).
| |
Adamantinomatous n= 45(%) |
Papillaryn=11(%) |
OR (CI 95%) |
P value |
| Age |
23-87(47ys) |
43-67(57yr) |
|
0.065 |
Female
Male |
20 (44.4)
22 (55.6) |
9(81.8) 6(18.2) |
0.18(0.03-0.92) |
0.026 |
| Time |
3-24 (9mo) |
8-23(12mo) |
--- |
0.000 |
| Tumor size |
23-49 (37mm) |
23-36(28mm) |
--- |
0.040 |
Partial exeresis
Total exeresis |
17/38)
28(62) |
3(27)
8(73) |
0.61(0.14-2.6) |
0.515 |
| Recurrence |
54(53.3) |
3(11) |
3.0(0.71-13) |
0.121 |
| Death |
6(13.3) |
2(18.2) |
0.69(0.12-4.0) |
0.680 |
| Follow-up |
12-54(36mo) |
34-56(54mo) |
--- |
0.007 |
OD: odds ratio; IC: 95% confidence interval. Descriptive statistics (frequency distribution) were determined for continuous and categorical variables. Odds ratios, 95% confidence interval and Phi correlations were performed in relation to the inflammatory response. Survival analysis was made by means of Kaplan-Meier method. A p-value of p<0.05 was considered statistically significant.
Table 2: Clinical and epidemiological characteristic associated with histological type.
| |
Adamantinomatous N=45 |
PapillaryN=11 |
OR(CI 95%) |
P value |
|  Gliosis |
30(67) |
3(27.3) |
5.3(1.2-23) |
.017 |
|  Brain infiltration |
24(53) |
2(18) |
5.1(.99-26.5) |
.036 |
| DystrophicCalcification |
39(87) |
1(9) |
65(7-603) |
.000 |
| Wetkeratin |
28(62) |
0(0) |
.37(.26-.55) |
.000 |
Inflammation
Round
Perivascular
mixed
Macrophages |
30(67)
8(18)
9(20)
13(29)
17(38) |
2(18.2)
9(82)
1(9)
0
1(9) |
9(1.7-47)
0
0
0
6(.71-52) |
.004
.029
0
0
.069 |
OD: odds ratio; IC: 95% confidence interval. Descriptive statistics (frequency distribution) were determined for continuous and categorical variables. Odds ratios, 95% confidence interval and Phi correlations were performed in relation to the inflammatory response. Survival analysis was made by means of Kaplan-Meier method. A p-value of p<0.05 was considered statistically significant.
Table 3: Histological features associated with histological type
craniopharyngiomas (ACF) and eleven (19.6%) were papillary craniopharyngiomas (PCF) (p=0.005). Twenty-nine (51.8%) samples were from female subjects and twenty-eight (48.2%) from male subjects. Age ranged from 23 to 87 years (mean 49.89). Time elapsed from onset of symptoms was 3-24 months (mean 10.89mo).
Tumor size ranged from 23 to 49 mm (mean 34.5). The followup period for the patients ranged from 12 to 56 months (mean 35.50).
Brain infiltration was observed in twenty-six (46.4%) cases, gliosis in thirty-three (58.9%), dystrophic calcifications in forty (71.4%), wet keratin in twenty-eight (50%) cases (Figure 1a). Evident inflammation was observed in thirty-two (57.1%) cases (Figure 2b and 2c); in nine (16.1%) of these inflammation was present around the outer epithelium, ten (17.9%) cases showed perivascular inflammation, and in thirteen (26.2%) cases, inflammation involved both, the area around the outer epithelium and the perivascular region (Figure 1e). Macrophages were observed in eighteen (32.1%) cases (Figure 1f).
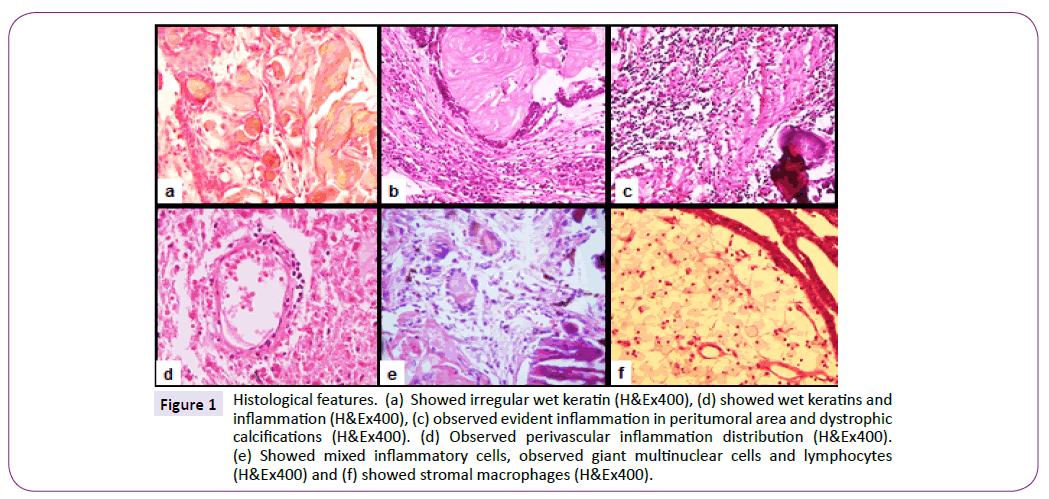
Figure 1: Histological features. (a) Showed irregular wet keratin (H&Ex400), (d) showed wet keratins and inflammation (H&Ex400), (c) observed evident inflammation in peritumoral area and dystrophic calcifications (H&Ex400). (d) Observed perivascular inflammation distribution (H&Ex400). (e) Showed mixed inflammatory cells, observed giant multinuclear cells and lymphocytes (H&Ex400) and (f) showed stromal macrophages (H&Ex400).
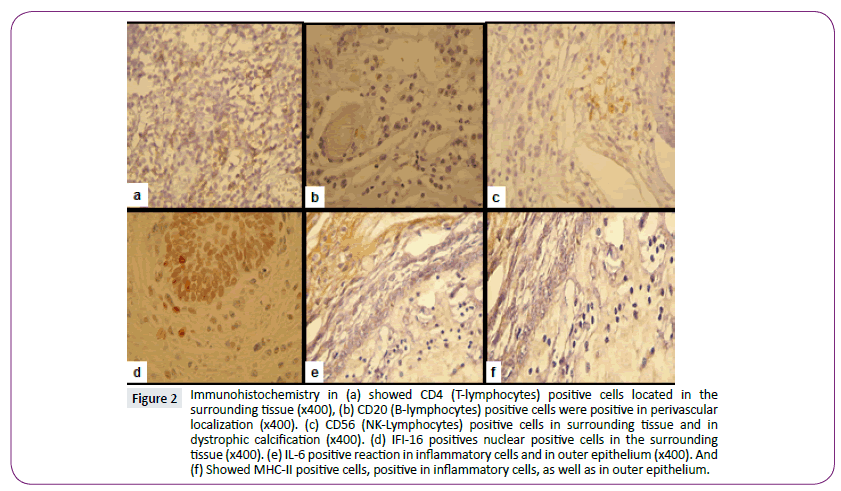
Figure 2: Immunohistochemistry in (a) showed CD4 (T-lymphocytes) positive cells located in the surrounding tissue (x400), (b) CD20 (B-lymphocytes) positive cells were positive in perivascular localization (x400). (c) CD56 (NK-Lymphocytes) positive cells in surrounding tissue and in dystrophic calcification (x400). (d) IFI-16 positives nuclear positive cells in the surrounding tissue (x400). (e) IL-6 positive reaction in inflammatory cells and in outer epithelium (x400). And (f) Showed MHC-II positive cells, positive in inflammatory cells, as well as in outer epithelium.
Results from immunohistochemical analysis are shown in Table 4. Inflammatory cells demonstrated positive immunohistochemical staining for CD4+ T cells (T Lymphocytes) were detected in 11 cases, in 10 (17.9%) of these cases the location was the surrounding tissue and in only 1 case (1.8%) they were restricted to perivascular locations; 45 (80.4%) were CD4 negative cases (Figure 2a). Ten (17.9%) cases were CD20 (B lymphocytes) positive with a perivascular distribution, thirteen (23.2%) were positive with inflammatory mixed location, and thirty-three cases were CD20 negative (Figure 2b).
| |
Adamantinomatous N=45 |
PapillaryN=11 |
OR(CI 95%) |
P value |
| CD68 |
17(38) |
1(9) |
6(.71-52) |
.069 |
| CD4 |
36(80) |
9(89) |
.88(1.6-4.8) |
.892 |
| CD20 |
23(49) |
1(9) |
9.5(1.1-81) |
.016 |
| CD56 |
41(91) |
0(0) |
.3.7(1.6-8.7) |
.000 |
| Il-6 |
44(98) |
0(0) |
12(1.8-78) |
.000 |
| MHC-II |
44(98) |
0(0) |
440(25.3-7649) |
.000 |
| IFI-16 |
29(64) |
0(0) |
1.6(1.2-2.3) |
.000 |
| TNFa |
44(100) |
0(0) |
440(25.3-7649 |
.000 |
| TNF? |
36(80) |
1(9) |
.88(1.6-4.8) |
.892 |
| Fascin |
44(98) |
1(9) |
440(25.3-7649) |
.000 |
| Plasma cells |
36(80 |
0(0) |
.88(1.6-4.8) |
.892 |
IL-6.- interleukin, IFI-16.- Interferon, gamma-inducible protein. MHC-II.- Major Histocompatibility complex class II. CD56.-Natural killer (NK) cells, CD45. CD4.- T lymphocytes, CD23.- B-lymphocytes. CD68.- macrophages. TNFa
OD: odds ratio; IC: 95% confidence interval Descriptive statistics (frequency distribution) were determined for continuous and categorical variables. Odds ratios, 95% confidence interval and Phi correlations were performed in relation to the inflammatory response. Survival analysis was made by means of Kaplan-Meier method. A p-value of p<0.05 was considered statistically significant.
Table 4: Antibodies used associated with histological type.
Three CD56-positive (NK lymphocytes) cases (5.4%) with distribution in the tissue surrounding the outer epithelium, only one case (1.8%) with perivascular distribution, mixed distribution in two cases (6%), in twenty-seven (48.2%) cases around wet keratin, and around dystrophic calcification in nine (16.1%) cases; fourteen cases (25%) were CD56-negative (Figure 2c).
Seventeen (30.4%) CD68-positive cases in stromal macrophages were found.
IL-6 was expressed in the nucleus of inflammatory cells in 29 (51.8%) cases, in outer epithelial cells and wet keratin in 5 (8.9%) cases and in dystrophic calcification in 10 (17.9%) cases (Figure 2d). IL-6 was positive in inflammatory cells in 23 (41.1%) cases, and positive in the outer epithelium in 17 (30.4%) cases, in gliosis in 2 (3.6%) and around wet keratin in 14 (25%), and was negative in stroma cells and dystrophic calcifications (Figure 2e).
MHC class II expression was detected in inflammatory cells in 32 (57.1%) cases, in outer epithelium, gliosis and wet keratin in 7 (12.5%) cases, and negative in 5.4% of the samples (Figure 2f). Macrophages were observed everywhere in the tumor and therefore the outside (Figure 3a). TNFα was positive in inflammatory cells (Figure 3b) and arrangements world like arrays (Figure 3c), in adaCP type and papillary type was positive between vascular spaces (Figure 3d), TNFγ was positive in some lymphocytes (Figure 3e) as well as plasma cells (Figure 3f) were observed in the boundary of the tumor.
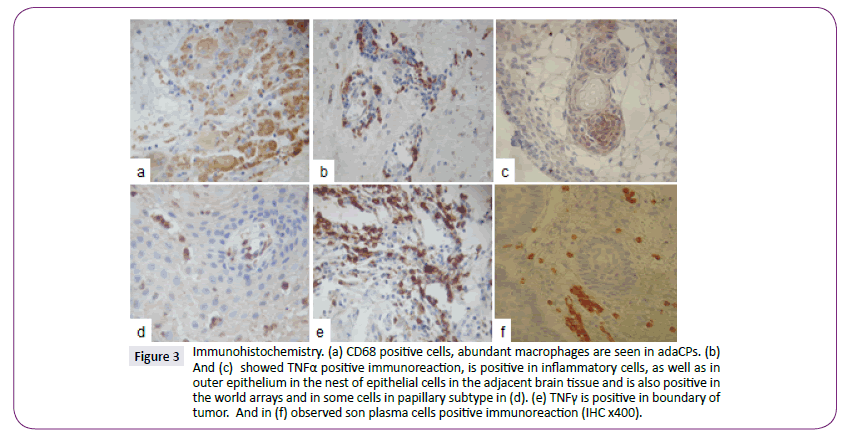
Figure 3: Immunohistochemistry. (a) CD68 positive cells, abundant macrophages are seen in adaCPs. (b) And (c) showed TNFa positive immunoreaction, is positive in inflammatory cells, as well as in outer epithelium in the nest of epithelial cells in the adjacent brain tissue and is also positive in the world arrays and in some cells in papillary subtype in (d). (e) TNF? is positive in boundary of tumor. And in (f) observed son plasma cells positive immunoreaction (IHC x400).
There was a statistical difference when histological type was associated with tumor size (p=0.004), gliosis (p=0.017), dystrophic calcification, wet keratin, follow-up and death (respectively p=0.000), brain infiltration (p=0.037), and inflammation (p=0.003). There were no statistical differences between gender, age, time of onset of symptoms, tumor size and tumor location. However, tumor size was associated with brain infiltration (p=0.014), inflammation (p=0.013), macrophages (p=0.030).
There was a statistically significant correlation between inflammation in craniopharyngioma and; CD4 (Phi=0.338, p=0.041), CD23 (Phi=0.652, p=0.000), CD68 (Phi=0.493, p=0.000), NK (Phi=0.456, p=0.040 MHC-II (Phi=0. 476, p=0.014), TNFα ( p=008, and IFI-16(Phi=0.436, p=0.031). However, there was no correlation with IL-6 (Phi=0.257, p=0.297) immunoexpression and inflammation.
Rrecurrence was correlated with DC (p=0.016). The range for the recurrence curves and follow-up data was 42.6 mo (38.8- 46.4) (Log Rank text p=0.03, IC95%) (Figure 4), and it shows that patients with stronger inflammation had a higher probability of recurrence than patients with no inflammation.
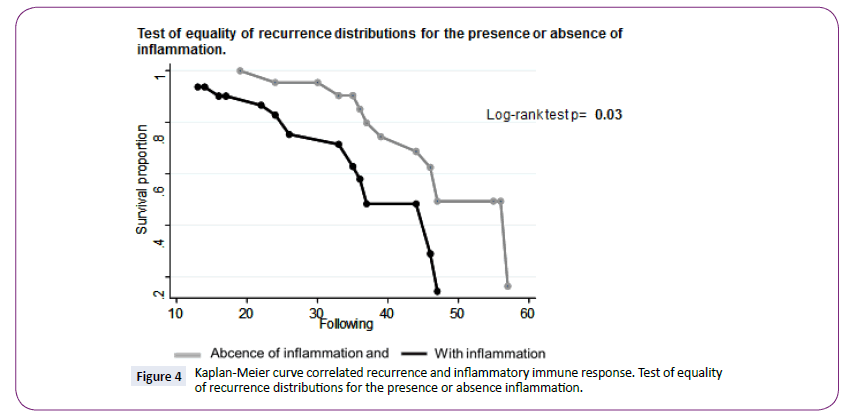
Figure 4: Kaplan-Meier curve correlated recurrence and inflammatory immune response. Test of equality of recurrence distributions for the presence or absence inflammation.
However, the test for equality of survival distributions for the presence or absence of inflammation showed a greater followup period for cases lacking inflammation than that for cases in which inflammation was present. (Log Rank text p=0.07, IC95%) (Figure 5).
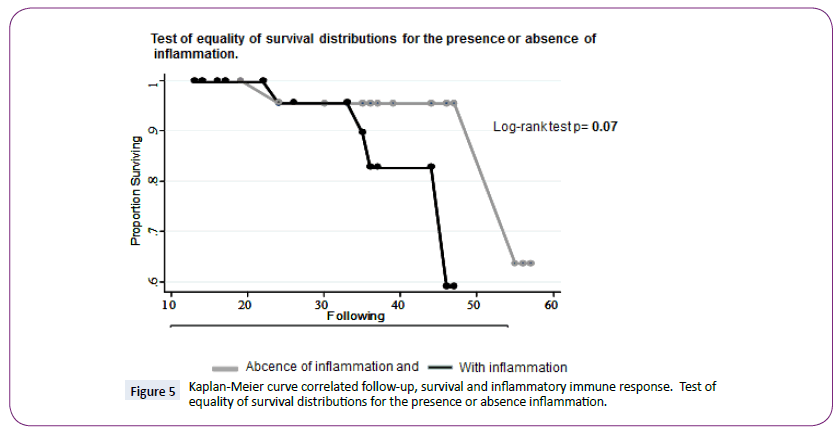
Figure 5: Kaplan-Meier curve correlated follow-up, survival and inflammatory immune response. Test of equality of survival distributions for the presence or absence inflammation.
Discussion
The total removal of craniopharyngiomas can be difficult because of their tendency to adhere to surrounding vital structures. Frequently, adjacent brain tissue is infiltrated by leukocytes and cytokine(s) produced by tumor cells may play a pivotal role in tissue reaction [3,7,10]. Two subtypes could be distinguished: an adamantinomatous (Ada) form that is more common in children, and a papillary (Pa) form that is observed almost exclusively in adults [1]. The tumour is usually part cystic and part solid. Besides these histological differences, these two variants differ in some molecular features that have been recently identified and could have important therapeutic implications [3,5]. It has been demonstrated that inflammation and macrophages can be present within the tumor and in the surrounding tissue, but to our knowledge this is the first clinico-pathologic and immunohistochemical report considering inflammation as biological behaviour [8].
Bleeding and escape of keratinous material from the CPs can result in yellow foci of xanthogranulomatous inflammation. Microscopic examination of these yellow foci of xanthogranulomatous inflammation reveals foamy macrophages and giant multinucleated cells surrounding cholesterol clefts [8]. Only a few studies have addressed the molecular classification of the cyst fluid so far and the mechanisms of action of intracystic agents are not clearly understood yet [8,9]. More recent studies suggest that the cystic element of CPs could be also the result of an active production of the fluid by the epithelial cells of tumor [2,3]. The pattern of embryological formation of the hypothalamic-pituitary axis plays a major role in its propensity for developing cystic epithelial lesions. Subsequent inflammatory, metaplastic, and neoplastic processes may promote further progression along the pathological continuum, ranging from benign epithelial cysts to aggressive neoplastic cystic CPs [1-3]. The numerous pathology reports on craniopharyngioma describe the classical features of squamous epithelium and keratin. Relatively little attention has been paid to its stroma and the surrounding tissue and immune response [7,8]. The tumor stroma have reactive changes and commonly showed cyst formation and calcification [9]. Much recent research aims to characterize the cyst fluid to understand the mechanism of formation and to refine treatments able to reduce cyst volume and inhibit the formation of new cysts. Indeed, the formation mechanism of cystic fluid has always been debated: it could be the result of blood-brain barrier impairment [2], but surely an active secretory process takes part in its formation [3,4], or is due to epithelial cells secretion [6,7], rupture of those structures [8,9], oil machinery fluid components of the cyst structures or is secondary to an immune response that produce and spontaneous involution [11]. Spontaneous rupture of craniopharyngioma cysts can be divided into three subtypes: cyst rupture associated with chemical meningitis; cyst rupture with improvement in symptoms related to the craniopharyngioma and not associated with chemical meningitis; and asymptomatic rupture [6,7]. Signs of aseptic meningitis, including neutrophilic pleocytosis, the presence of cholesterol crystals, macrophages, granulomas formation and inflammatory response. Inflammation is likely to produce greater tumor adherence to and infiltration of adjacent brain tissue [3]. The inflammatory cytokines has been examined at various inflammatory levels of AdaCps [12]. IL-1alpha, IL-6 and TNF-alpha has been reported significantly higher in the cyst fluid than in cerebrospinal fluid (CSF) [12], Mori et al. [13], observed that IL-6 plays an important role in the inflammatory reaction that occurs in the interface between the craniopharyngioma and the brain parenchyma. α-defensins 1-3 and other proteins and peptides have been involved in inflammation, mineralization processes and lipid transport [10]. Apolipoprotein A-I, A-II, C-I and J, haemoglobin fragments, ubiquitin, α-2-HS-glycoprotein or fetuin A, α-1-antichymotrypsin, vitamin D binding protein, and α-1-acid glycoprotein were also have been characterized in the oil machinery fluid [10]. Thymosins β4 and β10 peptides has been also identified in the intracystic fluid AdaCPs [14]. The presence of β-thymosins in the intracystic fluid of the tumour confirmed the secretion of these proteins in the extracellular environment [14]. Due to activity and antiapoptotic and anti-inflammatory properties could be strictly involved in both tumour progression and cyst development and growth [14]. Galectin-3, p53, and the macrophage movement inhibiting factor (MIF) are known to play crucial roles in these processes [15]. The central nervous system (CNS) is considered as an "immunologically privileged" site [16]. However, this notion has been slowly overturned by the realization that the neuroimmunologic interface is, in fact, dynamic and not absolved of immunity's ability to recognize danger. Numerous studies have investigated the immune system of brain tumour patients, mainly in the context of malignant glioma. Brain tumour patients suffer from extensive immunosuppression due to lymphopenia, decreased lymphocyte proliferation, and diminished cytotoxic activity, reduced MHC class I expression on monocytes and predominance of anti-inflammatory T-helper 2-type (Th2) cytokine production [16]. Derivatives of the monocyte/macrophage lineage appear to enter and take up residence in various structures of the CNS as part of normal ontogeny and physiology [16]. Leukocyte migration into and through tissues is fundamental to normal physiology, immunopathology and host defence. Leukocyte entry into the CNS is restricted, in part, because of the blood-brain barrier (BBB) [16]. Immunocompetent cells, such as T-lymphocytes of both CD4 and CD8 positive. Currently, this immune system-tumor interaction is represented by the concept of cancer immunoediting, which emphasizes that immunity may sub serve either classical cancer immunosurveillance functions or promote the eventual outgrowth of immunoevasive cancer cells [16]. Moreover, cancer patients mount both cellular and humoral adaptive, as well as innate, immune responses to the tumors they harbour. In concert with adaptive immune recognition, recent provocative data suggest that the innate immune system also discriminates between tumor cells and normal cells. Eisele et al. [17], showed that human glioma cells express MICA/B as well as ULBP1-3 and that the expression of these ligands in glioma may be influenced by TGF-β. Moreover, Wu et al. [18] Provided evidence that tumor-initiating cells within gliomas−also referred to as glioma stem cells and characterized by their CD133 cell surface marker expression−express NKG2D and NKp30/44/46 ligands. Our results show a perivascular infiltrate of CD20+ cells, and the following statistical differences when associating it with tumor size (p=0.004), histological type (p=0.013), brain infiltration and mode of inflammatory cell infiltration (p=0.000 respectively). CD4 positive immunoreaction was expressed in peritumoral area and was associated with tumor size (p=0.028), histological type (p=0.000), brain infiltration and mode of inflammatory cell infiltration (p=0.000 respectively). The inflammation labelled by CD45 has been identified commonly in the craniopharyngioma tissues involving the third ventricular floor [8]. Liu et al. [19] suggested that the difference in the inflammation between the two types of craniopharyngioma may affect the prognosis of the patients, and that ACF showed markedly higher CD45 and ICAM-1 expressions than PCF.
Stevens et al. [20] characterized the immune infiltrates in gliomas, carcinoma metastases, CP and meningiomas. Infiltrates in gliomas consist almost exclusively of T-cells of the suppressor/ cytotoxic type whereas infiltrates in carcinoma metastases and CPs contain considerable numbers of CD4+ T cells and B-cells.
TIL display, antigen-specific activated (TIL) must be able to move to relevant tumor sites in the brain. Given the anatomical complexity of the CNS, it has been proposed that there are three methods by which immune cells may access the CNS: from blood to the CSF via the choroid plexus, from blood to the subarachnoid space, and from blood to parenchyma [20]. However, the bloodbrain barrier (BBB) and its integrity in the setting of glioma before addressing how lymphocytes may traffic from the blood to tumor sites in the CNS parenchyma [21].
T cells homing to the brain first slow down by slowly tethering to capillary endothelium in a "rolling" step mediated by interactions between endothelial cell E- or P-selectins and P-selectin glycoprotein ligand-1 (PSGL-1) [21]. Subsequently, lymphocyte integrin molecules become "activated" when chemokines, likely present on the vascular endothelium, engage cognate G protein-coupled chemokine receptors on lymphocytes. Enhanced adhesion is likely mediated through interactions between integrin: ligand pairs α4,β1/7, VCAM-1 and LFA: ICAM- 1 expressed on lymphocytes and endothelium, respectively [21]. Lymphocytes ultimately transmigrate to the CNS parenchyma in the "diapedesis" step, which may occur via trans- or para-cellular endothelial transport [21].
Natural killer (NK) cells are large granular lymphocytes of the innate immune system. These cells are able to directly lyse infected or transformed cells without specific immunization [16], they also are the least abundant immune cell population within the brain tumour microenvironment. NK cells can also recognise the Fc part of antibodies via low affinity FcγRIIIA (CD16) receptor and perform antibody dependent cellular cytotoxicity (ADCC) of antibody-coated cells Moreover, they secrete various cytokines and chemokines, such as interferon gamma (IFN-γ) [21,22].
Hypoxia inducible factor-1 alpha (HIF-1α), which is one of the hypoxia inducible factor-1 subunits [19], and plays an important role in tumor cells adaptation to hypoxic microenvironment by regulating its downstream genes, including vascular endothelial growth factor (VEGF) and carbonic anhydrase 9 (CA9). Proescholdt et al. [23] Reported HIF-1α expression was largely absent in CP cysts. In addition, HIF-1α expression promotes tumor growth, angiogenesis and progression. However, adapting to the hypoxic conditions, HIF-1α and VEGF mRNA are largely expressed in recurrent CP [23]. Therefore, preventing the tumor cells from adapting to the hypoxic conditions may be an effective way to obviate the relapse of craniopharyngioma. Various studies have shown that the formation of new blood vessels, the angiogenesis, plays a significant role in tumor progression [24]. Possibly the cellular adaptation to hypoxia is a key factor of angiogenesis under hypoxic microenvironment within craniopharyngioma [19,23,24]
In Craniopharyngiomas, the role of the immune response is even more complex. An alternative explanation could be the involvement of the innate immune response which would account for some specific features of the cystic fluid such as its inflammatory properties responsible for the chemical meningitis sustained by craniopharyngioma fluid spill into the subarachnoid space [7,8]. The vasospasm induced by the contact of the cyst fluid to arteries evidence of local synthesis of IgG and elevated lactate levels in cyst fluid, which do not correlate with serum lactate levels, and finally, the strong affinity of natural killer (NK) cells to craniopharyngioma [13].
Macrophage-inhibiting factor is another molecule probably involved in the ontogenesis of craniopharyngioma. Macrophage migration inhibitory factor (MIF) roles as a pleiotropic protein, participating in inflammatory and immune responses [25]. MIF was originally discovered as a lymphokine involved in delayed hypersensitivity and various macrophage functions, including phagocytosis, spreading, and oncogenesis activity. It was demonstrated that anti-MIF antibodies effectively suppress tumor growth and tumor-associated angiogenesis, suggesting that MIF is involved not only in inflammatory and immune responses but also in tumor cell growth [26]. The MIF expression level seems to correlate with the risk of recurrence in CP, as it was significantly lower in rapidly recurring CPs than in the slowly recurring or nonrecurring lesions [25,27]. Analogous to MIF, galectin-3 levels of expression has been studied and had a significantly lower expression in rapidly recurrent CPs. In view of the antiapoptotic role of galectin-3, its low level of expression in recurring CPs seems to be related more to its role in phagocytosis. In this context the low levels of galectin-3 could correlate the ontogenesis of craniopharyngioma with defects in the normal biological elimination of embryonal tissue remnants. Tumorassociated macrophages (TAMs) are called as “corrupted” by neoplasms cells and subsequently facilitate, rather than inhibit, tumor metastasis, which reside in the tumor mass, play central roles in this intra tumoral interchange. A prevalent hypothesis suggests that macrophages are corrupted by cancer cells and subsequently contribute to tumor progression instead of tumor inhibition [27]. This mechanism comprises a paracrine signalling loop between tumor cells and TAMs that involves various chemokines and cytokines [28]. These factors secreted by cancer cells contribute to down-regulation of both expression of major histocompatibility complex class II and macrophage ability to present antigen. Pukrop et al. [29] suggested that TAMs influence the tumor microenvironment by modulating Wnt signaling. They showed that TAMs secrete Wnt-5a, which induces MMP- 7 expression in cancer cells and facilitates successful invasion. Previously, it was shown that macrophage-derived Wnt molecules promote vascular remodelling and that tumor cells are highly mobile and intravasate around perivascular TAM clusters. Taken together, we guesswork that invasive TAMs link angiogenesis and tumor invasion and which Wnt-signaling plays a role in mediating their activity [28,29]. Certainly, a molecular guarantee of adaCP is the activated Wnt signaling pathway maintained by nuclear β-catenin accumulation in a subset of these tumor cells, supplemented by a peculiar growth pattern in a specific tumor stem cell (TSC) population in human CPs, which represents a tumor stem cell niche and adds to tumor recurrence [29].
The presence of these antimicrobial peptides could suggest a possible involvement of the innate immune response in the formation and maintenance of the CPs associated cyst or simply be expression of an inflammatory response taking place within the CPs cyst. Detection of α-defensins 1–3 excludes that cyst fluid formation can derive from disruption of blood–brain barrier and suggests the involvement of innate immune response in pathology of CPs cyst formation. The reduction of α-defensins could derive both from direct antitumoral effect of interferon-α on squamous epithelial cells of craniopharyngioma cyst and from its immuno-modulatory effects on the recruitment of cells of innate immune systems [30].
The reduction of α- defensins, could derive from the direct antitumoral effect on the squamous epithelial cells of the CPs cyst, which reduces their secretory activity, from its immunomodulatory effects on the recruitment of cells of the innate immune systems, from its anti-angiogenetic activity or a combination of the above mentioned mechanisms [16].
Interferons are glycoproteins pertaining to the cytokine family and are related to cell growth factor beta and tumor necrosis factor, which are responsible for the control of cell differentiation and proliferation [30]. INF-α belongs to a family of proteins with antiproliferative and immunomodulatory functions but it is used as antitumoral agent. A recent study on the use of INF-α against CPs suggests a possible role of the Fas-induced apoptosis. Pro-apoptotic and anti-angiogenetic activities, rather than the recently described effects on immune cells [31]. The antitumoral activity of interferons is due to their anti-proliferation, cytotoxic and maturational effects, with the simultaneous modulation in patient immune response.
TAMs to secrete proteases within the tumor microenvironment, such as urokinase plasminogen activator [32], cathepsins B and D and matrix metallopeptidases 2 and 9. It is believed that these TAM-derived enzymes digest the tumor basement membrane, facilitating tumor cell escape [33,34].
Conclusion
There was a statistically significant correlation between inflammation in craniopharyngioma and; CD4, CD23, CD68, NK, TNFα, TNFγ, MHC-II, and IFI-16, fascin and plasma cells immunoexpression. However, there was no correlation between IL-6 immunoexpression and inflammation. Tumor size, histological type, mode of inflammation and inflammatory cells are important in biological behaviours for the follow-up of CPs. Inflammation in CP was correlated with a bad prognosis and we suggested that tumor-associated macrophages (TAMs) play an important role in craniopharyngioma, further studies are needed. AdaCP could express high levels of secreted mitogenic signals, including members of several families, as well as chemokines, cytokines with pro-inflammatory action, and their corresponding receptors, suggesting an important autocrine/paracrine role of these cells in the pathogenesis of adaCPs.
Conflict of Interest
The authors declare no conflict of interests in terms of employment or leadership position, stock ownership, honoraria, research funding, expert testimony or other remuneration.
7357
References
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, et al. (2009) The 2007 WHO Classification of Tumours of the Central Nervous System. ActaNeuropathol (Berl) 114:97-109.
- Hirano A, Ghatek NR, Zimmerman HM (1973)Fenestrated blood vessels in craniopharyngioma. ActaNeuropathol 26:171-17710.
- Petito CK, De Girolamo U, Earle KM (1967)Craniopharyngiomas. A clinical and pathological review. Cancer 37:1944-1952.
- Arefyeva IA, Semenova JB, Zubairaev MS, Kondrasheva EA, Moshkin AV (2002)Analysis of fluid in craniopharyngiomarelated cysts in children: proteins, lactate and pH.ActaNeurochir 144:551-554.
- Karavitaki N, Brufani C, Warner JT, Adams CB, Richards P, et al. (2005) Craniopharyngiomas in children and adults: Systematic analysis of 121 cases with long-term follow-up. ClinEndocrinol (Oxf) 62:397-409.
- Szeifert GT, Julow J, Szabolcs M, Slowik F, Balint K, et al. (1991) Secretory component of cystic craniopharyngiomas: a mucino-histochemical and electron-microscopic study. SurgNeurol 36:286-293.
- Szeifert GT, Julow J, Szabolcs M, Slowik F, Balint K, et al. (1991) Secretory component of cystic craniopharyngiomas: a mucino-histochemical and electron-microscopic study. SurgNeurol 36:286-293.
- Kumar A, Kasliwal MK, Suri A, Sharma BS (2010) Spontaneous asymptomatic rupture of cystic craniopharyngioma in a child: case report and review of the literature. Child Neurol 25:1555-1558.
- Kasai H, Hirano A, Llena JF, Kawamoto K (1997) A histopathological study of craniopharyngioma with special reference to its stroma and surrounding tissue. Brain TumorPathol 14:41-45.
- Martelli C, Iavarone F, Vincenzoni F, Rossetti DV, D'Angelo L, et al. (2014) Proteomic characterization of pediatriccraniopharyngiomaintracystic fluid by LC-MS top-down/bottom-up integrated approaches. Electrophoresis 35:2172-2183.
- Kinoshita Y, Tominaga A1, Usui S, Kurisu K (2014) A craniopharyngioma with spontaneous involution of a gadolinium-enhanced region on magnetic resonance imaging. SurgNeurolInt 5:128.
- Zhou J, Qi ST, Chen LG, Huang CR, You J, et al. (2013) Expression pattern of inflammatory cytokines at various inflammatory levels of adamantinomatouscraniopharyngioma. Zhonghua Yi XueZaZhi 93: 2499-2501.
- Mori S, Yamaguchi K, Morita H, Mohri N (1985) Distribution of HNK-1+ cells in malignant lymphomas. ActaPatholJpn 35: 339-350,
- Desiderio C, Martelli C, Rossetti DV, Di Rocco C, D'Angelo L, et al. (2013) Identification of thymosins β4 and β 10 in paediatric craniopharyngioma cystic fluid. Childs NervSyst 29:951-960.
- Lefranc F, Chevalier C, Vinchon SBM, Dhellemmes P, Schüring MP, et al. (2003) Characterization of the levels of expression of retinoic acid receptors, galectin-3, macrophage migration inhibiting factor, and p53 in 51 adamantinomatouscraniopharyngiomas. J of Neurosurgery 98:145-153.
- Parney I (2012) Basic concepts in glioma immunology. In: Yamanaka R, editor. Glioma. New York: Springer42-52.
- Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, et al. (2006) TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain129: 2416-2425.
- Wu A, Wiesner S, Xiao J, Ericson K, Chen W, et al. (2007) Expression of MHC I and NK ligands on human CD133(+) glioma cells: Possible targets of immunotherapy. J Neurooncol83:121-131.
- Liu H, Liu Z, Li J, Li Q, You C, et al. (2014) Relative quantitative expression of hypoxia-inducible factor 1α messenger ribonucleic acid in recurrent craniopharyngiomas. Neurol India62: 53-56.
- Stevens A, Kloter I, Roggendorf W (1988) Inflammatory infiltrates and natural killer cell presence in human brain tumors. Cancer61:738-743
- Ransohoff RM, Kivisäkk P, Kidd G (2003) Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol3: 569-581.
- Engelhardt B, Ransohoff RM (2005)The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends Immunol26:485-495.
- Proescholdt M, Merrill M, Stoerr EM, Lohmeier A, Dietmaier W, et al. (2011) Expression of carbonic anhydrase IX in craniopharyngiomas. J Neurosurg115:796-801.
- Vidal S, Kovacs K, Lloyd RV, Meyer FB, Scheithauer BW (2002) Angiogenesis in patients with craniopharyngiomas: Correlation with treatment and outcome. Cancer94:738-745.
- Nishihira J (2000) Macrophage Migration Inhibitory Factor (MIF): Its Essential Role in the Immune System and Cell Growth. Journal of Interferon & Cytokine Research20: 751-762.
- Pettorini BL,Inzitari R,Massimi L, Tamburrini G, Caldarelli M,et al. (2010) The role of inflammation in the genesis of the cystic component of craniopharyngiomas. Childs NervSyst26:1779-1784.
- Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer4: 71-78
- Oljavo LS, Whittaker CA, Condeelis JS, Pollard JW (2010)Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol184: 702-712.
- Pukrop T, Klemm F, HagemannTh, Gradl D, Schulz M, et al.(2006) Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. PNAS103: 5454-5459
- Amaguchi K, Morita H, Mohri N (1988) Distribution of HNK-1+ cells in malignant lymphomas. ActaPatholJpn35:339-350,
- Hölsken A, Gebhardt M, Buchfelder M, Fahlbusch R, Blümcke I, et al. (2011) EGFR signaling regulates tumor cell migration in craniopharyngiomas. Clin Cancer Res17:4367-4377.
- Tagliaferri P, Caraglia M, Budillon A (2005) New pharmacokinetic and pharmacodynamics tools for interferon-alpha (IFN-alpha) treatment of human cancer. Cancer ImmunolImmunother54:1-10
- Vasiljeva O, Papazoglou A, Krüger A, Brodoefel H, Korovin M, et al. (2006) Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res66:5242-5250.
- Hagemann T, Robinson SC, Schulz M, Trümper L, Balkwill FR, et al. (2004) Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-α dependent up-regulation of matrix metalloproteases. Carcinogenesis25:1543-1549.










