Keywords
|
| Western ghats; Streptomyces; L-asparaginase; Anti leukemia |
Introduction
|
| India contains a great wealth of biological diversity in its forests, wetlands and marine area. India ranks sixth among the 12 mega biodiversity centers of the world, and is a home for an unusually large number of endemic species. The majority of earth’s microbes remain unknown, and that their potential utility cannot be exploited until they are discovered and characterized. They provide wide scope for the development of new strains as well as biotechnological uses. The Western Ghats are a chain of hills that run along the western edge of peninsular India. Western Ghats of India are one of the thirty four biodiversity hotspots in the world known for their fauna and flora [1]. It’s a mystical land for microbiologists as few reports are document about microbial forms. The present study is concentrated on the isolation and characterization of actinomycetes isolates with L-asparaginase potential in Western Ghats regions of Agumbe, Karnataka, India. |
| Actinobacteria are well known for their ability to produce secondary metabolites and have proved to be a good source of antibiotics. Especially terrestrial Streptomycetes are isolated and screened for their biologically active metabolites including antibiotics, antitumor compounds, immunosuppressive agents and enzymes [2,3]. It has been reported that around 23,000 bioactive secondary metabolites are produced by microorganisms and over 10,000 of these compounds are from Actinomycetes [4]. Among these around 7,600 compounds are produced from Streptomyces species [5]. The rate of discovery of novel bioactive metabolites from terrestrial actinomycetes is decreasing with time, and new sources of therapeutic compounds are needed to be explored much attention has been focused on screening of actinomycetes from diverse environments [6]. In order to meet the increasing demand of new medicines for cancer due to its rapid development of resistance of existing drugs, search for new therapeutic enzymes is sought [7]. |
| Acute Lymphoblastic Leukemia (ALL) is one of the leading types of malignancies seen in mostly children’s between the ages of 2 to 4 years. ALL is a malignant disorder characterized by excessive production of Leukocytes that result in overcrowding of normal cell and spreads to other organs such as liver, spleen and lymph nodes. Exposure to chemical carcinogens, genetic factors and viral infections are some of the factors responsible for the causes of ALL [8]. |
| ASP is an effective chemotherapeutic agent that catalyzes the hydrolysis of L-asparagine. Human lymphoblast relies on exogenous asparagine, and ASP induces a relative asparagine deficiency, resulting in cell death. In ALL malignant cells are unable to synthesize the non-essential amino acid asparagine like normal cells so require high amount of asparagine. Leukemic cells depend on circulating asparagine. The enzyme L-asparaginase catalyzes the conversion of L-asparagine to aspartic acid and ammonia depriving the leukemic cell from circulating asparagine, which leads to cell death [9]. |
| The L-asparaginase from Escherichia coli and Erwinia cartovora are currently used as a first line of treatment against acute lymphoblastic leukemia. The prolonged administration of L-asparaginase leads to hypersensitivity reactions and produces corresponding antibodies against E. coli asparaginase, lead to inactivation of the drug effect or anaphylactic shock [10]. Therefore, there is a continuing need to screen microorganisms having potentiality for L-asparaginase which is serologically different with a similar therapeutic effect. |
| The search for therapeutic enzymes throughout the world stimulated a variety of different approaches for the isolation of therapeutic enzymes producing microbial strains. Among the actinomycetes, species of Streptomyces such as S. karnatakensis, S. venezuelae, and S. longsporusflavus are known to produce detectable amount of L-asparaginase. Still the actinomycetes are recognized as less explored source for L-asparaginase [11]. Hence, present inquest accords with the isolation and identification of a high L-asparaginase potential strain of actinomycetes from Western Ghats regions of Agumbe. |
Materials and Methods
|
|
Soil sampling
|
| The soil samples were collected from rhizosphere of plants from various locations of Agumbe province (Latitude 13.5087° N; Longitude 75.0959° E) in Western Ghats of Karnataka, India. The soil samples were collected from a depth of 12-15 cm from the soil surface in sterilized polyethylene zip lock covers and transported to the lab. Soil samples were air dried aseptically for 3 to 4 days to remove moisture [12]. Air dried soil samples were ground aseptically with pestle and mortar to make fine particles and sieved to remove gravel and debris for isolation of actinomycetes [13]. |
|
Isolation of actinomycetes from soil samples
|
| Actinomycetes were isolated from the soil sample by serial dilution plating technique. Soil dilutions were plated on to various media viz., Starch Casein Nitrate Agar (SCN) [14], Actinomycetes Isolation Agar (Himedia Laboratories, India) and Oat Meal Agar media [15], supplemented with Fluconazole 75 μg/ml and the plates were incubated at 30 ± 2°C for 7 to 10 days [16]. The colonies showing characteristics features of Actinomycetes (rough, chalky, powdery appearance, radiating growth and leathery texture etc.) were preserved on SCN agar slants at 4°C for further studies and were labeled as V1, V2, V3….. V25. Spore suspensions of purified isolates were also preserved in 10% glycerol (v/v) at -20°C for long term use [17]. |
|
Characterization of Actinomycetes isolates
|
| Isolated colonies were identified in accordance with the guidelines established by the International Streptomyces Project (ISP) described by Shirling and Gottileb. Morphological characterization of the isolated actinomycetes colonies was done by observing colony morphology, color of aerial and substrate mycelium, texture, pigmentation and appearance on the medium [15]. |
| Microscopic examination was performed by Coverslip culture method. Spore suspension of the actinomycetes isolate was inoculated on to the surface of Starch Casein Nitrate Agar blocks kept on sterile glass slide and covered with sterile coverslip and incubated in sterile humid chamber for 2 to 3 days and formation of aerial and substrate mycelium, spore chain arrangement on the mycelium of the isolates was studied by Gram’s Staining and Acid fast staining procedures under light microscope at 1000X magnification and identified up to genus level by referring to Bergey’s manual of determinative bacteriology [16,18,19]. |
| Biochemical characterization was done by performing tests such as starch hydrolysis, gelatin hydrolysis, casein hydrolysis, catalase test [20], oxidase test, H2S production test [21], nitrate reduction test, urease and melanin pigment production and carbohydrate utilization tests. (Dextrose, maltose, mannitol, D-xylose, fructose and starch) [22- 24]. |
|
Screening for L-asparaginase activity
|
| For the determination of L-asparaginase activity of actinomycetes isolates for the selection of potential strain, both qualitative and quantitative assay were performed. All the actinomycetes isolated were screened for their L-asparaginase activity by rapid plate assay method as described by Gulati et al. [25]. |
| Qualitative assay (Rapid Plate Method): This is a semi-qualitative assay to determine potentiality of actinomycetes having L-asparaginase activity. The isolates were streaked on to the modified M-9 media supplemented with L-asparagine as a sole source of carbon [The composition of M-9 medium is (g/L): Na2HPO4.2H20, 6; KH2PO4, 3; NaCl 0.5; L-asparagine, 5; 1M MgSO4.7H20, 2 ml; 0.1M CaCl2 2H20, 1 ml; 20% Glucose stock solution 10 ml, Agar, 20 and pH 7.0 in distilled water to 1 L] with phenol red (2.5%) as pH indicator, for the growth and incorporated with phenol red as pH indicator and incubated at 30 ± 2°C for 7 days [26]. The active strains cleave L- asparagine to L aspartic acid and ammonia leading to alkaline pH which shows change in color of medium from yellow to pink. Colonies with pink zone were considered positive strains for L-asparaginase and selected for further studies [25,27]. |
| Quantitative assay (Nesslerization method): Quantitative estimation of L-asparaginase activity was carried out for active strains identified from rapid plate assay. The test strains of actinomycetes were inoculated into Erlenmeyer flasks (250 ml) containing 100 ml of M9 medium (pH 7.0) and incubated at 30 ± 2°C in a controlled shaker incubator at 250 rpm for 7 days. Medium without inoculum was kept as control. The culture broth was filtered using Whatman filter paper No.1 and centrifuged at 10,000 rpm for 15 min. The supernatant obtained was used as crude extract of L-asparaginase. The growth of actinomycetes was measured as dry weight and the L-asparaginase activity was measured as the rate of hydrolysis of L-asparagine by measuring the ammonia released in culture filtrate by Nesslerization and expressed as IU/ml [28]. |
| Assay of L-asparaginase activity was initiated by adding 0.1 ml of enzyme extract to 0.2 ml of 0.05M Tris-HCl buffer (pH 8. 6), and 1.7 ml of 0.01M L-asparagine and incubated for 10 min at 37°C. The reaction was stopped by the addition of 0.5 ml of 1.5 M trichloroacetic acid (TCA) and precipitated protein was removed by centrifugation at 10,000 rpm. 0.5 ml of the supernatant was diluted to 7 ml with distilled water and treated with 1 ml of Nessler’s reagent and incubated for 10 min at room temperature. The absorbance was recorded at wavelength of 450 nm with UV/Visible Spectrophotometer (ELICO SL159, India). The amount of ammonia liberated in the reaction mixture was extrapolated from the ammonium sulphate standard curve. The reaction mixture from each strain was assayed in triplicates [23,29]. The enzyme activity was expressed in IU. One international unit of L-asparaginase activity is the amount of enzyme which catalyzes the formation of 1 μmole of ammonia per ml per minute (μ mole/ml/min) under the condition of the assay [30]. Based on the results obtained in Qualitative assay for the enzyme, potential actinomycetes strain with high unit of enzyme activity (IU) was chosen for further study [31,32]. |
Results and Discussion
|
|
Collection of soil samples
|
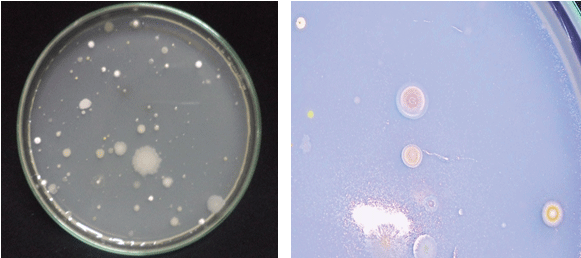
| Actinomycetes are one the major components of the microbial population present in soil. They form important class of bacteria present in rhizosphere and known to influence the growth of plant [32]. Since soil is major repository of microorganisms, in this study 15 rhizosphere soil samples were collected at different locations from forest areas of Agumbe, Western Ghats of Karnataka and processed for isolation. Actinomycetes are the most biotechnologically valuable prokaryotes responsible for the production of bioactive secondary metabolites with biological activities such as antibiotic, antifungal, antiviral, anticancer enzyme, immunosuppressant and other industrially useful compounds [33]. Previous studies have shown that diverse group of Actinomycetes are present in rhizosphere soil rich in organic matter [34]. Actinomycetes have been isolated and studied in several unexplored environments, niche, and extreme habitats in various parts of the world (including India) in the last few years [35]. But still so many different areas have to be explored to find novel species and fewer reports are documented regarding isolation of L-asparaginase active actinomycetes from Western Ghats of Karnataka. It has been reported that Actinomycetes dwelling in the terrestrial environments are diverse and unique in their ability to produce different chemical entities useful to humans [36]. With this insight, for isolation of promising actinomycetes in the current study, soil samples from forest area away from human intervention, rich in organic matter were chosen from rhizosphere region in Agumbe province. |
|
Isolation and characterization of Actinomycetes isolates
|
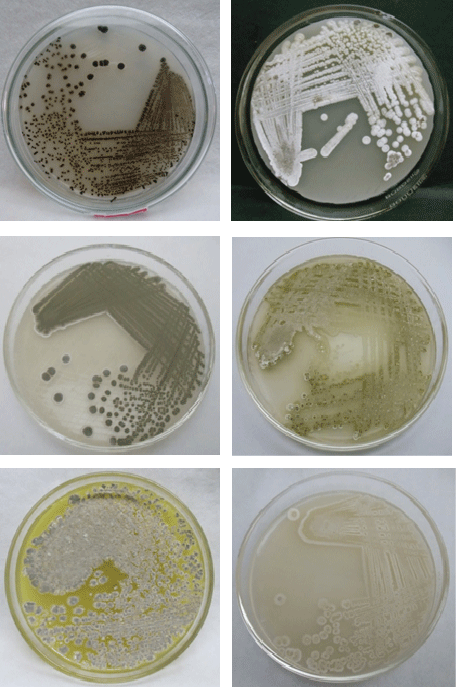
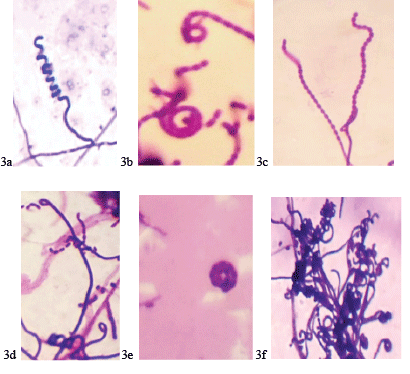
| A total of 25 actinomycetes were isolated based on the colony morphology from 15 soil samples collected and were designated as V1, V2, and V3…V25 isolates (Figure 1). The actinomycetes isolates were morphologically, biochemically characterized following classical taxonomical pathways with Bergey’s manual of determinative bacteriology [37] as summarized in Table 1 and Figure 2. Studies carried out on morphological (Table 1), biochemical characterization [37] (Tables 2 and 3) and sporulation pattern as observed with the aid of microscope by coverslip method (Figure 3), it has been confirmed that out of 25 isolated actinomycetes strains 24 strains (96%) were found to be the members belonging to Streptomyces genera with varied patterns of spore chain arrangements (Figure 3). |
| Similar kind of studies carried out by researchers revealed that members of Streptomyces were abundant and accounted for about 90% of actinomycetes isolated from rhizoshpheric soil samples [38]. It has been reported by the researcher that morphology plays an important role in characterization of Streptomyces species along with biochemical features and assist in classifying members of actinomycetes to Streptomyces genera, based on spore chain arrangement Gautham et al. [39] reported that SCN media yielded high number of actinomycetes of about 42 isolates followed by ISP media. |
| The morphological characteristics were found to be divers with varied colony morphology, color of aerial and substrate mycelium, surface texture with diverse range of chromogenic metabolites. The varied patterns of spore chain arrangements such as rectus, flexibilis, retinaculum aperatum, spira and mono-verticillus spira have been observed by coverslip studies (Table 1 and Figure 3) and all the isolates were found to be Gram positive and Nonacid fast by microscopic studies. |
| Studies on biochemical characterization of the isolates exhibited varied biochemical potentialities. All the isolates were catalase positive. Only 5 isolates were positive for H2S production. 12 isolates were positive for casein hydrolysis. 20 isolates hydrolyzed starch and 19 isolates hydrolyzed gelatin and positive for oxidase. Nitrate reduction was found be positive in 14 isolates. Only 8 isolates were positive for Urease production. 21 isolates exhibited melanin pigment production on L-tyrosine agar media (Table 2). |
| Carbohydrate utilization test showed diverse results with respect to acid, gas and alkali production. Most of the isolates were found to be positive for alkali production in all the sugars tested, and was considerably high in disaccharides and polysaccharides (Table 3). |
|
Screening of L-asparaginase activity
|
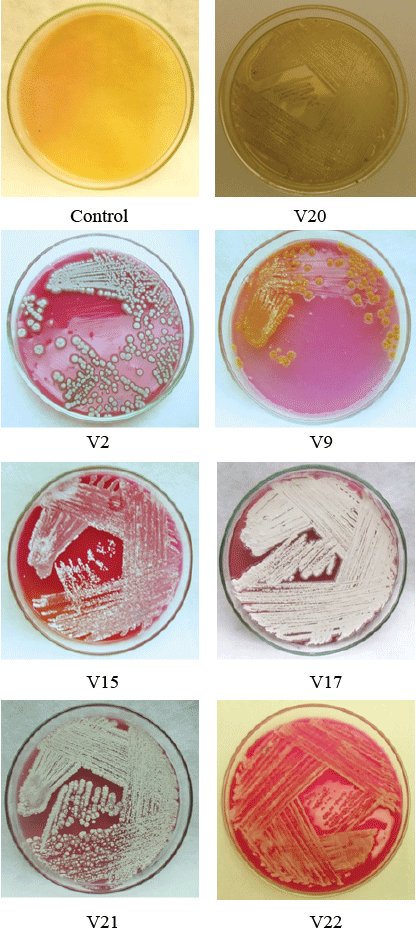
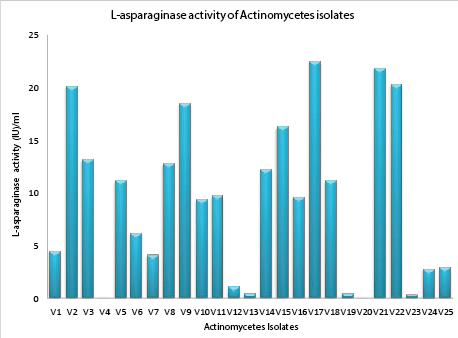
| Qualitative assay: Rapid plate assay is a semi quantitative simple and quick method for the detection of L-asparaginase production from actinomycetes by direct visualization of plates. Out of 25 isolated actinomycetes screened for L-asparaginase activity, 23 isolates showed positive results exhibiting pink zones (Figure 4) in rapid plate assay except the strains V4 and V20. All the positive strains were considered as active strains and when analyzed further for asparaginase enzyme activity, active strains V2, V9, V15, V17, V21, and V22 (Figure 2) showed highest activity as shown in Figure 4. |
| It has been documented earlier by the researchers about biological potency of Streptomyces species isolated from soil of Agumbe. Gautham et al. [39] reported Streptomyces GOS 01 isolate has exhibited capacity to produce protease, amylase and gelatinase enzymes, but reports on L-asparaginase ability of actinomycetes have not been documented. Studies on L- asparaginase biosynthesis in actinomycetes isolates were carried out to avoid the hypersensitive effect produced from bacteria. It has been shown that isolates from Egyptian soil have ability to produce L-asparaginase, especially the strain Streptomyces halstedi [40]. Jayam et al. [41] reported that out of 8 colonies isolated from Periyar Lake, Kumily only 4 isolates are found to be positive (50%) for L-asparaginase activity. |
| In this view present study revealed that Agumbe soil is a rich source for L-asparaginase active Streptomycetes, as 92% of isolates were found to be positive in rapid plate assay and was found to be higher when compared to previous reports available. L-asparaginase activity of the active strains was further quantified for the selection of a potential strain among them. |
| Qualitative assay for L-asparaginase: Amount of L-asparaginase enzyme production by active strain was determined by qualitative assay by Nesslerization. It was revealed that enzyme productivity of 23 active strains studied was ranging from 0.276 IU/ml (lowest) to 22.45 IU/ml (highest) as indicated in Figure 5. Six isolates V2 (20.11 IU/ml), V9 (18.45 IU/ml), V15 (16.22 IU/ml), V17 (22.45 IU/ml), V21 (22.82 IU/ ml), and V22 (20.30 IU/ml) found to have produced higher amounts of L-asparaginase compared to other isolates studied. The strain V17 exhibited high productivity of 22.45 IU/ml. |
| Previous studies on the analysis of L-asparaginase production, reported that the maximum production of L-asparaginase was 11.0 IU/ml at optimum condition by Streptomyces albioflavus [11]. Similar kind of scientific reports by Meena in 2015 [42] indicated that, potent actinomycetes isolate Streptomyces griseous exhibited L-asparaginase productivity of 5.361 IU/ml. Hence the strain V17 was selected as the potential strain for further studies [43]. |
Conclusion
|
| Findings of the present study conclude that Agumbe is the potential ecosystem for L-asparaginase active actinomycetes which deserves for bio prospecting. Isolated L-asparaginase appears to be a promising agent and requires further investigation of its potential antileukemic activity. Enzymes which degrade amino acids should receive greater attention as potential therapeutic agents. Further studies are under progress to study antioxidant and anti-cancerous properties of L-asparaginase purified from the potent strain, in cancerous cell lines. |
Tables at a glance
|
|
|
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|











