Introduction
Leptospirosis is a disease of animals and humans which has a worldwide distribution [17]. It is also emerging as important public health problem [14]. Leptospirosis is caused by pathogenic spirochetes of the genus Leptospira, belongs to the family Leptospiraceae and order spirochaetales and is manifested by different syndromes including haemolytic crisis, chronic interstitial nephritis, decreased milk production, mastitis, death of young adults due to haemolytic aneamia and mastitis.
Leptospirosis is also an important worldwide cause of abortions, still births and reproductive failure in cattle and pigs in particular and there is an increasing prevalence of leptospirosis in humans resulting from contact with infected livestock. In humans the disease ranges from sub-clinical infection to sever syndromes of multi organ infection with high mortality. The seroprevalence of leptospirosis was reported from Tamilnadu [8, 4]; Kerala [1]; Karnataka [15]; Andaman [13]; Uttar Pradesh [2]; North eastern states [7]; Haryana [5]; Orissa [16]; Uttaranchal [10] and from West Bengal [9]. The disease has been reported earlier from Andhra Pradesh [11, 4]. The endemicity of the disease in Tamilnadu, Kerala & Karnataka, the adjoining states of Andhra Pradesh and inadequate information on leptospirosis in animals in Andhra Pradesh made us to study the seroepidemiology of leptospirosis among various species of animals and humans in different districts of Andhra Pradesh. Hence seroepidemiological study was conducted species wise and data was analyzed and presented during the investigation.
Materials and Methods
A total of 2,320 serum samples were collected randomly from apparently healthy animals of different species includes Bovine (1499), sheep (316), goats (205), Pigs (133), dogs (99) were collected from different districts of Andhra Pradesh during the period 2006-2010. Similarly 258 serum samples were also collected from clinically suspected cases of Leptospirosis showing pyrexia and abortions in cattle (38), sheep showing fever, haemoglobinurea and abortions (83) Pigs having pyrexia and abortions (35), Dogs showing pyrexia, anorexia, hepatic dysfunction, jaundice (34) and from Humans with fever, jaundice, renal failure (68) and processed during the period of study. All the serum samples were inactivated at 56oC for 30 minutes and stored at – 200C till used for analysis.
Microscopic agglutination test (MAT)
Serum samples were tested by using Microscopic agglutination test (MAT) according to the method followed at regional Medical research centre (RMRC), Port Blair, Andaman and Nicobar Islands. The serum samples were screened using a panel of 9 live leptospiral reference serovars namely L. autumnalis, L. canicola, L. grippotyposa, L.icterohaemorragae, L.hardjo, L.hebdomedis, L.javanica, L. pomona and L.patoc, received from RMRC, port Blair, Andaman and Nicobar islands and maintained in our laboratory.
Leptospira reference antisera
Reference antiserum was procured from Regional Medical research center, Andaman & Nicobar Islands, Port Blair are presented in the table.1 was used for testing antigens.
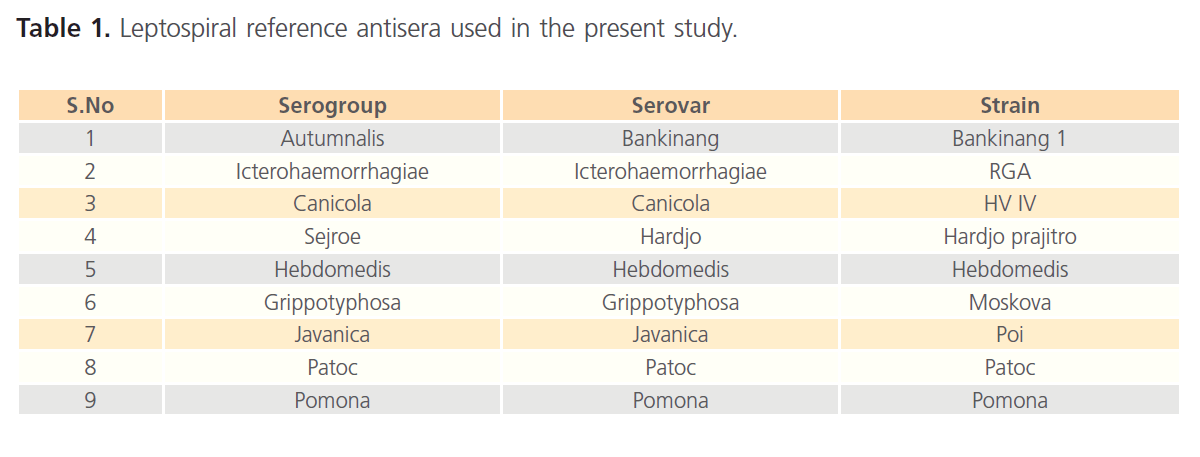
Table 1: Leptospiral reference antisera used in the present study.
Preparation of antigen for MAT
The reference serovars strains were grown in Ellinghausen, McCullough, Johnson and Harris (EMJH) liquid medium. The cultures were incubated at 300C for 5-7 days and examined for the growth of the organisms. The live cultures with density of 2-3 x 109organisms/ml were used as antigens. Reactivity of the cultures for the MAT was tested using the homologous antisera. The panel of antigens used in MAT was presented in Table 2.
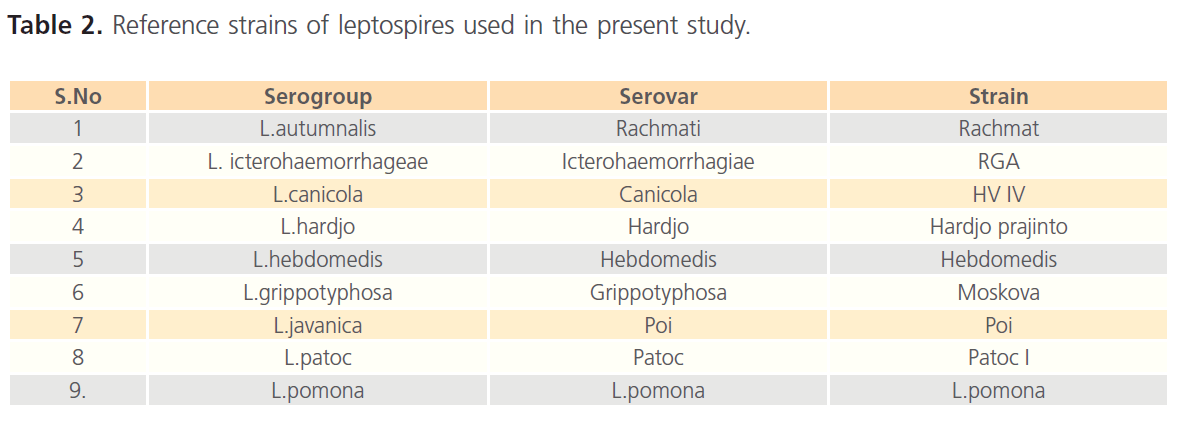
Table 2: Reference strains of leptospires used in the present study.
Method for conducting MAT
The protocol followed at Regional Medical research center (RMRC), WHO collaborating center for diagnosis, reference and training on leptospirosis, Port Blair, Andaman and Nicobar islands was used for conducting MAT. In brief the MAT was carried out in the U shaped microtitre plate consisting of 96 wells. 50ul of PBS added to all wells except 2nd column in which 90ul was taken. Then 10ul of serum samples were added to all the wells of 2nd column of plate mixed thoroughly and transferred 50ul to 3rd column in the same row and so on. Dilute serially and discard 50ul from the last well, which gives the dilution 1:10, 1:20, 1:40, 1:80, 1:160 and so on. Then added 50ul of fresh leptopsiral culture with a density of 2-3 x 109 organisms/ml to all the wells of the plate. Mixed thoroughly in shaker and incubated at 370C for 2 hours. Later serum antigen mixtures from each dilution were examined fewer than 20x dark field microscope. The reciprocal of the highest dilution of the serum where 50 % agglutination of leptospires occurs was considered as MAT titer. Test samples were compared with known positive and negative controls. The cutoff titer to differentiate positive and negative serum samples was determined using known positive and negatives serum samples collected from Andhra Pradesh. MAT titer of 1:80 was found optimum to differentiate positive and negative samples. Hence, MAT titer of 1:80 is considered as cut off point to differentiate positive and negative samples used in the study (Fig. 1).
Figure 1: Results of microscopic agglutination test.
Results
A total of 2,320 serum samples collected randomly from apparently healthy animals of different species Viz. cattle, buffaloes, sheep, goats, pigs, dogs, 485 (20.9 %) (Table 3) samples were positive for leptospiral antibodies. Similarly of the 258 serum samples from clinically suspected cases of bovine, sheep, pigs, dogs and humans, 99 samples (38.37 %) were found positive for antibodies to leptospira on MAT (Table 4).
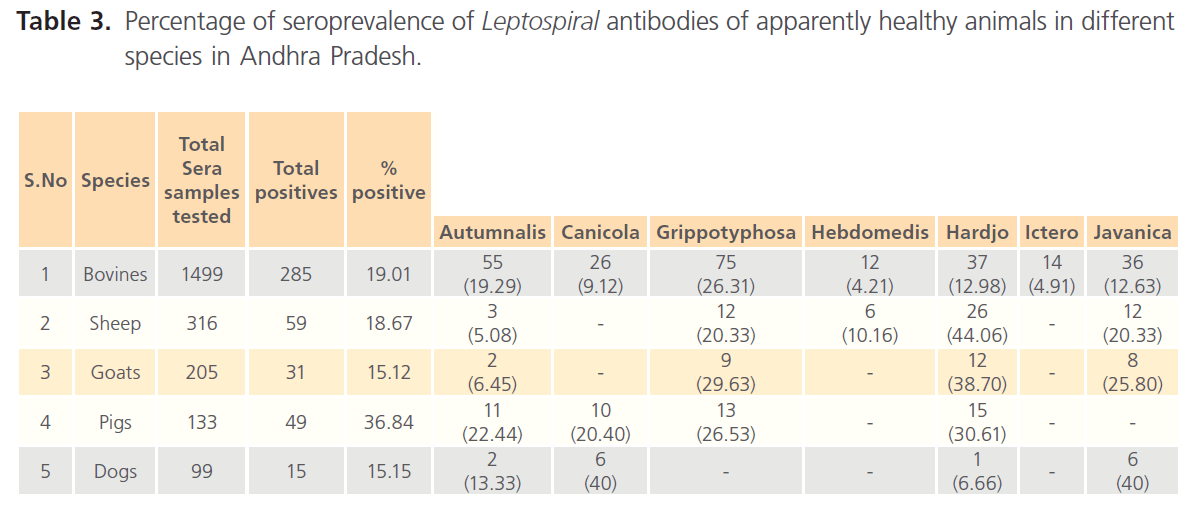
Table 3: Percentage of seroprevalence of Leptospiral antibodies of apparently healthy animals in different species in Andhra Pradesh.
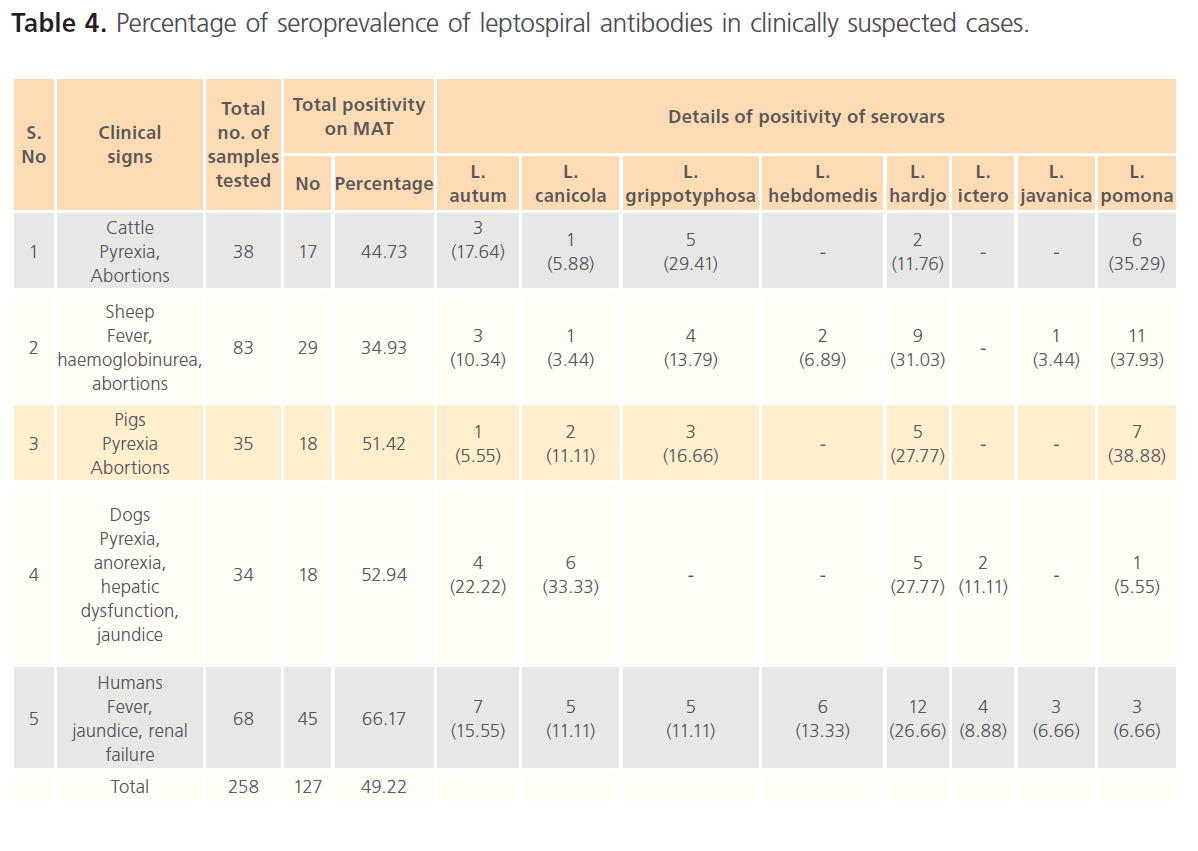
Table 4: Percentage of seroprevalence of leptospiral antibodies in clinically suspected cases.
Cattle
Out of 1,499 serum samples collected from apparently healthy cattle and buffaloes, 285 (19.1%) serum samples were found positive for leptospiral antibodies on MAT. On MAT seroprevalence of L. grippotyposa (26.31 %) was found to be highest followed by L. autumnalis (19.29 %), L. hardjo (12.98 %), L. javanica (12.63 %), L. icterohaemorrhagiae (4.91 %) and L. hebdomedis (4.21 %) respectively.
Of the 38 serum samples collected from cattle showing pyrexia and abortions subjected for MAT, the seropositivity was reported to be 44.73 %. The predominant serovar was L. Pomona (35.25 %), followed by L. autumnalis (17.64 %) and L. hardjo (11.76 %).
Sheep
The seroprevalence of leptospira in apparently healthy sheep in the present study was 18.67 %. Serovar analysis of the results revealed the predominance of the serovar hardjo (44.06 %) and L. autumnalis (5.08 %) in sheep of Andhra Pradesh.
Seroprevalence of leptospirosis in sheep (83) showing the clinical signs of fever, haemoglobinurea, found to be 21.00 %. The high prevalence of L. pomona (37.93 %) followed by L. hardjo (31.03 %), L. autumnalis (10.34 %), L. hebdomedis (6.80 %) and L. javanica and L. canicola (3.44 %). In addition to the serovars reported from apparently healthy sheep, L. hebdomedis and L. icterohaemorragiae were found circulating in sheep showing clinical signs.
Goats
Among 205 serum samples, the sero positivity was found to be 15.12 %. Serovar hardjo (38.7%) followed by L.grippotyphosa (29.63%), L.javanica (25.8%) and L.autumnalis (6.45%) were reported.
Pigs
The seropositivity in healthy pigs (133) was found to be 36.84 %. The predominance of serovar L. hardjo was 30.61 % followed by L. grippotyphosa (26.53 %), L. autumnalis (22.44%) and L. canicola (20.4%). Seroprevalence of leptospira in clinically suspected cases (35) was 51.12 % (18). The predominance of serovar L. pomona (38.88 %) was noticed followed by L. hardjo (27.77 %), L. grippotyphosa (16.66 %), L. canicola (11.11 %) and L. autumnalis (5.55%).
Dogs
Over all seropositivity of 15.15 % (99) was recorded in apparently healthy dogs compared to the clinically suspected cases of 52.94 % (34). The serovar L. canicola and L. javanica (40%) dominated by L. autumnalis (13.33%) and L. hardjo 6.66 % in apparently healthy dogs, whereas, the dominanace of L. canicola (33.33 %) followed by L. hardjo (27.77 %), L. autumnalis (22.22 %) and L. pomona (5.55%) were observed in clinically suspected cases. In addition the serovar L. icterohaemorrhagiae (11.11%) was also recorded in clinically suspected dogs.
Humans
Out of 68 serum samples screened from clinically suspected cases, 66.17 % seropositivity was reported. The predominance of serovar L. hardjo (26.66 %) followed by L. autumnalis (15.55 %), L. hebdomadis (13.33 %), L. canicola and L. grippotyphosa (11.11%), L. javanica and L. pomona (6.66%) and L. icterohaemorrhagiae (8.88 %) were noticed.
The seroprevalence of leptospiral antibodies in different regions of Andhra Pradesh was studied during the period (Fig. 2). The coastal region had the highest seroprevalence of 23.09% (1433) followed by Rayalseema with a seropositivity of 21.51% (703) and 16.03 % (184) in Telangana region.
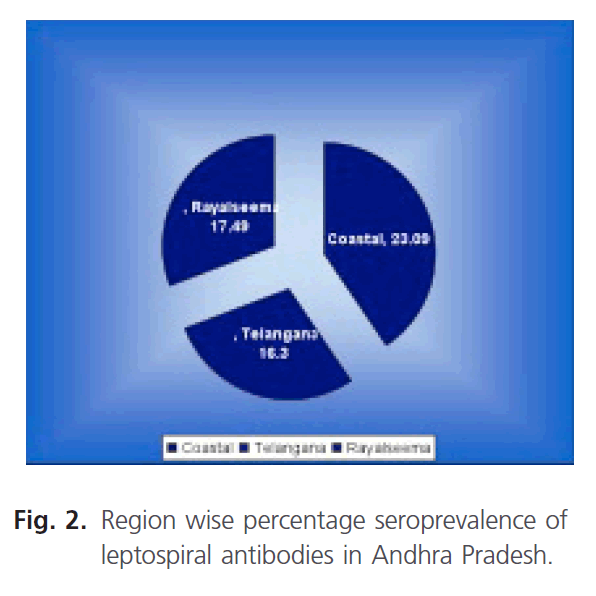
Fiagure 2: Region wise percentage seroprevalence of leptospiral antibodies in Andhra Pradesh.
Similarly the prevalence of leptospiral antibodies in different seasons of Andhra Pradesh was also studied during the period (Fig. 3). The highest seroprevalence of 28.29% during the South West monsoon followed by 21.45% in North east monsoon period. The lowest seroprevalence of 7.26% during summer followed by 12.22% in winter was observed.
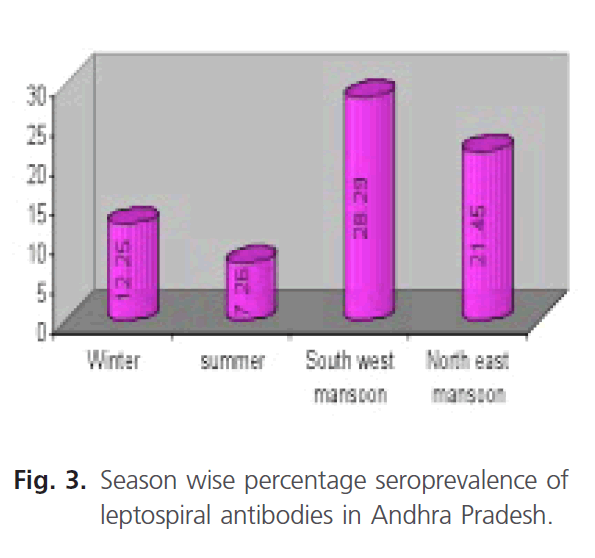
Fiagure 3: Season wise percentage seroprevalence of leptospiral antibodies in Andhra Pradesh.
District wise analysis of the results of the seropositivity of Leptospira in Andhra Pradesh revealed high seropositivity rate in west Godavari (34.00 %) followed by East Godavari (28.72 %) and low seropositivity of 4.83 % in Anatapur District was observed during the period of study (Fig. 4).
Fiagure 4: District wise percentage seroprevalence of leptospiral antibodies in Andhra Pradesh.
Discussion
A total of 2,320 serum samples representing different climatic zones, different species of animals and humans were collected during the period under study and subjected to MAT. Overall seropositivity of 20.09 % was observed in the state. The data was analyzed species wise, region wise and seasonal wise to understand the distribution of disease.
Seropositivity in case of clinically suspected cases of bovine, sheep, pigs, dogs and humans was reported to be higher compared to the apparently healthy animals due to active infection.
District wise analysis revealed the high prevalence in West Godavari district, due to natural vegetation with marshy lands and small ponds along with low humidity and temperature which is very much optimum for leptospiral growth, survival and perpetuation in the environment. Further, the habit of bathing of cattle in water bodies mostly contaminated with infected urine in the area helps in transmission of leptospires resulting in high seropositivity [11].
The second highest prevalent district is East Godavari (28.72%), due to the presence of more number of rice field infested with rats that acts as carriers for leptospira [6]. Also the warm wet conditions with a pH close to neutral and slightly alkaline provides an optimum survivability of leptospira [19, 20]. After that comes Chittoor District (15%) followed by Prakasam and Nellore Districts (13.3%). Lowest seroprevalence was reported in Anantapur (4.83%) and Kadapa districts (5.63%). The low prevalence was attributed to low rainfall with high temperature prevailing in the districts. The maintenance of animals in households separately for milking purpose under clean hygienic conditions could also be one of the factors for low prevalence in the area. These conditions did not favor the transmission of the disease [3].
Significant difference in seropositivity was observed between the rainy seasons, in southwest and north east monsoons. The difference is due to favorable environmental conditions such as low temperatures and water logged areas. Overflow of sewages mixing and spreading of contaminated urine etc suitable for survival and transmission of leptospira. Similarly, the favorable conditions at Andaman and Nicobar Islands (55 %age) reserved in high endemicity of the disease [14]. More number of cases was also reported from Tamilnadu during the rainy season [21].
Conclusion
The seroprevalence of leptospira in Andhra Pradesh was analyzed by Microscopic Agglutination Test (MAT). A total of 2,320 serum samples randomly from apparently healthy animals of cattle, buffaloes, sheep, goats, pigs, dogs and 258 serum samples from clinically suspected cases of both animals and humans from different districts of Andhra Pradesh were collected and subjected for MAT. The seropositivity of apparently healthy cattle, sheep, goats, pigs and dogs was found to be 19.0%, 18.67%, 14.63%, 36.84% and 15.15% respectively. Similarly seropositivity in clinically suspected cattle, sheep, pigs, dogs and humans was recorded as 28.94%, 21.69%, 31.43%, 50.00% and 61.76% respectively. The serum samples were screened against 9 reference serovars procured from ADMAS, Bangalore. Grippotyphosa followed by autumnalis and hardjo in cattle, hardjo followed by grippotyphosa in sheep, hardjo followed by grippotyposa and javanica in goats, hardjo followed by grippotyphosa and autumnalis in pigs, canicola, javanica, autumnalis and hardjo in dogs due to environmental, pH of the soil, season, grass lands and rice fields of the region, small ponds with stagnated water and presence of rodents in particular areas are the main factors for the prevalence of respective serovars in different species of animals. In humans, hardjo, autumnalis, grippotyposa and canicola were found to be commonly circulating serovars. The main predominant serovar in humans was observed during the study in Chittor district because of dairying and animal husbandry activities. The percentage of seroprevalence of leptospirosis in clinically suspected cases (38.37%) was high compared to the apparently healthy ones (20.9%) indicating acute infections.
The prevalence of leptospirosis was noticed high during south west and North east monsoons. High prevalence related to the congenital environment for the survival of leptospires during the monsoons. The seroprevalence of leptospirosis in coastal regions was high (23.09 %), followed by Rayalaseema (20.90 %) and Telangana (16.30 %) regions.
83
References
- Adinarayanan, N., James, PC. Studied on leptospirosis in farm stock and wild life in Kerala. Isolation of leptospires form divergent classes of animal hosts with an outbreak of cultural procedure. Ind Vet Jour. 1980; 57: 495-500.
- Adinarayanan, N., Jain, NC., Chandiramani, NK., Hajela, SK. Studies on leptospirosis among bovines in India. Ind Vet Jour. 1960; 37: 251-254.
- Ashford, DA., Kaiser, RM., Spiegel, RA., Perkins, BA., Weyant, RS. et al. Ame Jour of Trop Med and Hyg. 2000; 63: 249.
- Balakrishnan, G., Govindarajan, R., Meenambigai, TV., Jayakumar, V., Manohar, BM. Ind Vet Jour. 2008; 85: 551-552.
- Batra, M., Khadar, TG., Rao, R., Raghavan, N. Survey of Leptospirosis in farm animals in Haryana. Ind Jour of Ani Sci.1990; 60: 755-760.
- Collares–Pereira, M., Korver, H., Caothi, BV., Santos-Reis, M., Bellenger, E., Baranton, G., Terpstra, WJ. FEMS Micro Letters. 2000; 185: 181-187.
- Ghosh, SS., Srivatsava, SK., Gupta, BR. Seroincidence of leptospirosis in organized cattle farms in Northeastern hill region. Ind Jour of Compar Microb and Immun and Infe Dise. 1989; 10: 48-50.
- Koteeswaran, A. Seroprevalence of leptospirosis in man and animals in Tamil Nadu. Ind Jour of Medical Micro. 2006; 24: 329-331.
- Mandal, S., Joudar, SM., Chakravarthy, D., Sardar, N. Seroepidemiological study of bovine Leptospirosis in West Bengal. Ind Jour of Compar Microb, Immun and Inf Dise. 2008; 29: 42-44.
- Mariya, R., Srivastava, SK., Thangapandiyan, E. Seroprevalance of leptospiral antbodies in bovines. Ind Vet Jour. 2007; 84: 547-548.
- Ramanipushpa, RN., Punya Kumari, B. Seroprevalence of leptospirosis is domestic animals. Ind Vet Jour. 2005; 82: 670-671.
- Ratnam, S. Leptospirosis: An Indian perspective. Ind Jour of Med Microb. 1994; 12: 228-239.
- Sharma, S., Vijayachari, P., Sugunan, SC. Leptospiral carrier state and seroprevalence among animal population. A cross-sectional sample survey in Andaman and Nicobar Islands. Epidemial Infectious 2003; 131: 985-989.
- Sehgal, SC., Sugunan, AP., Vijayachari, P. Outbreak of leptospirosis after the cyelone in orissa. The Nat Medi Jour of India 2002; 15: 22-23.
- Upadhye, AS. Leptospirosis an etiological agent of abortion in bovines. Ind Jour of Compar Microb Immun and Inf Dis. 1982; 3: 235-237.
- Vaid, J., Lal, K., Kaushal, RS. Seroprevalence of animal disease in Himachal Pradesh. Ind Vet Jour 1991; 68: 705-707.
- Zuerner, RL., Bolin, CA. Repetitive sequence element cloned from Leptospira interrogans serovar hardjo type hardjobovis provides a sensitive diagnostic probe for bovine leptospirosis. Jour of Clin Microbi. 1988; 26: 2495-2500.
- atnam, S. Leptospirosis: An Indian Perspective. Ind Jour of Med Micro. 1994; 12: 228-239.
- Sehgal, SC., Vijayachari, P., Sharma, S., Sugunana, AP. Lepto dipstick: A rapid and simple method for serodiagnosis of acute leptospirosis. Trans Royal Soci of Trop Medi Hy. 1999; 93: 161-164.













