Background
Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis, Rural health, Gastroenterology
Introduction
Inflammatory Bowel Disease (IBD) is a common disease in western countries [1] and is often associated with high morbidity and a reduction in health related quality of life [2]. In Australia around 61,000 people were affected in 2005 and with an increasing prevalence [3] it stands to be a major workload burden for the health care system and a global economic burden. Whilst IBD is reported to be more common in some urban areas [4-8], a significant proportion of IBD patients live in rural areas. There is evidence that these patients have to access their care from urban centres, with one such centre reporting that over 40% of their patients on biological therapies live in rural locations [9].
Because of the complexity of IBD, along with the high morbidity, chronicity and the potential for complications, close and continued collaboration between patients and specialist medical care and other holistic, multi-disciplinary facilities is vital [3]. Challenges in obtaining optimal care for patients with IBD are encountered on a day-to-day basis in tertiary centres [10]; however, additional distance-related obstacles exist in the rural setting [11], which have the potential to influence disease outcomes.
Despite multiple studies documenting differences in health outcomes and survival rates between rural and urban patients in a number of diseases, minimal published data exists regarding the relative level of disease burden in rural patients with IBD compared to their urban counterparts. There is little known with respect to rural healthcare professionals’ knowledge of and exposure to IBD. Patient and health practitioner perceptions of barriers to optimal care in the rural setting have also not been previously described.
This study therefore aimed to firstly describe the disease burden and treatment experience of IBD in patients living in a rural area as compared to metropolitan locations. We then aimed to identify the level of IBD exposure among rural practitioners and their attitudes to and knowledge of IBD care and to identify any perceived barriers to the care and management of patients in the rural setting compared with their urban counterparts.
Methods
Overview: This cross-sectional study was conducted in three parts:
• Firstly, disease outcomes were compared between IBD patients living in rural as compared to metropolitan locations within an existing IBD database at a metropolitan teaching hospital. To determine whether any possible difference in outcomes might be a reflection of referral bias or real, these data were subsequently compared to a second rural IBD cohort gathered from a clinical database at a large rural General Practice (GP) site.
• Secondly, the rural IBD patient cohort (identified via the rural GP practice) was surveyed regarding patient perspectives on the potential barriers to optimal care of rural IBD patients.
• Finally, rural healthcare practitioners (HCPs) who care for rural IBD patients and IBD nurses were identified and surveyed regarding their knowledge of and attitude to rural IBD care and their perspectives on the potential barriers to optimal care of rural IBD patients.
IBD Outcomes
An existing Southern Adelaide IBD database held at Flinders Medical Centre (FMC) (a 580 bed public teaching hospital servicing the southern population of Adelaide and major regional rural centres) was reviewed and two cohorts (rural and metropolitan) were obtained. Cohorts were matched for diagnosis, age and gender. Patients on this database had previously consented to participate in IBD research. Disease characteristics and disease outcomes were extracted from a previously completed survey, titled ‘Inflammatory Bowel Disease Survey’ (Appendix 1). Questions related to disease severity, medication use, investigations, surgical interventions and complications.
The second rural IBD GP based cohort was collected from Mount Gambier, South Australia (SA). This location was chosen as it does not have a resident gastroenterologist and is located a significant distance (440 km) from the nearest Adelaide tertiary hospital. One large GP practice was contacted and agreed to participate in patient recruitment. Following interrogation of their database, patients with a diagnosis of IBD were identified and invited to participate by completing the same questionnaire as the existing Southern Adelaide patients. Questionnaires were posted accompanied by a letter of invitation, information sheet, consent form and opt-out form. A reminder letter was sent at 2 months except for “return to senders” and opts-out candidates. All completed surveys were de-identified.
Patient perspectives
The IBD cohort from Mount Gambier was asked to complete a second survey titled ‘Perceived barriers to IBD care in the rural setting’. These questions related to their perception(s) of current barriers the optimal care of rural IBD patients (Appendix 2). This survey was identical to Section C of the Rural Practitioner survey (referred to below).
Rural practitioner IBD exposure and perspectives
An invitation to participate via completion of a questionnaire was sent out to 1,130 Australian rural surgeons and physicians, SA rural GPs and metropolitan Australian IBD nurses. Rural was defined as practicing in an area with a Rural, Remote and Metropolitan Areas (RRMA) classification [12] of 3 or above. Consequently capital cities (RRMA 1) and other metropolitan centres with urban population of >100,000 (RRMA 2) were not included. Australian IBD nurses who are metropolitan based but often involved in assisting in the management of rural patients9, were identified through their contact list and invited to participate via email. Australian rural surgeons were identified using a list of attendees at the Annual Rural Provincial Surgeons meeting over the last 5 years and contacted via email. Australian rural physicians and SA rural GPs were identified through a Pharmaceutical company’s commercial database Janssen database and were contacted via mail. For all HCPs, if no reply was received, reminder letters were sent after 2 weeks and 2 months. Questionnaires were completed and returned via mail or online.
The questionnaire (Appendix 3) was divided into three sections. Section A sought information regarding IBD exposure and service provision, interest in IBD and attitudes towards current IBD speciality services. Section B examined the level of IBD knowledge (via previously validated questionnaire [13]. Section C investigated perceived barriers to optimal care of rural IBD patients. Overall level of rural health care and barriers experienced compared with metropolitan care formed the basis of questions with the final questions relating to suggested solutions. Return of a completed questionnaire was taken as consent.
Data analysis
IBD knowledge and attitudes were compared and assessed using appropriate statistics (Mann-Whitney). The reported perceived barriers to care by rural patients and health care providers were reviewed for common themes within each group.
Ethics approval
The study was approved by the Southern Adelaide Clinical Human Research Ethics Committee.
Results
IBD outcomes
A review of the FMC IBD database identified 53 rural IBD patients and sixty six metropolitan patients in the comparison cohort. No statistically significant differences were found in disease characteristics or outcomes between cohorts (Table 1).
| |
IBD data base: Rural Cohort |
IBD data base: Metropolitan Cohort |
P value |
Type
Crohn’s Disease
Ulcerative Colitis
Indeterminate colitis |
34/53 (64%)
19/53 (34%)
0/53(0%) |
39/65 (60%)
26/65 (40%)
0/65(0%) |
0.705
0.705
1.000 |
| Symptom duration before diagnosis (median) (months) |
9 |
12 |
0.684 |
Extra-intestinal manifestations
Episcleritis
Erythema nodosum
Spondyloarthropathy
Primary sclerosisng cholangitis |
5/53 (9%)
2/53 (4%)
1/53 (2%)
4/53 (8%) |
8/66 (12%)
6/66 (9%)
6/66 (9%)
5/66 (8%) |
0.771
0.297
0.130
1.000 |
Other extra-intestinal manifestations
Addison’s disease
Idiopathic thrombocytopenic purpura
Polymyalgia rheumatica
Alopecia/vitiligo
Autoimmune Haemolyticanaemia |
2/52 (4%)
1/52 (2%)
2/52 (4%)
2/52 (4%)
2/52 (4%) |
1/66 (2%)
2/66 (3%)
3/66 (5%)
4/66 (6%)
3/66 (5%) |
0.582
1.000
1.000
0.693
1.000 |
Weight loss prior to diagnosis
Yes
No
Unsure |
29/53 (55%)
14/53 (26%)
10/53 (19%) |
31/66 (47%)
21/66 (32%)
14/66 (21%) |
0.462
0.550
0.821 |
Height increase prior to diagnosis
Yes
No
Unsure
N/A |
3/53(6%)
11/53 (21%)
3/53(6%)
36/53 (68%) |
5/66(8%)
11/66 (17%)
1/66(2%)
49/66 (74%) |
0.731
0.638
0.322
0.541 |
Diagnosis
Investigations
Colonoscopy
Flexible sigmoidoscopy
Endoscopy
CT abdomen
Small bowel x-ay
MRI
Practitioner who diagnosed IBD
Gastroenterologist
GP
Surgeon
Other |
46/53 (87%)
13/53 (25%)
21/53 (40%)
17/53 (32%)
18/53 (34%)
6/53 (11%) 42/53 (79%)
5/53 (9%)
5/53 (9%)
1/53 (2%) |
59/66 (89%)
21/66 (32%)
25/66 (42%)
37/66 (56%)
26/66 (39%)
19/66 (29%) 54/66 (82%)
4/66(7%)
3/66(5%)
4/66(7%) |
0.777
0.4198
0.852
0.447
0.026
0.024 0.817
0.509
0.464
0.380 |
Previous surgery
Ileal resection
Duodenal/Jejunal resection
Colectomy
Ileostomy or colostomy
Pouch procedure
Stricturoplasty
Drainage of abscess
Fistulae repair |
12/53 (23%)
2/53 (4%)
12/52 (23%)
11/53 (21%)
2/53 (4%)
1/53 (2%)
9/53 (17%)
13/53 (25%) |
13/66 (20%)
4/66(6%)
18/66 (27%)
19/66 (29%)
5/66 (8%)
6/66 (9%)
12/66 (18%)
12/66 (18%) |
0.821
0.691
0.672
0.397
0.459
0.044
1.000
0.498 |
Medication Use (current or past)
5 amino-salicylic acid
Immunomodulator
Azathioprine
6MP
Methotrexate
Other
Hydrocortisone
Cyclosporin
Infliximab
Adalimumab |
47/53 (75%) 28/53 (73%)
8/49 (16%)
9/50 (18%) 29/53 (55%)
3/53 (6%)
17/53 (32%)
12/53 (19%) |
57/65 (88%) 45/63 (71%)
7/54(13%)
18/58 (31%) 35/66 (53%)
8/66 (12%)
24/66 (36%)
12/66 (17%) |
1.000 0.668
0.781
0.181 1.000
0.342
0.670
0.647 |
| Number of steroid courses |
3 |
2 |
0.982 |
History of Iron deficiency
Oral replacement
Iron infusion |
35/53 (66%)
35/35 (100%)
17/35 (49%) |
43/66 (65%)
42/43 (98%)
25/43 (58%) |
1.000
1.000
0.495 |
Bone densitometry performed
Normal
Mild osteoporosis
Moderate-severe osteoporosis
Unsure |
23/52 (44%)
17/23 (74%)
5/23 (22%)
1/23 (4%)
2/23 (9%) |
37/66 (56%)
14/37 (39%)
8/37 (22%)
7/37 (19%)
8/37 (22%) |
0.026
0.009
1.000
0.138
0.291 |
| Number of hospitalisations (median) |
2 |
2 |
0.777 |
| Member of IBD support group |
10/53 (19%) |
16/66 (24%) |
0.512 |
| Family History of IBD |
20/52 (38%) |
17/66 (26%) |
0.012 |
Table 1: Disease characteristics and outcomes of the FMC data base rural IBD cohort and metropolitan IBD comparison cohort.
Interrogation of the Mount Gambier GP practice data base found 114 patients with a diagnosis of IBD. There were 17 optouts and 4 return-to-senders. Thirty three people returned the initial disease outcomes survey (response rate 30%) with results outlined in (Table 2).
| |
Rural prospective Cohort |
IBD data base: Metropolitan Cohort |
P value |
Type
Crohn’s Disease
Ulcerative Colitis
Indeterminate colitis |
18/33 (55%)
13/33 (39%)
2/33(6%) |
39/65 (60%)
26/65 (40%)
0/65(0%) |
1.000
1.000
1.000 |
| Symptom duration before diagnosis (median) (months) |
6 |
12 |
0.684 |
Extra-intestinal manifestations
Episcleritis
Erythema nodosum
Spondyloarthropathy
Primary sclerosisng cholangitis |
2/33 (6%)
2/33 (6%)
3/33 (9%)
0/33 (0%) |
8/66 (12%)
6/66 (9%)
6/66 (9%)
5/66 (8%) |
0.489
0.715
1.000
0.166 |
Other extra-intestinal manifestations
Addison’s disease
Idiopathic thrombocytopenic purpura
Polymyalgia rheumatica
Alopecia/vitiligo
Autoimmune Haemolyticanaemia |
1/33 (3%)
1/33 (3%)
2/33 (6%)
2/33 (6%)
0/33 (0%) |
1/66 (2%)
2/66 (3%)
3/66 (5%)
4/66 (6%)
3/66 (5%) |
1.000
1.000
1.000
1.000
0.549 |
Weight loss prior to diagnosis
Yes
No
Unsure |
18/33 (55%)
11/33 (33%)
4/33 (12%) |
31/66 (47%)
21/66 (32%)
14/66 (21%) |
1.000
1.000
0.408 |
Height increase prior to diagnosis
Yes
No
Unsure
N/A |
0/33 (0%)
8/33 (24%)
2/33 (6%)
23/33(70%) |
5/66(8%)
11/66 (17%)
1/66(2%)
49/66 (74%) |
0.166
0.421
0.257
0.633 |
Diagnosis
Investigations
Colonoscopy
Flexible sigmoidoscopy
Endoscopy
CT abdomen
Small bowel x-ay
MRI
Practitioner who diagnosed IBD
Gastroenterologist
GP
Surgeon
Other |
23/33 (70%)
8/33(24%)
10/33 (30%)
10/33 (30%)
3/33(9%)
1/33(3%) 12/33 (36%)
8/33(24%)
12/33 (36%)
1/33(3%) |
59/66 (89%)
21/66 (32%)
25/66 (42%)
37/66 (56%)
26/66 (39%)
19/66 (29%) 54/66 (82%)
4/66(7%)
3/66(5%)
4/66(7%) |
0.023
0.490
0.510
0.019
0.002
0.003 0.0001
0.018
0.0001
0.662 |
Previous surgery
Ileal resection
Duodenal/Jejunal resection
Colectomy
Ileostomy or colostomy
Pouch procedure
Stricturoplasty
Drainage of abscess
Fistulae repair |
7/33 (21%)
3/33 (9%)
5/33 (15%)
6/33 (18%)
3/33 (9%)
1/33 (3%)
7/33 (21%)
10/33 (30%) |
13/66 (20%)
4/66(6%)
18/66 (27%)
19/66 (29%)
5/66 (8%)
6/66 (9%)
12/66 (18%)
12/66 (18%) |
1.000
0.683
0.214
0.329
1.000
0.419
0.789
0.204 |
Medication Use (current or past)
5 amino-salicylic acid
Immunomodulator
Azathioprine
6MP
Methotrexate
Other
Hydrocortisone
Cyclosporin
Infliximab
Adalimumab |
30/33 (91%) 17/33 (52%)
2/33 (6%)
1/33 (3%) 6/33 (18%)
0/33 (0%)
5/33 (15%)
2/33 (6%) |
57/65 (88%) 45/63 (71%)
7/54(13%)
18/58 (31%) 35/66 (53%)
8/66 (12%)
24/66 (36%)
12/66 (17%) |
0.746 0.070
0.473
0.001 0.001
0.049
0.036
0.133 |
| Number of steroid courses |
3 |
2 |
0.982 |
History of Iron deficiency
Oral replacement
Iron infusion |
21/33 (64%)
15/21 (71%)
5/21 (24%) |
43/66 (65%)
42/43 (98%)
25/43 (58%) |
1.000
0.004
0.016 |
Bone densitometry performed
Normal
Mild osteoporosis
Moderate-severe osteoporosis
Unsure |
17/33 (52%)
6/17 (35%)
6/17 (35%)
3/17 (18%)
0/17 (0%) |
37/66 (56%)
14/37 (39%)
8/37 (22%)
7/37 (19%)
8/37 (22%) |
0.680
1.000
0.328
1.000
0.046 |
| Number of hospitalisations (median) |
2 |
2 |
0.936 |
| Member of IBD support group |
7/31 (23%) |
16/66 (24%) |
1.000 |
| Family History of IBD |
9/33 (27%) |
17/66 (26%) |
1.000 |
Table 2: Disease characteristics and outcomes of the rural IBD Mount Gambier cohort and FMC data base metropolitan comparison cohort..
No statistically significant differences were found in disease type, presence and duration of symptoms, extra-intestinal manifestations of IBD, number of hospitalizations or previous surgery between the Mount Gambier rural cohort and metropolitan FMC cohort. The majority of both cohorts had a colonoscopy as part of their diagnostic work up but a statistically significant larger proportion of metropolitan patients had a small bowel x-ray (26/66 vs 3/33 p=0.002) and MRI (19/66 vs 1/33 p=0.003). There was no statistically significant difference in investigation usage throughout the course of their disease. The majority of metropolitan patients (54/66, 82%) were diagnosed by a gastroenterologist, which was significantly higher than the Mount Gambier rural cohort (12/33, 36%). Statistically significant differences were found in medication use in methotrexate (Mount Gambier rural cohort 1/33 3% vs metropolitan FMC cohort 18/66 31% p=0.001) and hydrocortisone (Mount Gambier rural cohort 6/33 (18%) vs metropolitan FMC cohort 35/66 (53%) p=0.001). Furthermore, a greater usage of infliximab was noted in the metropolitan cohort (24/66 36% vs Mount Gambier rural cohort 5/33 15%) although this result was not significant (p=0.036). Whilst no difference was found in incidence of iron deficiency, statistically more metropolitan FMC patients had received oral iron replacement (42/43 98% vs 15/21 71% p=0.004) than Mount Gambier rural patients and a trend was noted for a greater rate of intravenous replacement (FMC metropolitan patients 25/43 58% vs Mount Gambier rural patients 5/21 24% p=0.16).
Patient perspectives
Of the 114 patients invited to participate (including 17 opt-outs and 4 return-to-senders) 32 people returned the perceived barrier survey (response rate 29%).
Eighty six percent (24/28) of respondents opined that rural IBD patients have worse quality of health compared with people living in metropolitan areas. Perceived barriers to rural IBD care are shown in Figure 1. Access to specialist care was only thought to be adequate by 6/30 (20%) and complications were thought to have been potentially preventable by 14/27 (52%) if access were improved. Communication between the patient’s gastroenterology specialist and primary care physician was felt to be poor in only 1/28, however 8/24 (33%) felt that the level of communication had negatively influenced their outcome(s) in the past. Access to multidisciplinary team members was reported to be low (specialist colorectal surgeon 9/29, IBD nurse 3/29, dietician 13/29 and psychologist 6/29). Interventions suggested to be most helpful in enhancing access to specialist gastroenterology care are shown in Figure 2.
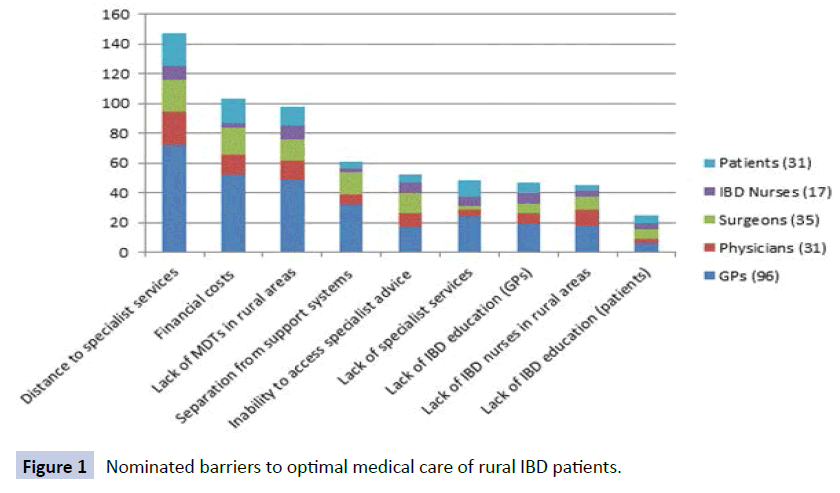
Figure 1: Nominated barriers to optimal Figure 1 medical care of rural IBD patients.
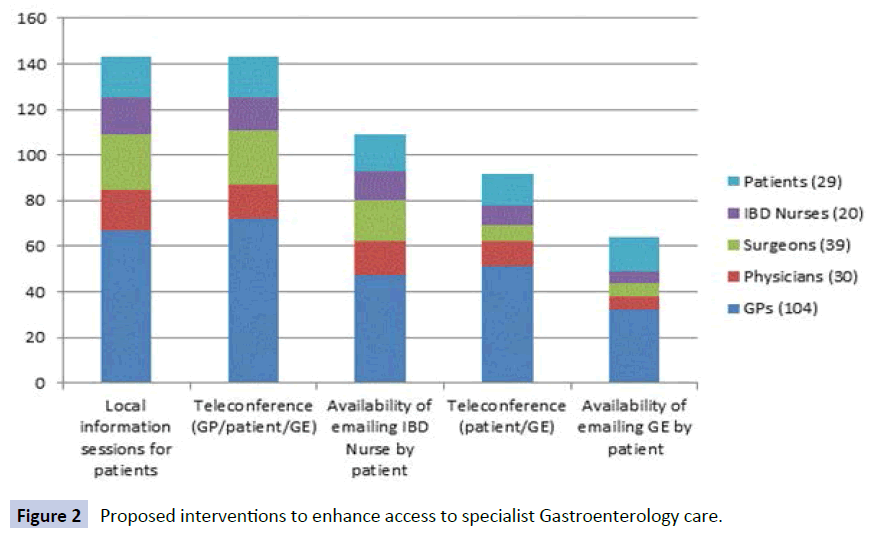
Figure 2: Proposed interventions to enhance access to specialist Gastroenterology care.
Rural HCPs’ IBD exposure and perspectives
A total of 233 completed questionnaires were obtained from the various HCPs surveyed, with not all participants completing all questions (Figure 3). This achieved an overall response rate of 21%. Demographic details of the cohort are outlined in (Table 3).
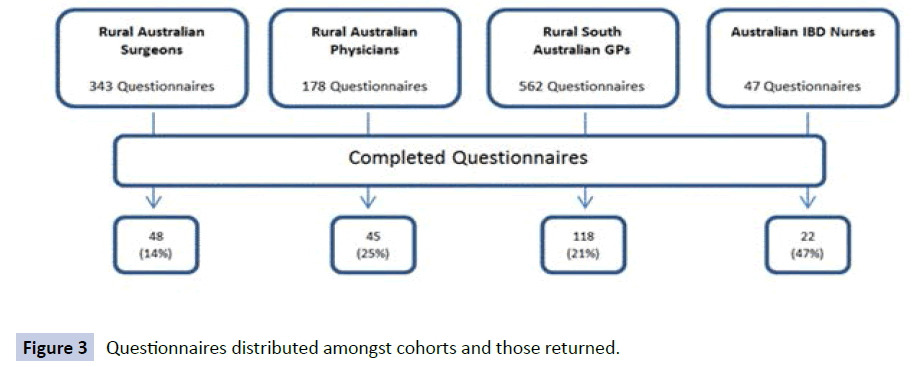
Figure 3: Questionnaires distributed amongst cohorts and those returned.
| |
GPs (n=118) |
Physicians (n=45) |
Surgeons (n=48) |
IBD Nurse (n=22) |
| Gender |
|
|
|
|
| Male |
75/117 (64%) |
36/42 (86%) |
43/47 (91%) |
0/22(0%) |
| Female |
42/117 (36%) |
6/42(14%) |
4/47(9%) |
22/22 (100%) |
| Age |
|
|
|
|
| Years (mean) |
48 |
52 |
53 |
48 |
| Training location |
|
|
|
|
| Australia |
76/101 (75%) |
21/38 (55%) |
28/48 (58%) |
17/22 (77%) |
| Overseas |
23/101 (23%) |
15/38 (39%) |
15/48 (31%) |
4/22(18%) |
| Both |
2/101(2%) |
2/38(5%) |
5/48(10%) |
1/22(5%) |
| Year of graduation |
|
|
|
|
| Year (median) |
1987 |
1982 |
1985 |
1981 |
| Nature of practice |
|
|
|
|
| IBD patients (total) |
|
|
|
|
| <5 |
68/117 (58%) |
14/45 (31%) |
20/45 (44%) |
0/22(0%) |
| 5-10 |
32/117 (27%) |
9/45(20%) |
15/45 (33%) |
1/22(5%) |
| 10-20 |
13/117 (11%) |
5/45(11%) |
7/45(16%) |
0/22(0%) |
| 20-50 |
4/117(3%) |
12/45 (27%) |
3/45(7%) |
0/22(0%) |
| >50 |
0/117(0%) |
5/45(11%) |
0/45(0%) |
21/22 (95%) |
| IBD patients per month |
|
|
|
|
| <1 |
48/117 (41%) |
16/45 (36%) |
22/46 (48%) |
0/21(0%) |
| 1-5 |
65/117 (56%) |
13/45 (29%) |
21/46 (46%) |
1/21(5%) |
| 5-10 |
3/117(2.5%) |
8/45(17.5%) |
2/46(4%) |
1/21(5%) |
| >10 |
1/117(0.5%) |
8/45(17.5%) |
1/46(2%) |
19/21 (90%) |
| Location of care |
|
|
|
|
| Inpatient |
0/115(0%) |
3/40(8.5%) |
3/26(12%) |
0/21(0%) |
| Outpatient |
56/115 (49%) |
5/40(12.5%) |
0/26(0%) |
5/21(24%) |
| Both |
59/115 (51%) |
32/40 (80%) |
23/26 (88%) |
16/21 (76%) |
| Attitudes to care of IBD patients |
|
|
|
|
| Comfortable |
73/106 (69%) |
23/35 (91%) |
26/42 (62%) |
21/21 (100%) |
| Uncomfortable |
33/106 (31%) |
3/35(9%) |
16/42 (38%) |
0/21(0%) |
| Referral to specialist |
|
|
|
|
| Always |
58/113 (51%) |
6/32(19%) |
18/45 (40%) |
N/A |
| Often |
39/113 (35%) |
7/32(22%) |
14/45 (31%) |
N/A |
| Sometimes |
15/113 (13%) |
19/32 (59%) |
13/45 (29%) |
N/A |
| Never |
1/113(1%) |
0/32(0%) |
0/45(0%) |
N/A |
Table 3: Demographics of IBD nurse and medical practitioner respondents.
Whilst the majority of doctors reported being comfortable and happy to manage IBD patients, the level of comfort varied when asked about using different medical therapies (Table 4).
| How comfortable are you at initiating/directing/using…? (1 = extremely comfortable to5= extremely uncomfortable) |
| |
1 |
2 |
3 |
4 |
5 |
| Maintenance therapy (agent not specified) (n=117) |
24 (20.5%) |
37 (31.5%) |
26 (22%) |
23 (20%) |
7(6%) |
| Therapy for acute flare (agent not specified) (n=117) |
14 (12%) |
39 (33%) |
30 (26%) |
32 (27%) |
2(2%) |
| Steroids (n=116) |
18 (15.5%) |
50 (43%) |
29 (25%) |
16 (14%) |
3 (2.5%) |
| Immunomodulators (n=117) |
9(8%) |
14 (12%) |
32 (27%) |
33 (28%) |
29 (25%) |
| Biologic agents (n=114) |
9(8%) |
11 (9.5%) |
12 (10.5%) |
24 (21%) |
58 (51%) |
Table 4: General Practitioner comfort with discrete therapies for IBD.
Nearly all GPs and physicians and half of surgeons order blood tests for monitoring patients on immunomodulators. Sixty percent (90/149) of all doctors reported ordering blood tests on a case by case scenario rather than having a formal protocol.
Good communication from gastroenterologists was reported by 18/19 (95%) IBD nurses, 85/112 (76%) GPs, 19/34 (56%) physicians and 23/43 (53%) surgeons. When asked directly what the level the communication between the gastroenterologist and primary care physician was thought to be, 33/109 (30%) of GPs rated it excellent, 73/109 (67%) satisfactory and 3/109 (3%) poor. Several GPs commented that communication was better with private gastroenterologists than from those in the public system.
The level of support from public outpatient departments in relation to IBD was described as good and satisfactory in 30% of GPs (34/112). Opinions regarding personal experience with public IBD services were varied, whilst approximately half of responding medical practitioners (48/97, 49%) believed that IBD nurses were useful and valuable. A significant proportion of rural doctors however, still preferred to communicate directly with a gastroenterologist (29/53 55% GPs, 7/17 41% physicians and 10/27 37% surgeons).
The majority of participants (100% 20/20 IBD nurses, 78% 91/116 GPs, 52% 23/44 physicians and 80% 35/44 surgeons) were supportive of the idea of IBD action plans. GPs commented that they would be particularly useful for the management of acute flares and suggested that they could be incorporated into GP care plans.
With the use of a previously validated screening tool14, the average correct mean score for IBD knowledge was 8.8/11 for GPs, 9.3/11 for surgeons, 9.8/11 for physicians and 10.4/11 for IBD nurses. When asked directly about participants perceived level of IBD knowledge and training, 71/114 63% of GPs felt that they lacked in this area. This compared with 21/44 48% of surgeons and 10/40 25% of physicians. Of the 71 GPs who felt that they lacked in this area, 34 (48%) reported that this was a barrier to optimal care of this cohort of patients. Workshops, lectures and IBD symposiums focusing on updates in medical management were the most common types of training that were suggested as likely to improve their knowledge.
The majority of participants perceived that outcomes were worse for rural IBD patients compared with their metropolitan counterparts (18/20 90% IBD nurses, 70/96 73% GPs, 23/37 62% physicians and 22/44 50% surgeons). Of those who thought that rural health outcomes for IBD patients were the same in both groups several GPs, physicians and surgeons reported that this was secondary to the presence of a visiting or resident gastroenterologist in their region.
When given a range of options as examples of proposed barriers for optimal IBD care for rural patients 81% of respondents identified distance to specialist services, lack of local multidisciplinary teams and financial costs of accessing specialist services as the three most important barriers to optimal IBD care for rural patients (Figure 1).
The majority of IBD nurses (15/20 75%) reported that access to specialist IBD care was not adequate in rural areas. Rural doctor’s responses were generally evenly divided. Of those who thought that current access was adequate, many reported that they had a visiting or resident gastroenterologist and that access was better in private than in public. Sixty six percent of GPs (63/95) and 78% of physicians (32/41) and surgeons (32/41) felt that some adverse IBD patient health outcomes could have been prevented if access to specialist IBD care was better.
The level of access to members of a multidisciplinary team at the participants practice or within close proximity varied. Half of GPs (53/114, 46%) had direct access to a specialist colorectal surgeon, while 9/115 (8%) GPs had access to an IBD nurse. This was a similar finding among surgeons and physicians. Direct access to a dietician was available by the majority of all doctors (97/115 84% GPs, 38/42 90% physicians and 32/41 78% surgeons), but only 64% physicians (27/42) and surgeons (28/44) had direct access to a psychologist in their region compared with 87/115 (76%) GPs. Interventions thought to be most helpful in enhancing access to specialist gastroenterology care are shown in Figure 2. Of the doctors who did not list teleconferencing as being helpful, six GPs and two physicians commented that this method was overly time consuming. Others commented that a visiting or resident gastroenterologist in the area would be more helpful.
Discussion
This is the first study to simultaneously describe data regarding disease outcomes of rural IBD patients compared with their urban counterparts, rural practitioner IBD experience and knowledge and perceived barriers to optimal care of rural IBD patients from the perspective of both rural HCPs and patients.
Whilst the majority of our surveyed cohort identified rural IBD patients as having worse healthcare outcomes than their metropolitan counterparts, our data is the first of its kind to report no statistically significant differences in key IBD health outcomes such as disease complications, hospitalizations, surgery and steroid use. Certainly, in other areas of medicine such as cerebrovascular disease, alcohol and smoking rates and rates of hospitalization and falls, Australians living in regional and remote areas generally have poorer health than those living in major cities [14-16]. These current data is reassuring as despite sentiments from key stakeholders reflecting perceived negative outcomes due to their rural location, the barriers that may exist have not been shown to result in this conclusion. There was, however, variance in clinical practice with respect to methotrexate, iron replacement and hydrocortisone therapy as well as access to small bowel imaging and MRI and access to specialist gastroenterologist for diagnosis.
Tertiary referral centers have noted that a large burden of IBD exists outside of the metropolitan area, with rural patients accessing a substantial portion of metropolitan based services [17]. This was highlighted in an IBD Nurse study which demonstrated that in the 2011 period, non-metropolitan patients represented 498 of the 1211 patients on an IBD data base (41%), and were responsible for 2441 (49.6%) of the occasions of service[9]. Rural areas however, are regions where specialist gastroenterology services are absent and consequently the majority of long-term care is carried out by rural general practitioners, surgeons and physicians. Despite this, our data show that IBD exposure for individual rural practitioners is marginal with only a small proportion of total practice devoted to this field. Consequently, given the relative lack of exposure to IBD, the risk of deskilling and decline in confidence in managing this cohort in the outpatient setting with the use of various IBD therapies is high. This issue was highlighted by Tan et al who reported 37% of rural and metropolitan GPs in SA were uncomfortable with IBD management and 71% uncomfortable with the use of immunomodulators [18].
In addition to improving individual primary care physician training, practitioner support from gastroenterologists and specialist services is vital to maintain outpatient care. Whilst access to specialist services appeared be to be satisfactory in regions with resident or visiting gastroenterologists in our study, many regions in rural Australia (and other countries) do not have this luxury.
Despite many rural and remote initiatives over recent years, the health needs of many rural residents, both in Australia and overseas, are still not adequately met. Geographical location (accessibility to and availability of appropriate health services) and rural and remote environments (including socioeconomic status, lifestyles, and indigeneity) are undoubtedly the hallmark characteristics of rural and remote Australia and impact on health outcomes [19], with the presence of distance being the major impediment to accessing health care. Evidence indicates that there is no one model capable of overcoming identified barriers and servicing the health needs of diverse rural and remote communities, but rather service models must vary in order to take account of the specific geographical, social, economic and cultural contexts that differentiate the many rural and remote communities [19]. Models should also be guided by the resident medical workforce and community members who are key stakeholders in the care of rural IBD patients, in addition to specialist service providers, which is what our study has endeavored to do.
Respondents in our study identified teleconferencing and regional information sessions as potential worthwhile interventions in the future designed to overcome these perceived barriers of access and distance to specialized services and associated downfalls of obtaining this (financial cost and lack of personal support systems). Although, telehealth and telemedicine (the real time delivery of health and medical services at a distance between two or more locations using technology-assisted communications) have been widely used in Australia over recent years, evidence to date, shows that the utilization of this strategy remains patchy [19] with barriers existing in the domains of time economics (a point shared by a proportion of our respondents) funding priorities, infrastructure, and training [18]. Information sessions in region areas was the second intervention supported by our respondents, surprisingly given that this (lack of education) was not highlighted as one of the three most important barriers to care. Additionally, IBD action plans were acknowledged as a potential important tool. There is a near complete absence of IBD action plans available in the literature highlighted by a recent systematic review [20]; however, coincidentally we have devised an evidence based tool [21], which is freely available to download from online IBD support groups and this should be actively promoted.
The strengths of this study consist of the inclusion of opinions from a wide variety of stakeholders and the collection of both quantitative disease outcome data and qualitative participant perspective data, which has not been found in the literature. Each cohort has different roles in the management of rural IBD and consequently their opinions in addition to patient perspectives and data regarding practitioner exposure to and knowledge of IBD are invaluable. The study is also one of the first to collect data regarding this issue, which is vital to determine if and how interventions may be introduced that would aid in over-coming potential barriers and optimise care of this cohort in the long term.
We wish to acknowledge however, the limitations of the study. Whilst we matched urban IBD patients with rural IBD patients on the tertiary hospital data base, it could well be argued that rural patients who had been seen by the urban tertiary clinic were not representative of all rural IBD patients. The study was also hampered by low response rates from health care professionals and rural IBD patients. The numbers of rural patients recruited to the study may not have been enough to demonstrate variances of outcome between them and their urban counterparts.
In conclusion, our study is the first to compare IBD outcomes between a rural and metropolitan cohort. Whilst reassuringly, there were no significant difference in disease the gross outcomes of complications, hospitalisations, surgery and steroid use between cohorts, variance in clinical practice with respect to methotrexate and iron replacement therapy and access to small bowel imaging and MRI was found, suggesting that care is indeed unequal and that improvements might yield better outcomes. Furthermore, barriers to optimal care have been identified by key stakeholders, which should be viewed as areas for improvement. These data can be used to guide the development of appropriate interventions to enable equality of access and quality of care for patients with IBD living in regional and remote locations.
Acknowledgements
Alice Bennett received non-directed funding from Janssen Australia. We wish to thank Ruth Prosser, Sarah Clark and the Hawkins Medical Clinic for assistance in the distribution of questionnaires.
7826
References
- PricewaterhouseCoopers Australia (PwC) (2013) Improving Inflammatory Bowel Disease care across Australia.
- Pallis AG, Vlachonikolis IG, Mouzas I A (2002) Assessing health-related quality of life in patients with inflammatory bowel disease, in Crete, Greece. BMC Gastroenterology 2: 1-15.
- Gibson PR, Iser J (2005) Inflammatory bowel disease. Australian Family Physician. 34(4): 233-237.
- Ekbom A, Helmick C, Zack M, Adami HO (1991) The epidemiology of inflammatory bowel disease: a large, population-based study in Sweden. Gastroenterology 100: 350-358.
- Blanchard JF, Bernstein CN, Wajda A, Rawsthorne P (2001)Small-area variations and sociodemographic correlates for the incidence of Crohn’s disease and ulcerative colitis. American Journal Epidemiology 154: 328-335.
- Soon IS, Molodecky NA, Rabi DM, Ghali W, Barkema H, et al. (2012) The relationship between urban environment and the inflammatory bowel diseases: a systematic review and meta-analysis. BMC Gastroenterology 12:51.
- Mahmud N, Weir DG (2001) The urban diet and Crohn’s disease: is there a relationship? European Journal Gastroenterology Hepatology13: 93-95.
- Tezel A, Dokmeci G, Eskiocak M, Umit H, Soylu A (2003) Epidemiological features of ulcerative colitis in Trakya, Turkey. The Journal of International Medical Research 31(2):141-148.
- Leach P, De Silva M, Mountifield R, Edwards S, Chitti L, et al. (2013) The effect of an inflammatory bowel disease nurse position on service delivery. Journal of Crohn’s Colitis 8: 370-374
- Weizman AV, Nguyen GC (2013) Interventions and targets aimed at improving quality in inflammatory bowel disease ambulatory care. World Journal Gastroenterology 19(38): 6375-6382.
- Buzza C, Ono SS, Turvey C, et al. (2011) Distance is relative: unpacking a principal barrier in rural healthcare. Journal of General Intern Medicine 26 Suppl 2: 648-654.
- Leong RW, Lawrance IC, Ching J, Cheung C, Fung S, et al. (2004) Knowledge, quality of life, and use of complementary and alternative medicine and therapies in IBD: a comparison of Chinese and Caucasian patients. Digestive Diseases and Sciences 49(10): 1672-1676.
- Australian Institute of Health and Welfare (2010) Cardiovascular medicines and primary health care: a regional analysis. Canberra: AIHW.
- Australian Institute of Health and Welfare (2010) A snapshot of men’s health in regional and remote Australia. Canberra: AIHW.
- Tan M, Holloway RH, Lange K, Andrews JM (2012) General practitioners knowledge of, and attitudes to Inflammatory Bowel Disease. Internal Medicine Journal 42 (7): 801-807.
- Moffatt JJ, Eley DS (2014) Barriers to the up-take of telemedicine in Australia – a view form providers. Rural and Remote Health 11:1581- 2011.
- Humphreys J, Wakerman J (2013) rimary health care in rural and remote Australia: achieving equity of access and outcomes through national reform. A discussion paper.
- Bennett AL, Munkholm P, Andrews JM (2015) Tools for primary care management of inflammatory bowel disease: Do they exist? World J Gastroenterol 21: 4457-4465.
- Bennett AL, Buckton S, Lawrance I, Leong R, Moore G, et al. (2015) Ulcerative colitis outpatient management: Development and evaluation of tools to support primary care practitioners. Intern Med J 45:1254-1266.