Keywords
Ruptured AAA aneurysm, Common iliac artery aneurysm, Mycotic aneurysm, Salmonella infections
Introduction
Mycotic aneurysms of the abdominal aorta are rare and are associated with high morbidity and mortality. The mortality is increased further when it is associated with Salmonella infections [1] especially in the presence of risk factors for atherosclerosis [2]. The presentation of this disease can be varied from fever, mild abdominal symptoms to hypotensive shock. Mycotic aneurysms carry a very high risk of aneurysmal rupture with haemorrhage, ongoing source of sepsis, embolic infarction. Survival from a ruptured aortic aneurysm is also a rare event in itself but ruptured iliac aneurysms are even less common with a significantly worse prognosis [1]. Early diagnosis requires a high degree of clinical alertness. A CT scan of the abdomen and pelvis with intra-venous contrast positively diagnosed it. Blood cultures as well as cultures of aneurysmal plaque can demonstrate the infected organism. Treatment consists of antibiotic therapy combined with aggressive surgical debridement of the infected tissue and vascular reconstruction, as needed. Endovascular therapies may have a role in the treatment of ruptured infected aneurysm and the treatment of patients at prohibitive risk for open surgery.
Case Report
A 38yr old known smoker and hypertensive female patient was referred from the OBGYN service of San Fernando teaching hospital to Vascular surgery after a non- contrast CT scan (Cr was 3.5) of the abdomen and pelvis findings of a possible ruptured aortic aneurysm near the aortic bifurcation into the iliac arteries (Figure 1). History revealed that the patient was admitted with one day history of progressively worsening lower abdominal pain with no associated nausea, vomiting, fever or bowel symptoms. The patient also claimed that one (1) month ago, she was admitted to internal medicine with fever, abdominal pain and diarrhoea and weight loss. She was treated for gastroenteritis and was found to be hypothyroid during that admission. The patient was also a known case of fibroid uterus and followed up in gynaecology outpatient clinic. Her urine pregnancy test was negative and urinalysis did not revealed any sign of urinary tract infection.
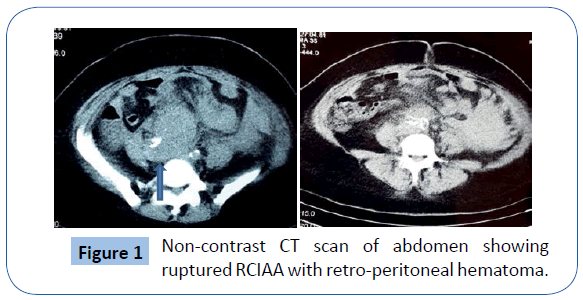
Figure 1: Non-contrast CT scan of abdomen showing ruptured RCIAA with retro-peritoneal hematoma.
On examination she was , hypotensive and her mucous membranes were pale. Abdominal examination revealed that she had an acute abdomen but an aneurysm could not be palpated. A Subsequent duplex scan of the abdomen and pelvis by the consultant radiologist confirm the presence of retro-peritoneal ruptured aortic aneurysm (Figures 2 and 3). After resuscitation, she was taken to theatre for an exploratory laparotomy and was found to have inflamed large bowel with the sigmoid colon looking like an inflammatory mass which had eroded into a major vessel. Careful dissection yielded the right common iliac artery remnants of what was an aneurysm with the bowel wall intimately attached to it. Further dissection revealed that the rupture was just beyond the aortic bifurcation on the right side at the very proximal common iliac artery and distal end was just proximal to the bifurcation of right common iliac artery into the external and internal iliac arteries. Both proximal and distal control was obtained and the area was thoroughly debrided (Figure 4). The area was then lavaged with normal saline; an in-situ bypass from the aorta to the right common femoral artery was performed using a size 18 woven gel-impregnated Dacron graft. The proximal anastomosis (graft to aorta/end to-side) was performed using 2/0 polypropylene sutures (Figure 5). The graft was passed through the pelvis below the inguinal ligament and anastomosed with common femoral artery with 4/0 poly propylene sutures in endto- side fashion (Figure 6). The graft was covered with free edge of mesentery and abdomen closed over a suction drain. Specimens were sent for histopathology as well for culture and sensitivity.
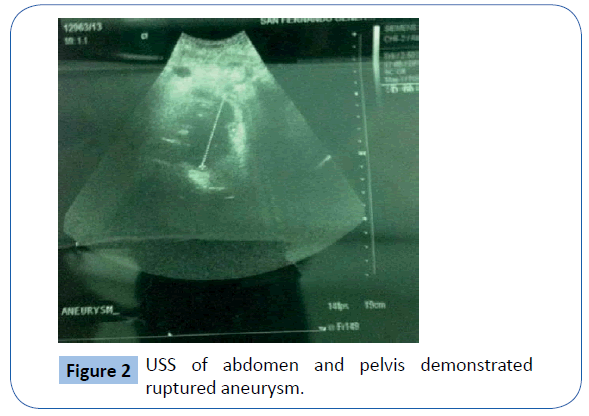
Figure 2: USS of abdomen and pelvis demonstrated ruptured aneurysm.
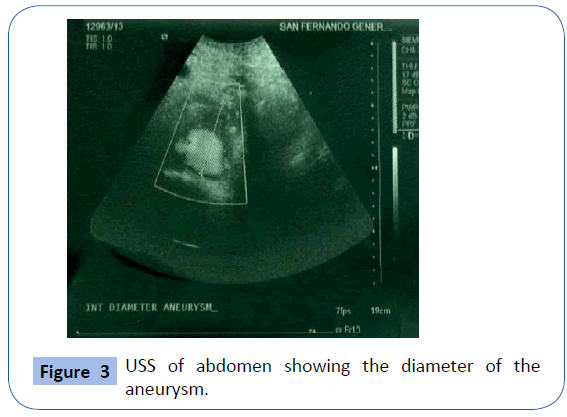
Figure 3: USS of abdomen showing the diameter of the aneurysm.
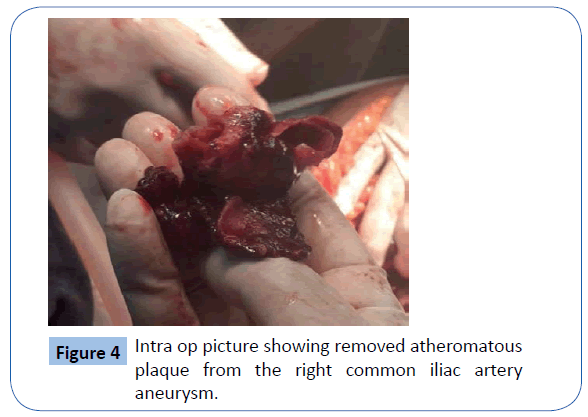
Figure 4: Intra op picture showing removed atheromatous plaque from the right common iliac artery aneurysm.
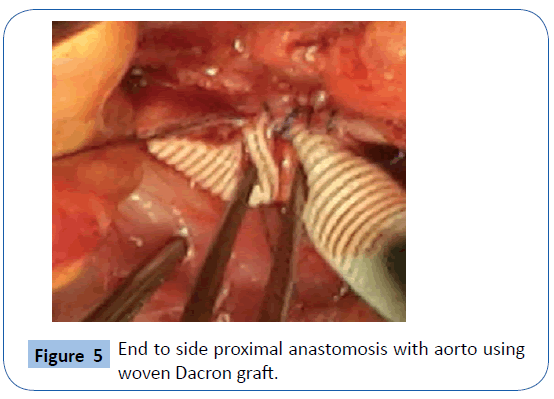
Figure 5: End to side proximal anastomosis with aorto using woven Dacron graft.
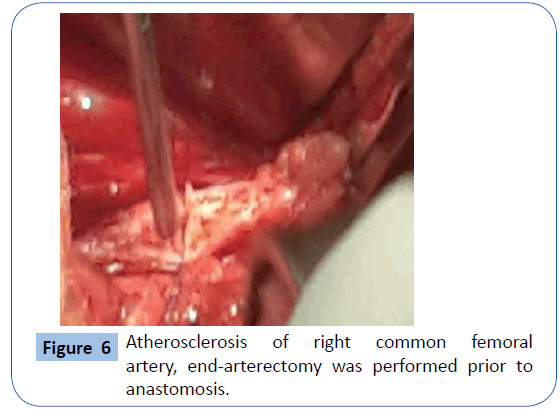
Figure 6: Atherosclerosis of right common femoral artery, end-arterectomy was performed prior to anastomosis.
After operation the patient was admitted into the Intensive Care Unit and then to the surgical ward for post-operative monitoring. Her postoperative recovery was otherwise uneventful except a small fluid collection over the groin wound which was drained under ultrasound guidance. The fluid was also send for culture and sensitivity and was negative for bacteria. The culture from her aneurysm surgery yielded Salmonella group D, non-typhi species. Blood cultures were negative as were subsequent stool cultures.
After discussion with the hospital microbiologist she was placed on an intravenous third generation cephalosporin (Ceftazidime) then switched to Ciprofloxacin. Due to presence of a synthetic graft in an infected aorto-iliac bed, Rifampicin was added to the antibiotic regimen. She was discharged home on the 14th postoperative day and was followed up in the vascular surgery outpatient clinic. A repeat CT scan of abdomen and pelvis with intravenous contrast after one year revealed good patency the aorto-femoral bypass with no evidence of any recurrent aneurysm or pseudo-aneurysm (Figure 7).
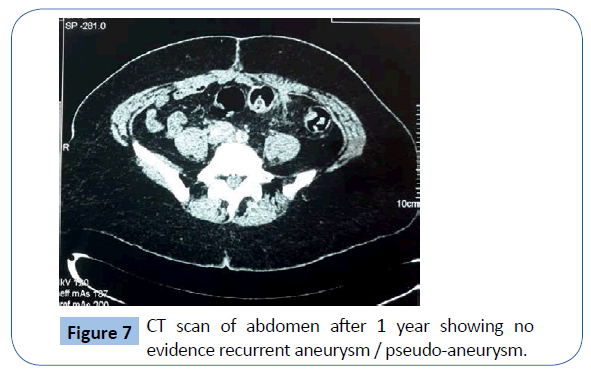
Figure 7: CT scan of abdomen after 1 year showing no evidence recurrent aneurysm / pseudo-aneurysm.
Discussion
The term "mycotic" (meaning fungus) was coined by Osler who was describing the gross pathological appearance of two small saccular aortic aneurysms, not the underlying pathological organism [3].
Mycotic aneurysms frequently found in atypical locations. The most common sites are thoracic and abdominal aorta, abdominal visceral arteries, lower extremity arteries, intracranial arteries (typically more peripheral arteries than berry aneurysms).
The reported incidence of endovascular infections in patients with non-typhoid Salmonella bacteraemia ranges from 9 to 10% in western countries [4,5] to 16.2% in a recent Taiwanese series [6].
Advanced age and diabetes mellitus, both of which were likely to contribute to significant atherosclerosis, as evidenced by the occurrence of ischaemic stroke, were risk factors predisposing our patient for Salmonella endovascular infection [4,5].
Risk factors are-bacteraemia, infective endocarditis, intravenous drug use, immunosuppression, iatrogenic aortic trauma, preexisting atherosclerotic plaque or native aneurysm, prosthetic arterial devices (stents, grafts). Patients with RA receiving immunosuppressive agents as well as immunocompromised (HIV infection) patients may develop fulminating bacteraemia, which leads to the formation of infected aneurysms [7-9].
The mechanisms of infection are septicaemia, septic emboli or contiguous spread from adjacent infection. Mycotic aneurysms arising from infection of the arterial wall are usually bacterial. It is a complication of the haematogenous spread of bacterial infection, classically from the heart. The vessel wall becomes infected with bacteria, is digested and a false aneurysm forms, which is unstable and highly prone to rupture [10].
The commonest organisms are Staphylococcus aureus and Salmonella species, Streptococcus pneumoniae, and Mycobacterium tuberculosis [11].
Salmonella infection of the abdominal aorta with mycotic aneurysm formation is rare and associated with high morbidity and mortality. Although rare, Salmonella is still the most common cause of (primary) mycotic aneurysms [11-13]. Nontyphoid Salmonella are food-borne pathogens that commonly cause a mild, self-limiting gastroenteritis but does have a predilection for damaged blood vessels, especially those injured by atherosclerosis. This predilection for invading damaged endothelium in the heart and arterial walls1 leads to a spectrum of cardiovascular infections including mediastinitis, pericarditis, endocarditis, aortitis, mycotic aneurysms, and infection of cardiac devices [14].
Transient bacteraemia leading to haematogenous infection of atherosclerotic vessels is also considered to be the next most common cause of mycotic aneurysms [15]. Previously, septic emboli from bacterial endocarditis was the major source but gram-negative sepsis in elderly patients, usually from the gastrointestinal tract, start the infection in atherosclerotic aneurysms [16].
Atherosclerosis is recognised as a major risk factor but mycotic aneurysms may arise from primary aortitis [17] induced by septic emboli or be related to a secondary adjacent infection such as a psoas abscess, pancreatitis, bowel infection(index patient) [18] or even pyogenic osteomyelitis of a lumbar vertebra spreading to the aorta, with subsequent rupture [19]. Rupture can be into the retro-peritoneum or into the adjacent structures such as the duodenum, inferior vena cava or the renal vein [20]. Indeed there are cases of the abdominal aorta with infective aortitis which have ruptured despite being non-aneurysmal [21] in nature with CT scanning now being used to diagnose even impending rupture of non-aneurysmal bacterial aortitis [22].
The disease process is regarded as a spectrum from one of early infectious aortitis to mycotic aneurysms. One classification divided them into (1) a post-embolic (mycotic) aneurysm group (2) an infective aortitis group and (3) an infected atherosclerotic aneurysm group [23].
Patients with end-stage renal failure undergoing hemodialysis who tend to show advanced atherosclerosis, can also present with mycotic aneurysms of the abdominal aorta [24] and rupture of abdominal aortic mycotic aneurysms has been reported [25] developing as a result of Staphylococcal infection in a patient receiving haemodialysis treatment.
In our patient the risk factors for aneurysm formation were uncontrolled hypertension, cigarette smoking, hyperlipidaemia and borderline renal dysfunction.
The presentation usually includes the presence of fever, lower abdominal or back pain, leucocytosis, a painful or tender pulsatile abdominal mass and a positive blood or tissue culture of Salmonella. Mycotic aneurysms of a Salmonella etiology are also known to also present with rapid expansion or rupture [11]. Accurate clinical findings lead to the correct preoperative diagnosis in 11 out of 13 cases (85%) in one study [26] and three out of four patients in another [16] series.
Abdominal ultrasonography, aortography and CT scans are the basic imaging methods used to diagnose this condition. CT was found to be the most sensitive tool [27] in the diagnosis of early-stage disease with findings that have been closely studied for the incidence of infective aortitis, mycotic aneurysms and subsequent evolution into a rupture [28]. CT features of mycotic aneurysms of the aorta include (a) hazy aortic wall with rupture, (b) gas-forming inflammation, (c) retroperitoneal para-aortic fluid collection (and vertebral erosion) and (d) thrombus formation within a false lumen after aneurysmal rupture [29]. CT-Scanning and aortography are considered to be the best combined examinations for the diagnosis of abdominal aortic mycotic aneurysms [30].
Once the diagnosis is made, treatment is by way of surgery which involves wide debridement of the infected area and either immediate or staged revascularisation [31,32] via an in situ graft, an extra-anatomic bypass such as an axillary-femoral or a femoral to femoral bypass [33] or even use of a natural conduit such as the superficial femoral vein [34]. This has given rise to some controversy over the merits of each technique, since passing a synthetic graft through an infected or potentially infected area may lead to an infected graft with serious consequences. Lee postulated [24] that in afebrile patients or those responding well to antibiotic therapy they should have an In-situ Bypass (ISB) done since the probability of an infection is low, once adequate antibiotic cover and rigid follow-up are instituted.
Extra-Anatomic Bypass Grafting (EABG) is an alternative technique if the bed of the aneurysm is heavily contaminated or purulent. Most authors agree that wide debridement of the infected aneurysm is essential and both ISB and EABG appear to have similar results [35] although some authors report higher infection rates in ISB [23] and others poor postoperative results with EABG [36] but either procedure is recognised as being surgically acceptable [37].
In order to decrease the risk of infection postoperatively, one team used an omental flap to cover the prosthetic graft [21] based on an experimental procedure done as far back as 1968 [38]. A review of the literature suggested that EABG should be the procedure of choice if there is diffuse widespread retroperitoneal sepsis with frank pus. In any case the bypass must be performed first to avoid prolonged limb and colonic ischemia and even then no guarantee can be made since aortic “stump blowout” can occur due to either persisting or recurrent infection [23].
Another option also available in this era of minimally invasive procedures involves endovascular aortic repair (EVAR) as a rapid short-term solution being used as a “bridge” to delayed open surgery [39]. However, EVAR remains a good alternative despite the theoretical risks of infection and the 24-month survival and aneurysm-related event-free rates were the same as open or conventional surgery, although this group had a higher late mortality rate [40].
Some predictors for mortality and morbidity related to mycotic aneurysms in this series were Salmonella species, leucocytosis, shock and aorto-enteric fistula [40] but not the EVAR or open surgery. Other studies listed the location, rupture, the presence of established infection and virulence of organism [26] as predictors. The incidence of aorto-duodenal fistulas in such aneurysms has been well established and is likely due to the continued presence of Group D Salmonella in the aortic walls [41].
Although arterial infection due to Salmonella is now rare, it is still one of the most common causes of primary mycotic aneurysms however, Staphylococcus, Bacteroides, and Pseudomonas have all been implicated in aneurysm formation, rupture and demise [26]. It is generally accepted that a six (6) week course of the bactericidal antibiotic as determined by sensitivity patterns should be adequate but the presence of an active infection, systemic dissemination, or a procedure such as the ISB may warrant a 3 or 6 month course (long-term) or even lifelong treatment [23,29] as in our patient.
Follow up is also recommended with regular CT scans [23] since graft infection is still a possibility [35] and the frequency is warranted by the clinical presentation, surgical procedure done and the status of the patient postoperatively.
Conclusion
Despite prompt diagnosis, good surgical technique and appropriate antibiotic therapy the mortality with gram negative aortic infection is high because of the chance of early rupture in a patient with extensive atherosclerosis and systemic infection due to virulent organisms. The treatment with wide debridement of the infected aorto-iliac tissue followed by in-situ graft bypass or an extra-anatomic bypass can be utilised. In a review of the literature, in situ reconstruction is a viable surgical procedure for Salmonella induced ruptured aortic or aorto-iliac aneurysms [1,42].
Acknowledgement
Informed consent was obtained from patient to publish this case.
7718
References
- Kwon TW, Shin ES, Kim DK, Lee SO, Kim GE (2003) Ruptured abdominal aortic aneurysms due to Salmonella, not of typhi species. Ann VascSurg 17: 464-467.
- De Marchis GM, Braunschweig M, Greuter S (2005) Nontyphoidal salmonellosis and mycotic aneurysm: a case report. Mt Sinai J Med 72: 351-353.
- Osler W (1885) The Gulstonian Lectures, on Malignant Endocarditis. Br Med J 1: 467-470.
- Nielsen H, Gradel KO, Schønheyder HC (2006) High incidence of intravascular focus in nontyphoid Salmonella bacteremia in the age group above 50 years: a population-based study. APMIS 114: 641-645.
- Benenson S, Raveh D, Schlesinger Y, Alberton J, Rudensky B, et al. (2001) The risk of vascular infection in adult patients with nontyphi Salmonella bacteremia. Am J Med 110: 60-63.
- Hsu RB, Lin FY (2005) Risk factors for bacteraemia and endovascular infection due to non-typhoid salmonella: a reappraisal. QJM 98: 821-827.
- Yang DH, Chang WC (2013) Nontyphoid Salmonella-infected mycotic aneurysm in a patient with rheumatoid arthritis. RheumatolInt 33: 3103-3104.
- Ando H, Minami R, Takahama S, Yamamoto M (2010) An infected abdominal aortic aneurysm due to non-typhoidal Salmonella in an HIV-1-infected Japanese patient. Intern Med 49: 1237-1241.
- Fielder J, Miriti K, Bird P (2010) Mycotic aneurysm of the inferior gluteal artery caused by non-typhi Salmonella in a man infected with HIV: a case report. J Med Case Rep 4: 273.
- Fernández Guerrero ML, Aguado JM, Arribas A, Lumbreras C, de Gorgolas M (2004) The spectrum of cardiovascular infections due to Salmonella enterica: a review of clinical features and factors determining outcome. Medicine (Baltimore) 83: 123-138.
- Flamand F, Harris KA, DeRose G, Karam B, Jamieson WG (1992) Arteritis due to Salmonella with aneurysm formation: two cases. Can J Surg 35: 248-252.
- Ewart JM, Burke ML, Bunt TJ (1983) Spontaneous abdominal aortic infections. Essentials of diagnosis and management. Am Surg 49: 37-50.
- MacLellan DG, Sandland AM, Spelman DW, Buxton B, Hardy KJ (1983) Ruptured abdominal aortic aneurysm: a complication of Salmonella bovis-morbificans infection. Aust N Z J Surg 53: 371-373.
- Salzberger LA, Cavuoti D, Barnard J (2002) Fatal salmonella aortitis with mycotic aneurysm rupture. Am J Forensic Med Pathol 23: 382-385.
- Oz MC, McNicholas KW, Serra AJ, Spagna PM, Lemole GM (1989) Review of Salmonella mycotic aneurysms of the thoracic aorta. J CardiovascSurg (Torino) 30: 99-103.
- McNamara MF, Roberts AB, Bakshi KR (1987) Gram-negative bacterial infection of aortic aneurysms. J Cardiovasc Surg (Torino) 28: 453-455.
- Ljungberg B, Braconier JH (1986) Abdominalaortitis and infected aneurysms due to salmonella. Scand J Infect Dis 18: 401-406.
- Chen IM, Chang HH, Hsu CP, Lai ST, Shih CC (2005) Ten-year experience with surgical repair of mycotic aortic aneurysms. J Chin Med Assoc 68: 265-271.
- Knipping L, Mangold G (1995) [Bacterial infections of the abdominal aorta]. Chirurg 66: 887-889.
- Sessa C, Farah I, Voirin L, Magne JL, Brion JP, et al. (1997) Infected aneurysms of the infrarenal abdominal aorta: diagnostic criteria and therapeutic strategy. Ann VascSurg 11: 453-463.
- Stephens CT, Pounds LL, Killewich LA (2006) Rupture of a nonaneurysmal aorta secondary to Staphylococcus aortitis. Angiology 57: 506-512.
- Mantello MT, Panaccione JL, Moriarty PE, Esposito WJ (1990) Impending rupture of nonaneurysmal bacterial aortitis: CT diagnosis. J Comput Assist Tomogr 14: 950-953.
- Fichelle JM, Tabet G, Cormier P, Farkas JC, Laurian C, et al. (1993) Infected infrarenal aortic aneurysms: when is in situ reconstruction safe? J Vasc Surg 17: 635-645.
- Lee CC, Ng YY, Chou YH, Tiu CM, Wang Z, et al. (2000) Mycotic aneurysm of the abdominal aorta in a patient undergoing hemodialysis: an unusual complication of Staphylococcus aureus bacteremia. Clin Infect Dis 30: 823-824.
- Diskin CJ, Stokes TJ, Dansby LM, LazenbyA (2004) Observe and be guarded: the development and rupture of an abdominal aortic mycotic aneurysm in an afebrile hemodialysis patient with normal angiogram, and CT scan, and sterile blood cultures. Clin Exp Nephrology 8: 388-391.
- Reddy DJ, Shepard AD, Evans JR, Wright DJ, Smith RF, et al. (1991) Management of infected aortoiliac aneurysms. Arch Surg 126: 873-878.
- Gomes MN, Choyke PL, Wallace RB (1992) Infected aortic aneurysms. A changing entity. Ann Surg 215: 435-442.
- Carreras M, Larena JA, Tabernero G, Langara E, Pena JM (1997) Evolution of salmonella aortitis towards the formation of abdominal aneurysm. Eur Radiol 7: 54-56.
- Lee MH, Chan P, Chiou HJ, Cheung WK (1996) Diagnostic imaging of Salmonella-related mycotic aneurysm of aorta by CT. Clin Imaging 20: 26-30.
- de Aguiar ET, Lobato Ade C, de Toledo JV, Campos Júnior W, Langer B (1992) [Abdominal aortic mycotic aneurysm due to Salmonella: case report and review of literature]. Rev HospClinFac Med Sao Paulo 47: 153-157.
- Dubois M, Daenens K, Houthoofd S, Peetermans WE, Fourneau I (2010) Treatment of mycotic aneurysms with involvement of the abdominal aorta: single-centre experience in 44 consecutive cases. Eur J Vasc Endovasc Surg 40: 450-456.
- Humphrey RW, Vercoutere AL, Abu-Dalu J (1983) Ruptured mycotic salmonella aortic aneurysm treated with combined cefotaxime antibiotic and staged surgical management. Angiology 34: 674-678.
- Müller BT, Wegener OR, Grabitz K, Pillny M, Thomas L, et al. (2001) Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg 33: 106-113.
- Aerts PD, van Zitteren M, Kotsopoulos AM, van Berge Henegouwen DP, Vriens PW, et al. (2012) Infected abdominal aneurysm due to Salmonella sepsis: report of a unique case treated using the superficial femoral vein. Ann Vasc Surg 26: 279.
- Lee CH, Hsieh HC, Ko PJ (2011) In situ versus extra-anatomical reconstruction for primary infected infra-renal abdominal aortic aneurysms. J Vasc Surg 54: 64-70.
- Trairatvorakul P, Sriphojanart S, Sathapatayavongs B (1990) Abdominal aortic aneurysms infected with salmonella: problems of treatment. J Vasc Surg 12: 16-19.
- Belz J, Gattermann M, Schroder HJ (1989) Extra-anatomic bypass as therapy of infected bacterial (mycotic) infrarenal aortic aneurysm. A comparative, literature supported analysis. Chirurg 60: 479-488.
- Goldsmith HS, De Los Santos R, Vanamee P (1968) Experimental protection of vascular prosthesis by omentum. Arch Surgery 97: 572-574.
- Fukunaga N, Hashimoto T, Ozu Y, Yuzaki M, Shomura Y, et al. (2012) Successful treatment for infected aortic aneurysm using endovascular aneurysm repairs as a bridge to delayed open surgery. Ann Vasc Surg 26: 280.
- Kan CD, Lee HL, Luo CY (2009) The efficacy of aortic stent grafts in the management of mycotic abdominal aortic aneurysm-institute case management with systemic literature comparison. Ann Vasc Surg 24: 433-440.
- Witz M, Lehmann J, Shnaker A, Gotesman BS, Korzets Z (2005) Delayed recurrence of group D salmonella mycotic aneurysm of the abdominal aorta presenting as an aorto-duodenal fistula. Isr Med Assoc J 7: 46-47.
- van Dam van Isselt EF, Moll FL, Bast TJ (1998) Cryptogenic Salmonella-infected ruptured aortic aneurysms. Cardiovasc Surg 6: 347-350.













