Cucunawangsih1*, Beti Ernawati Dewi2, Veli Sungono1, Nata Pratama Hardjo Lugito3, Bambang Sutrisna4, Herdiman T. Pohan5, Agus Syahrurachman2, Djoko Widodo5, Sudarto Ronoatmodjo3, Modastri K. Sudaryo3, Cicilia Windiyaningsih6 and T. Mirawati Sudiro2
1Microbiology Department, Faculty of Medicine, Pelita Harapan University, Tangerang, Indonesia
2Microbiology Department, Faculty of Medicine, Indonesia University, Jakarta, Indonesia
3Internal Medicine Department, Faculty of Medicine, Pelita Harapan University, Tangerang, Indonesia
4Epidemiology Department, Faculty of Public Health, Indonesia University, Depok, Indonesia
5Tropic Infection Division, Internal Medicine Department, Indonesia University, Jakarta, Indonesia
6Epidemiology Centre, Sulianto Saroso Hospital, Jakarta, Indonesia
- *Corresponding Author:
- Cucunawangsih
Microbiology Department, Faculty of Medicine, PelitaHarapan University
JendralSudirman Boulevard, LippoKarawaci, Tangerang, Banten, 15811, Indonesia
Tel: +6221 54210130
E-mail: cucunawangsih.fk@uph.edu
Keywords
Dengue; Scoring model; Diagnosis; Early phase of illness
Introduction
Dengue illness is a re-emerging mosquito-borne viral disease, caused by one of the four dengue virus serotypes (DENV-1, DENV- 2, DENV-3, and DENV-4) of the Flavivirus genus, Flaviviridae family and transmitted by Aedes aegypty [1]. It is estimated that there are approximately 50-200 million dengue cases, 500,000 severe dengue cases, and over 20,000 dengue related deaths annually worldwide [2]. Mapping of global dengue incidence published in April 2013 suggested that there were 390 million dengue infections annually worldwide [3]. The World Health Organization (WHO) reported that 75% of the global dengue disease burden originated from the Western Pacific and South East Asia regions [4,5]. In these regions there were 353,907 dengue cases and 1,073 deaths in 2010. In American region, there were 1.6 million dengue cases, 49,000 of them were severe dengue in 2010 [5]. Dengue illness is one of the major public health problems in Indonesia, and currently the leading cause of hospital admissions and deaths among children [2,3]. In 2009 there were 158,912 dengue cases, and the annual dengue incidence in 2013 was 35-40/100,000 person-year [6]. The number of dengue illness has increased among the patients aged 15 years and above [7]. Multiple virus serotypes are circulating, but severe illness is predominantly associated to DENV-3 [8-10].
The clinical spectrums of dengue illness are ranging from undifferentiated fever, self-limiting dengue fever (DF) to more severe, life threatening forms of the illness termed dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), which are characterized by plasma leakage as a result of increased vascular fragility and permeability [4]. In the early phase, this illness often shows similar clinical features of other febrile illnesses, such as headache, myalgia, arthralgia, and rash [7-14]. The main characteristics of DHF such as retro-orbital pain, petechiae or other clinical signs of bleeding, and plasma leakage are not seen in the early phase of illness in many patients. They typically appear after the third or fourth day of fever [15,16]. Definitive early dengue diagnosis is difficult and requires laboratory tests based on viral or antigen detection, such as real time polymerase chain reaction (RT-PCR), and virus isolation [4,17-19]. However, these examinations are not sufficiently rapid, costly and only available in research laboratories. Many dengue endemic areas in Indonesia lack the necessary laboratory infrastructures or supports to perform these examinations. Commercial non-structural-1 (NS-1) antigen detection kits are widely available nowadays and used for diagnosis of acute dengue infection [15,20,21]. However these assays are still under evaluation for diagnosis accuracy and cost-effectiveness in multiple settings [4].
Efficient and accurate dengue diagnosis in the early phase of illness is very important in clinical settings to reduce case fatality rate [22,23]. The 2009 WHO classification system, which divide dengue illness into several levels of severity (dengue with/without warning signs and severe dengue) is more sensitive to recognize severe dengue and designed to monitor illness progression for clinical management and epidemiological surveillance [15,23,24]. Thus, many of dengue cases in the early phase individually do not meet all of the criteria outlined by this classification system [25].
Because of limited resources of sophisticated laboratory facilities in Indonesia, we tried to develop a dengue scoring model using initial clinical features and simple hematology test. This scoring model could distinguish dengue from non-dengue illness, especially from other flavivirus, Japanese B encephalitis, and Chikungunya in the first 72 hours of illness.
Materials and Methods
Patients and clinical data collection
A prospective analytical observational study was conducted from July 2011 to March 2012 on adult patients in five primary healthcare facilities and one private hospital in Tangerang, Indonesia. This study had been approved by Ethical Review Board of Faculty of Public Health, University of Indonesia. Enrolment of study participants is conditional on appropriate informed consent administered by principal investigator. All biological materials collected were anonymized after completion of demographic and clinical data collection. Adult patients (age 15-60 year-old) with fever within 72 hours of data collection were eligible to participate. Patients with immunologic/haematologic disorder, severe lung/heart/liver illness, rhinitis/cough, and clinically obvious alternative diagnoses were excluded. Clinical data such as headache, nausea/vomiting/abdominal pain, retro-orbital pain, myalgia, arthralgia, skin rash, spontaneous bleeding, and positive tourniquet test were obtained from eligible participants. All eligible patients were screened with NS-1 antigen detection. The diagnosis of dengue illness was based on the presence of fever with two or more symptoms and signs with one or more laboratory criteria on haematology examinations. Patients with positive RT-PCR and/or NS-1 antigen detection results following either positive or negative of immunoglobulin M (IgM) antidengue antibody results were confirmed as dengue.
Laboratory methods
A complete haematological count was performed on 5 cc anticoagulated whole blood collected on the 1st visit by an automated haemocytometer (Sysmex®, Japan). NS-1 antigen, IgM and immunoglobulin G (IgG) anti-dengue antibodies were examined using commercially available Dengue Duo (Standard Diagnostic Inc®, Korea) according to manufacturer’s instruction. For RT-PCR, the ribonucleic acids (RNAs) were extracted from acute sera using QIAamp Viral RNA mini kit (Qiagen®, Germany) according to the manufacturer’s protocol. Detection of dengue viral RNA serotypes with RT-PCR was carried out using Lanciotti’s method [26,27].
Data analysis
The data were analysed using STATA version 11.00. Chi-Square and t test were used for categorical variables and continuous variables, respectively. A multivariate logistic regression analysis was used to develop the dengue scoring model. Sensitivity and specificity for each value of the scoring model for dengue diagnosis were determined. Performance of the dengue scoring model was assessed using the ROC curve. Validity of scoring model was evaluated based on sensitivity, specificity, negative and positive predictive value, likelihood ratio compared to 1997, 2009 and 2011 WHO dengue classification, using NS-1 antigen detection and/or positive RT-PCR results as gold standard [4, 28,29].
Results
Patient characteristics
Eighty four patients of acute onset febrile illness were included in this study. One patient was excluded due to mixed infection with Plasmodium vivax. The demographic characteristics, initial clinical features, and simple laboratory results were shown in Table 1. There was no significant difference between male and female. Nausea/vomiting/abdominal pain and headache were the most common reasons of patients with acute febrile illness went to healthcare facilities.
| Characteristics |
na |
Reference rangeb |
| Demographic information |
|
|
| Age (year) |
30.3 ± 10.6 |
|
| Female |
39 (46) |
|
| Male |
45 (54) |
|
| Initial clinical features |
|
|
| Fever |
84 (100) |
|
| Nausea/vomiting/abdominal pain |
73 (87) |
|
| Retro-orbita pain |
16 (19) |
|
| Myalgia |
59 (70) |
|
| Arthralgia |
34 (40) |
|
| Headache |
70 (83) |
|
| Positive torniquet test |
9 (11) |
|
| Spontaneous bleeding |
|
|
| Petechiae |
8 (9) |
|
| Epistacsis |
2 (2) |
|
| Gum bleeding |
3 (3) |
|
| Hematemesis-melena |
1 (1) |
|
| Simple laboratory results |
|
|
| Hemoglobin (g/dL) |
13.4 ± 1.9 |
13.3 – 16.2 (male); 12 – 15.8 (female) |
| Hematocrit (%) |
39.5 ± 5.5 |
40 – 52 (male); 35.4 – 44.4 (female) |
| Total WBC count (/μL) |
6,141 ± 3,834 |
3,540 – 9,060 |
| Platelet count (/μL) |
15,8179 ± 83,259 |
165,000 – 415,000 |
| Band neutrophils count (%) |
1 ± 1 |
0 – 5 |
| Segment neutrophils count (%) |
59.6 ± 17.3 |
40 – 70 |
| Lymphocytes count (%) |
28 ± 14 |
20 – 50 |
| Monocytes count (%) |
9.5 ± 4.2 |
4 – 8 |
*Data presented as n (%) or mean ± standard deviation.
WBC = white blood cell; atotal confirmed cases/total reported cases; bSource:Harrison’s manual of medicine, ed.17, 2009
Table 1: Demographic characteristics, initial clinical features and simple laboratory results of the patients*.
The mean hematocrit level was 39.49 ± 5.53%, indicating that there were no plasma leakages in early phase of illness (Table 1). Increased monocytes count showed infection process in the early phase of dengue illness. This result was appropriate with monocytes’ role in the immune system. Monocytes are one of the major target cells of dengue virus infection. In the early phase of illness, the mean platelet count was 157,895±82,803 /μL and total white blood cell (WBC) count was 6,141 ± 3,834/μL.
Risk factors of dengue illness
The bivariate association of independent variables and dengue illness was shown in Table 2. All variables with p value < 0.25 were considered for multivariate analysis. Clinical symptoms and signs of dengue illness was also considered in determining dengue illness risk factors in the multivariate analysis [13,18,30,31]. This study exclude haemoglobin and segment neutrophil in the analysis because of co-linearition and strong correlation with hematocrit level (r=0.90).
| Clinical and hematological characteristics |
Presumptive model* |
Probable model˜ |
| OR (95% CI) |
p value |
OR (95% CI) |
p value |
| Day of fever |
1.08 (0.26 ; 4.51) |
0.90 |
6.28 (0.91 ; 43.33) |
0.06 |
| Nausea/vomiting/abdominal pain |
1.01 (0.23 ; 4.28) |
0.98 |
0.75 (0.06 ; 8.09) |
0.82 |
| Positive torniquet test |
1.30 (0.24 ; 6.93) |
0.75 |
0.28 (0.00 ; 10.41) |
0.49 |
| Spontaneous bleeding |
5.5 (0.67 ; 45.13) |
0.11 |
0.77 (0.01 ; 39.08) |
0.89 |
| Retro-orbita pain |
2.07 (0.53 ; 8.02) |
0.29 |
2.28 (0.19 ; 26.13) |
0.50 |
| Myalgia |
0.66 (0.22 ; 1.43) |
0.45 |
0.15 (0.01 ; 1.29) |
0.08 |
| Arthralgia |
0.87 (0.33 ; 2.24) |
0.77 |
2.37 (0.32 ; 47.14) |
0.39 |
| Headache |
0.83 (0.23 ; 2.91) |
0.77 |
0.95 (0.12 ; 7.45) |
0.96 |
| Hemoglobin (g/dL) |
0.99 (0.99 ; 1.00) |
0.96 |
0.98 (0.95 ; 1.01) |
0.34 |
| Hematocrit (%) |
1.00 (0.99 ; 1.00) |
0.92 |
1.00 (0.94 ; 1.06) |
0.55 |
| Total WBC count (/mm3) |
0.09 (0.02 ; 0.30) |
0.00 |
2.76 (0.33 ; 22.56) |
0.34 |
| Platelet count (/mm3) |
0.99 (0.98 ; 0.99) |
0.00 |
1.49 (0.34 ; 6.57) |
0.59 |
| Band neutrophil count (%) |
1.40 (0.94 ; 2.08) |
0.09 |
0.98 (0.92 ; 1.05) |
0.71 |
| Segement neutrophil count (%) |
0.96 (0.93 ; 0.99) |
0.03 |
0.92 (0.81 ; 1.05) |
0.25 |
| Lymphocyte count (%) |
0.00 (3.03 ; 2.20) |
0.07 |
0.79 (1.27 ; 4.93) |
0.97 |
| Monocyte count (%) |
1.70 (0.83 ; 3.45) |
0.14 |
1.40 (0.34 ; 5.80) |
0.63 |
| Anti dengueIgM |
|
|
0.40 (0.03 ; 5.03) |
0.48 |
| Anti dengueIgG |
|
|
5.86 (0.43 ; 78.43) |
0.18 |
| NS-1 antigen |
|
|
67.18 (4.09 ; 1102.97) |
0.00 |
*Presumptive model is a model consisting of significant clinical and hematological variables for dengue diagnosis; ˜Probable model is a model consisting of significant clinical, hematological, and serology laboratory variables for dengue diagnosis.
OR = Odd ratio; 95% CI = 95% confidence interval; p value of bivariate analysis
IgM = immunoglobulin M; IgG = immunoglobulin G; NS-1 = nonstructural-1
Table 2: Clinical dan hematological characteristics of the presumtive and probable dengue models.
The NS-1 and IgG-IgM variables were added in the bivariate and multivariate analysis to develop the scoring model of probable dengue diagnosis. A Roctab analysis was used to determine the cut-off point of any numeric variables, such as days of fever, total WBC, monocytes, segment neutrophil, lymphocytes, and platelet count (Table 3).
| |
Cut-off |
p value |
OR (95% CI) |
Sen% |
Spe% |
LR (+) |
LR (-) |
| Day of fever |
2 |
0.11 |
2.25 (0.69 ; 7.07) |
96,67 |
0 |
0,96 |
- |
| Hematocrit (%) |
40.4 |
0.09 |
2.42 (0.77 ; 8.40) |
43,33 |
76 |
1,80 |
0,74 |
| Total WBC count (/mm3) |
4,300 |
0.00 |
0.61 (0.77 ; 0.42) |
50.00 |
12 |
0,56 |
4,16 |
| Monocyte count (%) |
9 |
0.01 |
3.36 (1.15 ; 10.30) |
65.00 |
48 |
1,25 |
0,72 |
| Band neutrophil count (%) |
1 |
0.04 |
2.83 (0.89 ; 9.93) |
50,91 |
72 |
1,81 |
0,68 |
| Lymphocyte count (%) |
46 |
0.01 |
3.36 (1.15 ; 10.30) |
65.00 |
48 |
1,25 |
0,72 |
| Platelet count (/mm3) |
150,600 |
0.03 |
2.85 (0.98 ; 8.56) |
38,33 |
36 |
0,59 |
1,71 |
Sen = sensitivity, Spe = specificity, LR (+) = likelihood ratio positive; LR (-) = likelihood ratio negative
WBC = white blood cells
Table 3: Cut-off point of numeric variables related to the incidence of dengue illness.
Using Roctab analysis method, prediction of presumptive and probable dengue illness were obtained with total score ≥ 14 and ≥ 7, respectively. With these total score, presumptive model had sensitivity and specificity of 79.7% and 60% respectively (Figure 1), and probable model had sensitivity and specificity of 79.7% and 68% respectively (Figure 2). The presumptive and probable models showed good performace as area under the curve (AUC) were 0.81 and 0.86 respectively (Figures 1 and 2). These results showed the dengue scoring model was a good predictor for dengue illness.
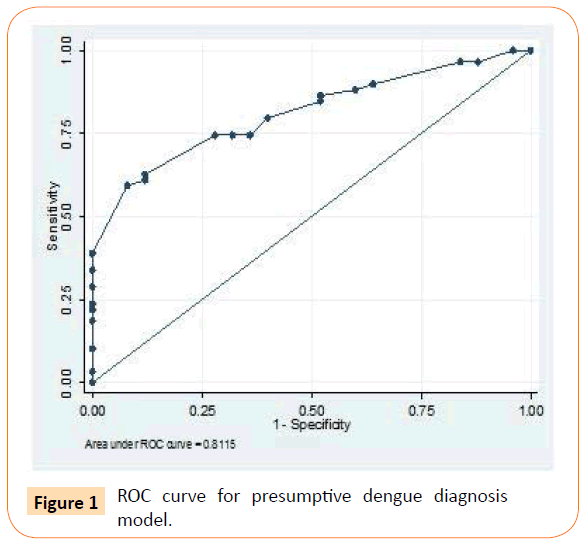
Figure 1: ROC curve for presumptive dengue diagnosis model.
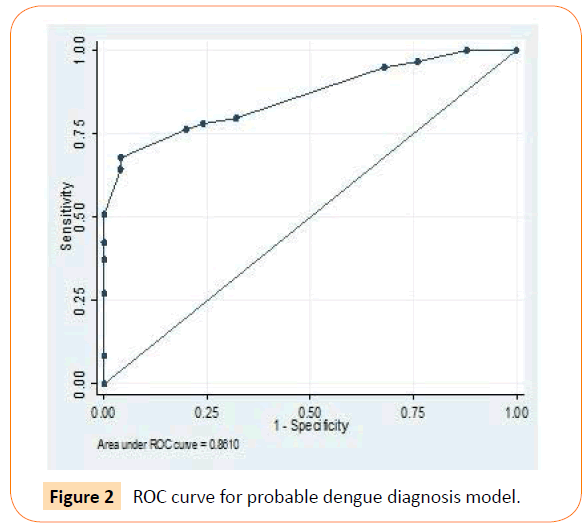
Figure 2: ROC curve for probable dengue diagnosis model.
For the final step, the dengue diagnosis scoring model, the 1997, 2009 and 2011 WHO classification were used on all study samples. NS-1 antigen detection and/or RT-PCR positive results was used as a gold standard to assess the validity of the dengue diagnosis scoring model. Compared to the gold standard examination, the 2009 WHO classification and probable model had sensitivity of 89.7% and 74.1% respectively, and specificity of 64% and 64% respectively. These results were higher than other dengue diagnostic tests results. Also the likelihood positive and negative ratio of these two tests were higher than other tests (2.60 and 6.21 for 2009 WHO classification, 2.20 and 2.50 for probable model). The results of this step are summarized in Tables 4 and 5.
| Presumptive model |
Probable model |
| Predictors |
Criteria |
OR (95% CI) |
p value |
Score^ |
Predictors |
Criteria |
OR (95% CI) |
p value |
Score^ |
| Clinical features |
| Day of fever |
≥ 2 |
3.75 (0.81 ; 15.70) |
0.09 |
8 |
Day of fever |
≥ 2 |
6.48 (1.06 ; 39.42) |
0.04 |
4 |
| < 2 |
0 |
< 2 |
0 |
| Torniquet test |
Positive |
1.54 (0.21 ; 11.29) |
0.66 |
5 |
Torniquet test |
Positive |
0.15 (0.00 ; 2.60) |
0.19 |
1 |
| Negative |
0 |
Negative |
0 |
| Myalgia |
Yes |
0.28 (0.05 ; 1.33) |
0.11 |
0 |
Myalgia |
Yes |
0.27 (0.04 ; 1.63) |
0.15 |
0 |
| No |
-4 |
No |
-2 |
| Simple laboratory |
|
| Monocyte (%) |
> 9 |
5.00 91.52 ; 16.47) |
0.00 |
10 |
Monocyte (%) |
> 9 |
1.97 (0.49 ; 6.50) |
0.37 |
3 |
| ≤ 9 |
0 |
≤ 9 |
0 |
| WBC (/mm3) |
≤ 4,300 |
7.38 (0.51 ; 36.03) |
0.01 |
10 |
NS-1 antigen |
Positive |
67.70 (5.49 ; 834.89) |
0 |
6 |
| > 4,300 |
0 |
Negative |
0 |
| Platelet (/mm3) |
≤ 150,600 |
1.38 (0.38 ; 4.93) |
0.61 |
5 |
|
|
|
|
|
| > 150,600 |
0 |
|
|
|
|
|
^Total score for presumptive model: ≥ 14; and probable model: ≥ 7
OR = Odd ratio; 95% CI = 95% confidence interval; p value of ROC analysis
WBC = white blood cell; NS-1 = non structural 1
Table 4: Scoring model of dengue diagnosis in the early phase of illness.
| |
NS-1 (+) and/or RT-PCR (+)* |
| |
Sen (%) |
Spe (%) |
PPV |
NPV |
LR (+) |
LR (-) |
| Presumptive model |
70,7 |
52 |
77,4 |
43,3 |
1,47 |
1,77 |
| Probable model |
74,1 |
64 |
82,7 |
51,6 |
2,20 |
2,50 |
| 1997 WHO classification |
56,9 |
48 |
71,7 |
32,4 |
1,09 |
1,11 |
| 2009 WHO classification |
89,7 |
64 |
85,2 |
72,7 |
2,60 |
6,21 |
| 2011 WHO classification |
51,7 |
56 |
78.0 |
42,4 |
1,17 |
1,15 |
*Detection of NS-1 dengue antigen and/or the presence of dengue virus using RT-PCR method; Sen = sensitivity, Spe = specificity, PPV = positive predictive value, NPV = negative predictive value, LR (+) = likelihood ratio positive; LR (-) = likelihood ratio negative
Table 5: Comparison of diagnostic value of the dengue diagnosis scoring model and WHO classification to NS-1 and RT-PCR.
Discussion
The clinical features of early phase of dengue illness such as headache, nausea/vomiting/abdominal pain were also found in non-dengue illness. These clinical features were also found in the previous study in 2004 and in a study by Daumas et al. [9,32] on the other hand, a study by Basuki et al. [24] showed that only 22-23% of the study participants had those complaints. Our study did not perform an ultrasound exam that could detect the presence of ascites or gall bladder edema as the cause of most gastrointestinal symptoms. Positive torniquet test was only detected in a few samples, and spontaneous bleeding was found only in 14 samples (Table 1) because the data were taken at the early phase of illness and most of dengue diagnosis was dengue fever or dengue without warning sign. Although an increased hematocrit level of 20% or more is the primary predictor to distinguish dengue fever with dengue haemorrhagic fever, this variable could not be entered into the scoring model because plasma leakage commonly occurs on 4th to 7th day of fever. Plasma leakage was proved by the presence of pleural effusion on chest x-ray and ultrasound, or hypoalbuminemia that were not done in this study [33,34]. Also there were many factors that affected hematocrit value such as fluid therapy, bleeding, anemia, etc.
This newly developed dengue scoring model used clinical symptoms and signs, and simple laboratory results that could be done in almost all primary health cares in Indonesia. Monocytosis (monocytes count above 9%) has become important predictor of immune response in the early phase of dengue illness. This result was also in line with other study by Khan et al. and Chan et al. [35,36].
There were different variables between presumptive and probable models because NS-1 dengue antigen test is a specific predictor for dengue illness. The high value of likelihood positive ratio abled the model to make a diagnosis with certainty and reduce false positives, making it a good tool in diagnosing dengue illness (p = 0.00). This model also had good validity compared to the 1997 and 2011 WHO classification. Dengue diagnosis scoring model was developed as a screening tool for the early phase of dengue illness, but not to rule of the illness. Unlike the WHO classification, the newly developed scoring model could detect dengue illness without increased hematocrit level, which is not always easy to obtain in primary care. The limitations of this study were sample collection was limited in adult patients who came to the health care facilities in a dengue endemic areas in our country. Validity and performance of the model would differ when applied to different populations. Clinicians should be cautious in applying the results of this study in daily practice. Further study involving samples from different settings is needed to generate representative data. The precision of the model in this study led to the lower reliability of the research results. It is possible that there was selection bias that potentially limits the ability to generalize it to dengue patients. The internal validity of the scoring model also considered clinical symptoms and signs which were related to the natural history of dengue illness.
In conclusion, this scoring model was able to distinguish dengue and non-dengue illness to the extent probable dengue. This scoring model was easy to implement, that it could help clinicians to determine the diagnosis of patients with acute febrile illness in the early phase and its appropriate treatment strategies.
Acknowledgement
This study was funded by Pelita Harapan University. We are grateful to Muliyanah Daya for collecting and compiling data over the study period. We thank to Prodia laboratory for their support in this study; Primary Health Cares in Tangerang and Siloam Hospital Lippo Village for samples collection.
Conflicts of Interest
The authors declare no potential conflict of interest.
7469
References
- Simmons CP, Farrar JJ, Nguyen vV, Wills B (2012) Dengue. N Engl J Med 366: 1423-1432.
- Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH (2011) Economic impact of dengue illness in the Americas. Am J Trop Med Hyg 84: 200-207.
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. (2013) The global distribution and burden of dengue. Nature 496: 504-507.
- WHO (2009) Dengue: Guidelines for diagnosis, treatment, prevention and control. New edition 2009. Geneva: World Health Organization.
- Ferreira GL (2012) Global dengue epidemiology trends. Rev Inst Med Trop Sao Paulo 54 Suppl 18: S5-6.
- Karyanti MR,Uiterwaal CS, Kusriastuti R, Hadinegoro SR, Rovers MM, et al. (2014) The changing incidence of dengue haemorrhagic fever in Indonesia: a 45-year registry-based analysis. BMC Infect Dis 14: 412.
- Setiati TE, Wagenaar J FP, de Kruif MD, Mairuhu A TA, van Gorp E CM, et al. (2006) Changing epidemiology of dengue hemorrhagic fever in Indonesia. Dengue Bull 30: 1-14.
- WHO. Situation of dengue/dengue haemorrhagic fever in the South-East Asian Region. Available: https://www.searo.who.int/en/section 10/section 332_1098.htm.
- Cucunawangsih (2004) IgM-IgG rapid immunochromatography diagnostic test and determination of dengue serotype using reverve transcriptase-polymerase chain reaction. Thesis.
- Prasetyo DS, Angky BA, Dewi BE, Cucunawangsih, Chandra R, et al. (2011) Association between dengue virus serotypes and type of dengue viral infection in Departement of Child Health, CiptoMangunkusumo Hospital, Jakarta, Indonesia. Dengue Bull 35: 205-213.
- Oishi K, Saito M, Mapua CA, Natividad FF (2007) Dengue illness: clinical features and pathogenesis. J Infect Chemother 13: 125-133.
- Phuong HL, de Vries PJ, Nga TT, Giao PT, Hung le Q, et al. (2006) Dengue as a cause of acute undifferentiated fever in Vietnam. BMC Infect Dis 6: 123.
- Kularatne S, Gawarammana IB, Kumarasiri PRV (2005) Epidemiological, clinical features, laboratory investigations and early diagnosis of dengue fever in adults: a descriptive study in Sri Lanka. Southeast Asian J Trop Med Public Health 35: 686-692.
- Chadwick D, Anis B, Wilder-Smith A, Paton N (2006) Distinguish dengue fever from other infections on the basis of simple clinical and laboratory feature: application of logistic regression analysis. J ClinVirol 35: 147-158.
- Chaterji S, Allen JC Jr, Chow A, Leo YS, Ooi EE (2011) Evaluation of the NS1 rapid test and the WHO dengue classification schemes for use as bedside diagnosis of acute dengue fever in adults. Am J Trop Med Hyg 84: 224-228.
- Setiati TE, Mairuhu ATA, Koraka P, Supriatna M, Mac Gillavry MR, et al. (2007) Dengue disease severity in Indonesia children: an evaluation of the World Health Organization classification system. BMC Infection Diseases 7.
- Dutra NR, de Paula MB, de Oliveira MD, d e Oliveira LL, De Paula SO (2009) The laboratorial diagnosis of dengue: applications and implications. J Glob Infect Dis 1: 38-44.
- Potts JA, Rothman AL (2008) Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health 13: 1328-1340.
- Kao CL, King CC, Chao DY, Wu HL, Chang GJ (2005) Laboratory diagnosis of dengue virus infection: current and future perspectives in clinical diagnosis and public health. J MicrobiolImmunol Infect 38: 5-16.
- Sekaran SD, SubramaniamEwCL, Kanthesh MB (2009) Sensitivity of dengue virus NS-1 detection in primary and secondary infections. African Journal of Microbiology Research 3: 105-110.
- Hunsperger EA, Yoksan S, Buchy P, Nguyen VC, Sekaran SD, et al. (2014) Evaluation of commercially available diagnostic tests for the detection of dengue virus NS1 antigen and anti-dengue virus IgM antibody. PLoSNegl Trop Dis 8: e3171.
- Suaya JA, Shepard DS, Siqueira JB, Martelli CT, Lum LC, et al. (2009) Cost of dengue cases in eight countries in the Americas and Asia: a prospective study. Am J Trop Med Hyg 80: 846-855.
- Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, et al. (2006) The WHO dengue classification and case definitions: time for a reassessment. Lancet 368: 170-173.
- Basuki PS, Budiyanto, Puspitasari D, Husada D, Darmowandono W, et al. (2010) Application of revised dengue classification criteria as a severity marker of dengue viral infection in Indonesia. Southeast Asian J Trop Med Public Health 41: 1088-1094.
- Guzman MG, Vázquez S, Kouri G (2009) Dengue: where are we today? Malays J Med Sci 16: 4-11.
- Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV (1992) Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. Journal of Clinical Micriobiology 30: 545-551.
- Sudiro TM, Ishiko H, Green S, Vaugn DW, Nisalak A, et al. (1997) Rapid diagnosis of dengue viremia by reverse transcriptase-polymerase chain reaction using 3’-noncoding region universal primers. Am J Trop Med Hyg 56: 424-429.
- WHO (1997). Dengue hemorrhagic fever: diagnosis, treatment, prevention, and control. 2nd ed. Geneva. World Health Organization pp. 12-23, 45-46.
- Laksono IS (2012) Classification 2011: The dengue guidelines 1997, 2009, 2011: How they different. Paper presentation at one day symposium all about dengue. Jakarta, Indonesia.
- Mayxay M, Phetsouvanh R, Moore CE, Chansamouth V, Vongsouvath M, et al. (2011) Predictive diagnostic value of the tourniquet test for the diagnosis of dengue infection in adults. Trop Med Int Health 16: 127-133.
- Gregory CJ, Lorenzi OD, Colon L, Garcia AS, Santiago LM, et al. (2011) Utility of torniquete test and the white blood cell count to differentiate dengue among acute febrile illness in the emergency room. Plos Neglected Tropical Diseases 5(12): e1400.
- Daumas RP,Passos SR, Oliveira RV, Nogueira RM, Georg I, et al. (2013) Clinical and laboratory features that discriminate dengue from other febrile illnesses: a diagnostic accuracy study in Rio de Janeiro, Brazil. BMC Infect Dis 13: 77.
- Zulkarnain I (2004) Gallbladder edema in Dengue hemorrhagic fever and its association with haematocrit levels and type of infections. Acta Med Indones 36: 84-86.
- Chen K (2004) Haemostatic disorder in Dengue hemorrhagic fever. Acta Med Indones 36: 55-56.
- Khan E,Kisat M, Khan N, Nasir A, Ayub S, et al. (2010) Demographic and clinical features of dengue fever in Pakistan from 2003-2007: a retrospective cross-sectional study. PLoS One 5: e12505.
- Tsai JJ, Chan KS, Chang JS, Chang K, Lin CC, et al. (2009) Effect of serotypes on clinical manifestations of dengue fever in adults. J MicrobiolImmunol Infect 42: 471-478.







