Keywords
Seabuckthorn oil; Intramuscular administration; Antioxidant Status; Rabbit; Histopathology.
Introduction
Seabuckthorn (Hippophae rhamnoides L. Family Elaeagnaceae) bearing small orange yellow-to-red coloured fruits and commonly named as a ‘wonder plant’, is generally found at high altitude of 2500– 4300 m in several Asian and European countries including India, China, Nepal, Britain, Germany, France, Finland, and Russia. In India it is found mainly in cold arid condition of Ladakh (Leh & Kargil), Lahul-Spiti, parts of Chamba and upper Kinnaur district of Himachal Pradesh, Sikkim and Arunachal Pradesh [1]. The seed oil contains high amounts of vitamins A, E, K, carotenoids, phytosterols, which act as antioxidants to neutralize free radicals by activating superoxide dismutase [2,3 & 4]. Seabuckthorn seed oil has a high amount of linoleinic acid and α-lenolenic acid, while pulp of SBT is a rich source of palmitic acid, palmitoleic acid and ß - sitosterol [5, 6 & 7]. The fatty acid content of oil acts as epidermal barrier system that stabilizes cell membrane structure [6 & 8]. As a natural immune enhancer, it maintains the stability of the immune system and keeps the supervisory role of the system normal [9]. Studies of seabuckthorn oil have suggested its usefulness in treating radiation damage, burns, eye, skin ailments, cardiovascular diseases, oral inflammation, mucous injuries and gastric ulcers [3, 6 & 10]. The oil has anti-oxidative, antimicrobial, immunomodulatory, cytoprotective, hepato-protective and tissue regenerative properties [8, 11 & 12].
Sunburns are common problems associated with exposure to ultraviolet rays in troops and it is impractical to cover all skin by protective clothing. Depending on the skin architecture, duration and intensity of exposure the adverse reactions may vary from simple blackening and irritation to outright ulceration, oozing and pain with enhanced neural end organ sensitivity. Seabuckthorn oil had obvious effects on anathrepis, eliminating inflammation and slough, easing pain, promoting immune function and strengthening body resistance [13].
Chromosomal studies tend to demonstrate skin DNA damage, disruptions and biochemical studies show local production of oxidative stress markers. Seabuckthorn oil supplementation increases the activation of glutathione peroxidase, superoxide dismutase, glucose-6-phosphate dehydrase, and membrane levels of sialic acid and the sulfydryl group in erythrocytes. The oil also protects against oxidative damage [14, 15, 12 & 16].
The intramuscular (i.m.) route of administration is commonly employed parenteral route for many pharmaceutical products and veterinary medicines especially in food animals [17]. The route provides sustained release effect of a drug via the depot formation. The advantages of using a long-acting depot injection include increased medication compliance due to reduction in the frequency of dosing, as well as more consistent plasma concentrations. The intramuscular route offers a faster rate of absorption than the subcutaneous route, and muscle tissue can often hold a larger volume of fluid without discomfort.
Seabuckthorn oil is well known to give skin protective action against sun exposure clinically [3 & 13]. Toxicity studies on other formulations of seabuckthorn oil are available, but no scientific study has been conducted to determine the efficacy and toxicity of proposed oil intramuscularly. The sustained release i. m. injection of seabuckthorn seed oil also enables weekly dosing that reduces repeated application. We studied the efficacy and safety of intramuscular injection of seabuckthorn oil. Since most of the herbal oils were not used as parenteral products, seabuckthorn seed oil proved as one of the crucial oil which has antioxidant and UV protective property [18 & 19]. It is required to evaluate its efficacy and safety data that can be utilized for commercial purposes. The present study was carried out to prepare an i.m. injection as a depot formulation by utilizing antioxidant and radioprotective properties of oil which can be used as a vehicle to deliver various drug candidates.
2. Materials and Methods
2.1. Chemicals and reagents
Seabuckthorn (Hippophae rhamnoides) seed oil was provided by Defence Institute of High Altitude Research (DIHAR), Leh, India. Total Antioxidant Status Kit was procured from Cayman Chemical Company, USA. All other chemicals and solvents used in the study were of analytical grade.
2.2. Animals
The experiments were conducted on male New Zealand white rabbits weighing 2.5–3.0 kg. The animals were obtained from experimental animal facility of Institute of Nuclear Medicine and Allied Sciences (INMAS), Delhi. All animal experiments were approved by the Institutional Animal Ethical Committee and confirmed to general national guidelines on the care and use of laboratory animals. The animals were maintained at controlled temperature and hygiene conditions and were provided food and drinking water ad libitum. They were housed individually in cages and maintained on 12 hours day and night cycle.
3. Treatment Regimen
To study the efficacy and intramuscular toxicity of seabuckthorn oil, 24 New Zealand white rabbits were divided into four groups. Group I served as control and received no treatment while animals of group II, III and IV were administered sterile seabuckthorn seed oil (0.5, 1 and 1.5 ml/kg body weight respectively) by intramuscular route in gluteus muscle. Hairs at injection sites were clipped with a clipper a day before giving the injection. The injections were made with a sterile needle and administered once a week for 7 consecutive weeks for evaluation of safety and tolerability of the oil. Blood samples from the ear vein of animals were collected at pre-determined time intervals and efficacy of the oil was measured in term of total antioxidants status using antioxidant assay kit. Animals were sacrificed after seven week of intramuscular administration of oil for toxicological evaluation.
4. In vitro antioxidant activity
Blood samples at 0, 4, 24, 48 and 96 hours were collected from the ear veins of the rabbits after first injection of seabuckthorn oil. The samples were kept at 25 ºC for 30 minutes for clotting then centrifuged at 3000, g for 15 minutes at 4 ºC. Serum was separated to analyze the total antioxidant status. Total antioxidant activity was assayed and changes in absorbance were recorded at 750 nm. Total antioxidant activity was calculated as mM and compared with the values of control group.
The assay relies on the activity of antioxidants in the samples to inhibit the oxidation of ABTS® (2, 2’- Azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS®·+ by metmyoglobin to assess total antioxidant capacity. The amount of ABTS® ·+ produced was monitored by taking absorbance at 750 nm using ELISA Reader (Biotek KC 400). Under the reaction conditions used, the antioxidants in the sample cause the suppression of absorbance at 750 nm to a degree which is proportional to their concentrations [20, 21 & 22]. The capacity of antioxidants in the samples to prevent ABTS® oxidation was compared with Trolox, a water-soluble tocopherol analogue, and was quantified as milli molar (mM) of Trolax equivalents.
5. Sub-chronic toxicity studies
Animals were sacrificed after seven week of i.m. administration of seabuckthorn oil and various toxicological parameters were investigated.
5.1. Physical Assessment
Physical parameters (body weight, food and water intake) and local injury were studied during treatment of animals. Mortality was also recorded during treatment of all groups. Autopsy was done if animals died during the course of treatment. The health status of rabbits was observed daily while body weight of animals were measured weekly during the experiment. Each injection site was examined each day for any morphological changes. Erythma, edema, swelling, cyanosis and paralysis in muscular tissue were also observed as a sign of toxicity throughout the study. At the end of the experiment animals were sacrificed and weight of various vital organs such as lung, liver, kidney, heart and spleen were determined.
5.2. Haematological assessment
As indicators to immune function status, blood samples were collected by cardiac puncture for hematological examination. Red blood cells (RBC), white blood cells (WBC), hemoglobin (Hb), hematocrit (HCT), mean corpuscular volume (MCV), mean hemoglobin concentration (MHC) and platelets (PLT) counts were determinate using hematological automatic analyzer (Roche Integra, 400 Plus, Diagnostic Systems).
5.3. Serum Biochemistry
Clinical chemistry parameters determined included blood levels of albumin, total protein, total bilirubin, urea, creatinine, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, glucose, total cholesterol, using a Hitachi 912 auto analyzer.
5.4. Histological examinations
The animals were sacrificed by chloroform anesthesia and exsanguinations and necropsied after the 7th day of last injection. Liver, kidney, Spleen and muscle tissues of site of injection were removed from the sacrificed animals and preserved in 10% buffered formalin for histological examination. A complete set of muscular tissues were examined for microscopic changes at the site of injection (damage by injecting needle and effect of oil volume, if any). The standard histopathological procedure was followed in which tissues were fixed in 10% formalin and embedded in paraffin. 5μm sections were cut from tissues of each group. The sections were deparaffinized using xylene and ethanol. The slides were washed with phosphate buffer saline (PBS) and permeabilized with permeabilization solution (0.1M citrate, 0.1% Triton X-100). The deparaffinized sections were stained with haematoxylin and eosin. Tissue sections were observed under a fluorescence microscope (Olympus BX 60), at a magnification of 40X and the findings were analyzed.
6. Statistical Analysis
Data from individual experiments were presented as Mean ± S.D. Differences between groups were analyzed using analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test. Minimum criterion for statistical significance was set at p<0.05 for all comparisons.
7. Results
7.1. In vitro antioxidant activity assay
Antioxidant activity was evaluated by total antioxidant assay following ABTS assay which is a common in vitro method to assess total antioxidant capacity of plant extracts. The results showed significant improvement in antioxidant activity when compared with control (Fig. 1.). The total antioxidant concentration in group II, III and IV was approximate 15-30 times higher to control group. The maximum antioxidant concentration was achieved at 48 hrs after that it starts to decline. At 96 hrs the antioxidant concentration was still higher as compare to control. Linear relationship between antioxidant status and dose was observed. Group IV treated with oil showed marked increase in antioxidant concentration (0.35 mM/L) than group II and III (0.25 and 0.3 mM/L). These results proved the seabuckthorn seed oil as effective source of free radical scavengers demonstrated antioxidant activity.
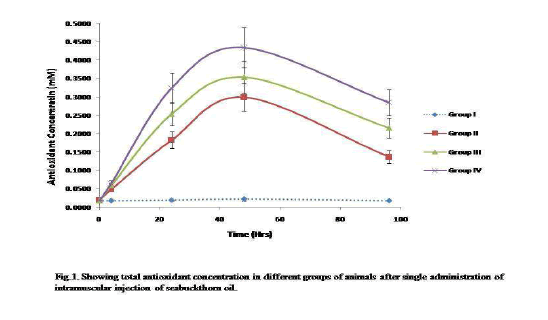
Figure 1: Showing total antioxident concentrationin different groups of animals after single administration of intramuscular injection of seabuckthorn oil.
7.2. Sub-chronic safety studies
7.2.1. Physical Parameters
No significant changes in general condition and body weights gains were seen in all groups of animals. The overall means for daily food consumption were similar for all groups throughout the study (data not shown). No deaths were observed in all groups until the end of the study. Tissue hardening and inflammatory reactions were observed at the site of administration in all the treated animals. However no other clinical sign of toxicity such as cyanosis and paralysis were observed.
7.2.2. Hematological Parameters
No toxicologically important hematological findings occurred in treated animals. Results showed that the values of hematological parameters (RBCs, WBCs, Hb, HCT, MCV, MCH and platelets) were within the normal ranges as compared to control group (Table 1).
| Parameters |
Group I |
Group II |
GroupIII |
Group IV |
| RBC(106 / µL) |
05.18±0.96 |
05.26±0.64 |
5.43±0.41 |
6.64±0.65 |
| WBC (106 /µL) |
10.43±1.80 |
11.32±1.63 |
9.90±1.50 |
17.30±2.55 |
| Hb (g / dL) |
12.00±1.08 |
13.40±2.54 |
12.73±0.67 |
10.67±2.67 |
| HCT (%) |
34.93±5.57 |
36.76±4.68 |
37.47±1.90 |
43.77±3.52 |
| MCV (fL) |
67.83±2.07 |
65.92±1.98 |
67.90±1.99 |
69.57±10.81 |
| MCH (pg) |
21.83±0.30 |
21.73±0.36 |
22.47±1.15 |
22.07±3.09 |
| Platelets(103 / µL) |
318.33±24.98 |
367.25±27.20 |
431.33±28.57 |
434.67±26.95 |
All the values are mean ± SD of six rabbits in each group.
Table 1- Effect of sub chronic intramuscularly administered seabuckthorn oil on haematological parameters
7.2.3. Serum Biochemistry
Seabuckthorn oil administration related effects on serum chemistry parameters consists no changes in protein, bilirubin, urea and alkaline phosphatase concentrations in test groups as compared to the control group. The differences were statistically not significant (Table 2).
| Parameters |
Group I |
Group II |
Group III |
Group IV |
| TP |
6.63± 0.61 |
6.37±0.76 |
6.55±0.53 |
6.72±0.35 |
| SGOT |
28.33±3.88 |
27.50±3.27 |
28.83±4.58 |
31.67±7.03 |
| SGPT |
46.83±5.67 |
48.00±7.40 |
49.67±8.50 |
51.50±8.02 |
| ALP |
135.17±7.08 |
135.00±7.07 |
134.33±9.91 |
139.67±11.72 |
| TG |
75.00±8.25 |
74.50±5.86 |
74.67±6.59 |
74.67±13.98 |
| CHO |
27.17±3.06 |
30.50±4.37 |
29.33±3.20 |
32.67±4.68 |
| Sugar |
154.17±18.55 |
157.17±10.32 |
156.83±11.39 |
156.50±13.81 |
| Urea |
21.67±3.88 |
22.17±3.76 |
24.67±4.76 |
25.17±3.31 |
| Creatinine |
0.67±0.12 |
0.65±0.10 |
0.65±0.10 |
0.67±0.12 |
| Bilirubin |
0.45±0.10 |
0.48±0.08 |
0.57±0.08 |
0.65±0.14 |
| Uric Acid |
2.63±0.35 |
2.73±0.34 |
2.82±0.26 |
2.83±0.38 |
| Triglycerides |
83.50±6.57 |
81.17±4.40 |
82.67±8.57 |
80.00±8.56 |
All the values are mean ± SD of six rabbits in each group.
Table 2- Effect of sub chronic intramuscularly administered seabuckthorn oil on biochemical parameters
7.2.4. Histopathological Findings
Gross necropsy demonstrated swelling of muscular tissue at the site of injection in the entire animal groups. Histological assessment also showed a mild lymphocytic infiltrate in the perimycium, and interstitial tissues inflammation in muscular tissues (Fig. 2) but which were not severe sign of toxicity. There were no significant treatment related histopathological changes observed in vital organs (kidney, liver and spleen) of all the treated groups of animals as compared to control (Fig. 3).
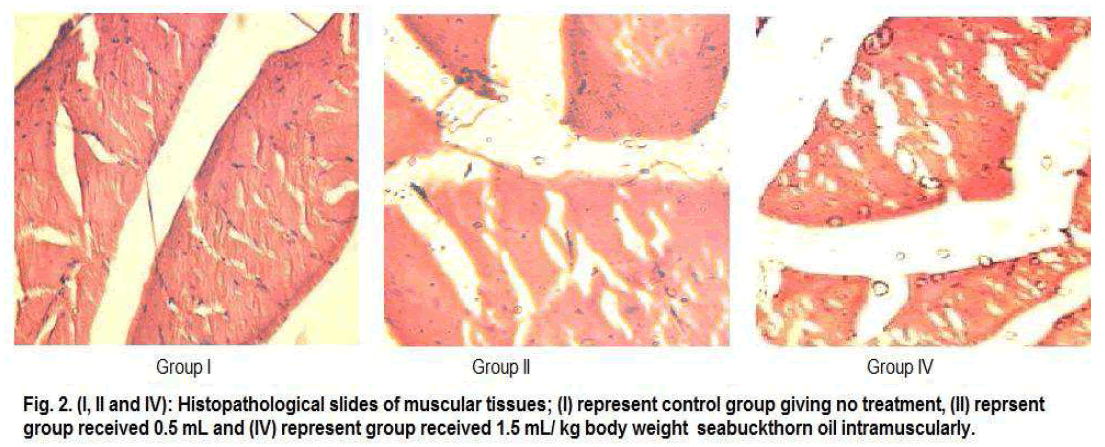
Figure 2: Histo pathological slides of muscular tissues;(I) represent control group giving no treatment,(II)represent group recieved 0.5ml and (IV)represent group recieved1.5ml/kg body wweight seabukthron oil intramuscularly.
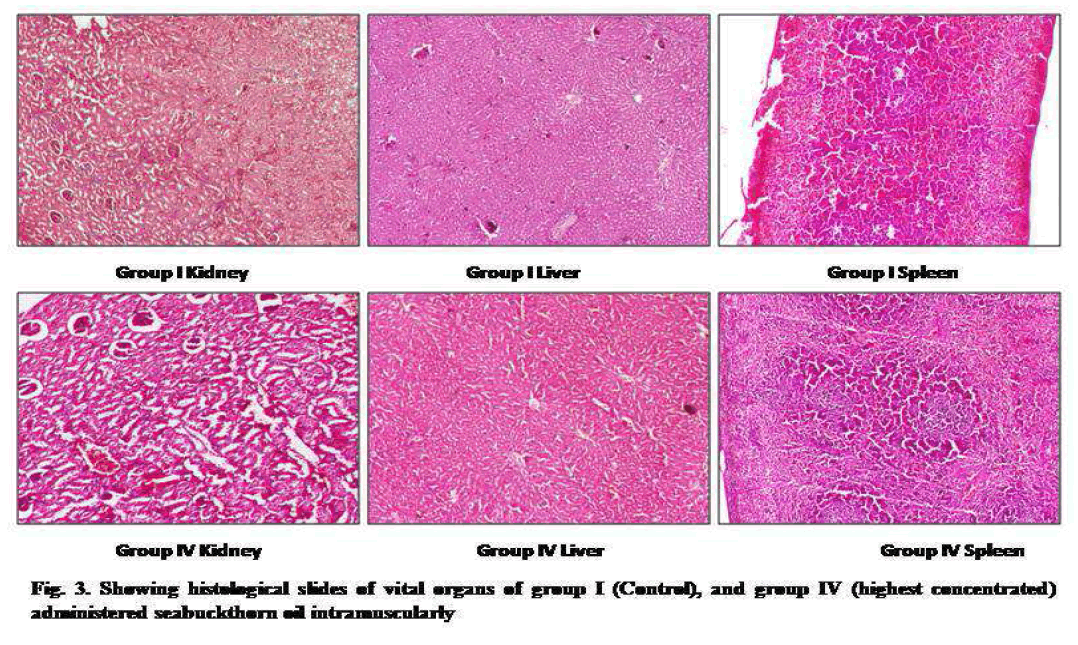
Figure 3: showing histological slides of vital organs of group I(Control),and group IV (highest concentrated) administered seabuckthron oil intramuscularly.
8. Discussion
Seabuckthorn, a well-known traditional medicinal herb, possesses diverse biological activities and pharmacological functions including treatment of radiation damage [23], burns, eye, skin ailments, cardiovascular diseases, oral inflammation, mucous injuries and gastric ulcers [3]. Free radicals and reactive oxygen species (ROS) are highly reactive molecules that are generated by normal cellular processes, environmental stresses, and UV irradiation. ROS react with cellular components, damaging DNA, carbohydrates, proteins, and lipids causing cellular and tissue injury. Excess production of reactive oxygen species can also lead to inflammation, premature aging disorders, and several disease states, including cancer, diabetes, and atherosclerosis. Organisms have developed complex antioxidant systems to protect themselves from oxidative stress; however, excess ROS can overwhelm the systems and cause severe damage. The antioxidant system of living organisms includes enzymes such as superoxide dismutase, catalase, and glutathione peroxidase; macromolecules such as albumin, ceruloplasmin, and ferritin; and an array of small molecules, including ascorbic acid, α- tocopherol, β-carotene, reduced glutathione, uric acid, and bilirubin. The sum of endogenous and food-derived antioxidants represents the total antioxidant activity of the extracellular fluid. Cooperation of all the different antioxidants provides greater protection against attack by reactive oxygen or nitrogen radicals, than any single compound alone. Thus, the overall antioxidant capacity may give more relevant biological information compared to that obtained by the measurement of individual components, as it considers the cumulative effect of all antioxidants present in plasma and body fluids. Our study demonstrates the effect of antioxidant formulation containing seabuckthorn oil as a scavenger that reduces the formation of free radicals and thereby provides protection effect when administered intramuscularly [24]. Moreover the proposed oil can also be used as a carrier in certain intramuscular formulations for prolonging their effects via depot formation.
The results of this study indicated that seed oil of seabuckthorn not only possessed significant antioxidant effect but also safe when injected intramuscularly in rabbits. Our study reveals the potential of seabuckthorn seed oil for use as a natural agent with both high antioxidant contents and safety. In this study, rabbits were administered i.m. injections of seabuckthorn seed oil over a period of 49 days. Total antioxidant status of oil was investigated which showed the higher amount of antioxidants in the treated animals when compared with control group that is attributed to the presence of vitamins A, E, K, carotenoids, phytosterols. Seed oil of seabuckthorn was rich in antioxidant components, such as carotene, ascorbic acid, thiamine, riboflavin, nicotinic acid, zeaxanthin, cryptoxanthin, and coumarin (scopoletin), which contributed to their antioxidant properties. Among these antioxidant components, there may be some interactions and synergistic effects for antioxidant properties. The results of total antioxidant capacity assay showed an increase in antioxidant status with increase in dose.
A comprehensive study of toxicological investigations revealed no evidence of a systemic effect of exposure to this oil at any of the concentrations administered. In the present study, no experimental rabbits administered with seabuckthorn seed oil were died or showed any adverse effects in sub-chronic i.m. toxicity studies. Tissue hardening was the most observed clinical sign in all the groups, although slight swelling at administration sites was also observed. Various hematological parameters were investigated which were within the normal ranges as compared to control group. The organ body weight ratio of vital organs was also comparable with control group. Our study described here includes observation of histological changes up to 7 weeks post administration of seabuckthorn oil, resulting from multiple intramuscular injections at the same site in rabbits. The only changes seen were histopathological changes which were observed both in control as well as treated group of animals that is due to rupturing of the muscles by the needle [25]. Local damage to skeletal muscle is one of the undesirable side effects of intramuscularly applied drugs and chemicals [26, 27]. Histological examination of other tissues and organs were normal indicating that the oil has no significant toxicity this was supported by the hematological data which were also normal. The oil has no apparent toxic effects on the liver, kidney, heart and spleen. Histopathological findings in safety studies of oil may therefore be limited to the local effect at the site of injection. Results of this sub-chronic study indicate that the potential for toxicity with seabuckthorn oil is very low. The nature and extent of the observed histological findings are consistent with local minimal trauma due to the needle penetration in muscles, together with possible effects of the injected saline. The results indicated that the intramuscular route is a safe route of administration, since it does not produces any serious inflammatory reactions. The use of SBT oil as dermal application for immunity enhancement and safety from sun exposure has been done from a long time but it required repeated applications. Intramuscular administration might be a better way for improving the patient compliance by virtue of its sustained effect.
The rabbit model was chosen because of the availability of a large and homogeneous muscle mass in the paravertebral area, allowing adequate muscle samples. Rabbit is also a key species for testing muscle tolerance of injectable formulations [28].
9. Conclusion
The present study has shown that seabuckthorn seed oil demonstrate strong antioxidant activity. The intramuscular toxicity study of seabuckthorn oil showed that this oil is safe to be used as a vehicle in different formulations. In vivo studies confirm no sub-chronic toxicity of seabuckthorn oil in designated doses on animal muscle tissues. The histopathological studies of muscle tissues did not show any significant changes in structure in treated groups of animals as compared to the control group confirming the safety. The intramuscular administration of oil is well tolerated and its immunity enhancing property indicates the potential to be used as carrier for different sustained release depot formulations.
Conflicts of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors are thankful to Defence Research and Development Organization, Ministry of Defence, Government of India for providing the funds to carry out this work.
Conflict of Interest: NIL
Source of Support: NONE
5614
References
- Rousi, A; 1971. The genus Hippophae L. Antaxonomic study. Ann. Bot. Fennici 8, 177–227.
- Weiss, J F; 1997. Pharmacologic approaches tonprotection against radiation-induced lethalitynand other damage. Environ. Health Perspect.n105 (Suppl. 6), 1473–1478.
- Yang, B. Kalimo, K. Mattila, L. Kallio, S.nKatajisto, J. Peltola, O. Kallio, H; 1999. Effect ofndietary supplementation with sea buckthornn(Hippophaë rhamnoides) seed and pulp oils onnatopic dermatitis. J. Nutr. Biochem. 10, 622-n630.
- Kasparaviciene, G. Briedis, V. Ivanauskas, L;n2004. Influence of seabuckthorn oil productionntechnology on its antioxidant activity. Medicina.n40(8), 753-757.
- Chen, Y. Jiang, Z. Qin, W; 1990. Chemistry,ncomposition and characteristics of seabuckthornnfruit and its oil. Chem. Industry of ForestnProducts (Chinese) 10, 163–175.
- Yang, B. Kalimo, K O. Tahvonen, R L. Mattila, LnM. Katajisto, J K, Kallio, H P; 2000. Effect ofndietary supplementation with sea buckthornn(Hippophaë rhamnoides) seed and pulp oils onnthe fatty acid composition of skin glycerophospholipids of patients with atopicndermatitis. J. Nutr. Biochem. 11(6), 338-340.
- Hong, Ge; 1992. GC-MS analyses on chemicalncomposition of sterol from seabuckthorn fruitnoil. Seabuckthorn. 5 (1), 7-15.
- Gao, Z L. Gu, X H. Cheng, F T. Jiang, F H; 2003.nEffect of seabuckthorn on liver fibrosis: anclinical study. World J Gastroenterol. 9, 1615-n1617.
- Alam Z.; 2004. Chemical and nutritionalnconstituents of seabuckthorn juice. Pak. J. Nutr.,n3, 99-106.
- Liu, B. Wu, Z. Liu, W; 1980. Preliminarynobservation on curing effects of Sea-buckthornnfruit juice for high blood cholesterol andncoronary heart disease. Acta AcademiaenMedicinae Sichua. 11(3), 178–182
- Geetha, S. Sai Ram, M. Singh, V. Ilavazhagan, G.nSawhney, R.C; 2002. Antioxidant andnimmunomodulatory properties of seabuckthornn(Hippophae rhamnoides) – An in vitro study. J.nEthnopharmacol. 79, 373–378.
- Negi P, Chauhan A, Sadia G, Rohinishree Y,nRamteke R; 2005. Antioxidant and antibacterialnactivities of various seabuckthorn (Hippophaenrhamnoides L.) seed extracts. Food Chem. 92,n119-124.
- Xu, Mingyu; 1993. A brief report on an antibacterialnexperiment using seabuckthorn oil.nHippophae 6 (2), 28-29.
- Rosch, D. Bergmann, M. Knorr, D. Kroh, L;n2003. Structure-antioxidant efficiencynrelationships of phenolic compounds and theirncontribution to the antioxidant activity ofnseabuckthorn juice. J Agric Food Chem. 51,n4233-4239.
- Rosch, D. Mugge, C. Fogliano, V. Kroh L W;n2004. Antioxidant oligomericnproanthocyanidins from seabuckthorn (Hippophae rhamnoides ) Pomace. J Agric FoodnChem. 52, 6712-6718.
- Wu, D. Meng, Z; 2003. Effect of sulfur dioxideninhalation on the glutathione redox system innmice and protective role of sea buckthorn seednoil. Arch Environ Contam Toxicol. 45, 423-428.
- Brazeau, G A. Cooper, B. Svetic, K A. Smith, C L.nGupta, P; 1998. Current perspectives on painnupon injection of drugs. J Pharm Sci 87, 667-77.
- Beveridge, T. Li, T S C. Oomah, B D; 1999. Seanbuckthorn products: manufacture andncomposition. Journal of Agriculture and FoodnChemistry 47, 3480–3488.
- Eccleston, C. Baoru, Y. Tahvonen, R. Kallio, H.nRimbach, G H. Minihane, A M; 2002. Effects ofnan antioxidant rich juice (Sea buckthorn) on risknfactors for coronary heart disease in humans.nJournal of Nutritional Biochemistry 13, 346–n354.
- Miller, N J. Rice-Evans, C. Davies, M J; 1993. Annew method for measuring antioxidant activity.nBiochem. Soc. Trans. 21, 955.n21. Miller, N J. Rice-Evans, C; 1997. Factorsninfluencing the antioxidant activity determinednby the ABTS·+ radical cation assay. Free Rad.nRes. 26, 195-199.
- Rice-Evans, C. Miller, N; 1994. Total antioxidantnstatus in plasma and body fluids. MethodsnEnzymol. 234 (24), 279-293.
- Goel, H C. Prasad, J. Singh, S. Sagar, R K.nKumar, I P. Sinha, A K; 2002. Radioprotectionnby herbal preparation of Hippophaenrhamnoides, RH-3 against whole body lethalnirradiation in mice. Phytomedicine 9, 15–25.
- Alam, Z.; 2006. Anticarcinogenic potential ofnlipids from Hippophae evidence from the recentnliterature. Asian Pac J Cancer Prev. 7 (1), 32–35.n25. Summan, M. McKinstry, M. Warren, G L.nHulderman, T. Mishra, D. Brumbaugh, K.nLuster, MI et. Simeonova, PP; 2003.nInflammatory mediators and skeletal muscle injury: a DNA microarray analysis. J InterferonnCytokine Res. 23: 237-245.
- Brumback, R A. Empting, L. Susag, M E. Staton,nR D; 1982. Muscle fibrosis associated withnintramuscular chlorpromazine administration.nA preliminary report. J. Pharm. Pharmacol. 34,n526-528.
- Svendsen, O; 1983. Local muscle damage andnoily vehicles: A study on local reactions innrabbits after intramuscular injection ofnneuroleptic drugs in aqueous or oily vehicles.nActa Pharmacol. Toxicol. 52, 298-304.
- Sutton, S C. Evans, L A. Rinaldi, M T. andnNorton, K A; 1996. Predicting injection sitenmuscle damage: Evaluation of immediatenrelease parenteral formulations in animalnmodels. Pharm. Res. 13, 1507–1513.









