Keywords
Guillain Barre Syndrome; Shingrix vaccine; Polyradiculoneuropathy; Cytoalbuminogenic dissociation
Introduction
Guillain-Barré syndrome (GBS) is a rare life threatening acute polyradiculoneuropathy with variable clinical presentations [1]. GBS has been documented to most commonly occur as a post-infective immune-mediated process. GBS has been associated with many vaccines and illnesses; H1N1 Swine Flu vaccine, pneumococcal vaccine, Campylobacter jejuni bacteria, cytomegalovirus, and herpes zoster virus [2-4]. One vaccine that has yet to be identified to cause GBS is the newly released Shingrix® vaccine. Shingrix® aims to prevent herpes zoster infection and the associated post-herpetic neuralgia. Here, we report a case of severe GBS after recent Shingrix® vaccine immunization.
Case Report
A 76-year-old female with a past medical history of hyperlipidemia, asthma, macular degeneration, and two previous episodes of pancreatitis of unknown origin, presented with a two-day history of bilateral proximal lower extremity weakness. The Patient denied recent camping, hiking, insect bites, and prior neuropathy/myopathy. However, the patient did report a mild upper respiratory tract infection ten weeks prior to admission. The Patient stated that she had received the new Shingrix® (Recombinant Zoster) vaccine ten days before hospitalization. The Patient reported that she had progressive weakness that initially started with inability to ascend stairs. This progressed to difficulty with standing and a subsequent non-traumatic fall. These described symptoms deviated significantly from her baseline. The Patient’s baseline consisted of independence with mobility and activities of daily living. The patient did not require any assistive devices.
On presentation, the patient was afebrile (98.4°F), had a normal respiratory rate (17 breaths/minute), had an oxygen saturation of 98% on room air, and was normotensive (136/76 mmHg). The Patient reported paresthesias and numbness on the dorsal region of her right lower extremity and difficulty lifting both legs. On examination, the Patient was alert and oriented, cranial nerves II-XII were intact, there was 5/5 bilateral upper extremity strength, and 5/5 bilateral lower extremity strength with dorsiflexion. The Patient had normal sensation bilaterally in upper and lower extremities. However, the Patient had 3/5 lower bilateral extremity strength with hip flexion. Furthermore, that Patient had bilateral absent Patellar and Achilles tendon reflexes.
On further assessment, the Patient’s labs were unremarkable. A computed tomography scan of the brain was performed, which revealed no evidence of acute intracranial abnormalities. The report did endorse microvascular ischemic disease involving the white matter. These results were found to be secondary to aging. Magnetic resonance imaging scan of the brain and lumbar spinal cord was only positive for degenerative antegrade spondylolisthesis deformity at L5-S1. Over the next 24 hours, the Patient reported worsening lower extremity paresthesias and weakness. The Patient could no longer stand without assistance. The Patient also stated that she was having neuropathic pain in bilateral lower extremities; burning, tingling, and electrical shock sensations. Furthermore, the Patient described new difficulties with swallowing. An endoscopy was performed, and the Patient was described to have esophageal dysmotility because she was unable to pass the barium pill during the examination. Consequently, a lumbar puncture was performed due to concern for disease progression. Lumbar cerebral spinal fluid studies revealed an elevated protein level (61 mg/dL), a normal white blood cell count (0 CUMM), and a cytoalbuminogenic dissociation. These results indicated acute inflammatory demyelinating polyneuropathy (GBS). Neurologic consultation was obtained. No electrodiagnostic studies were performed.
The Patient was diagnosed with Guillen Barre Syndrome likely secondary to Shingrix® vaccine. Treatment with intravenous immunoglobulin (2 g/kg divided over five days) was initiated. The Patient showed gradual improvements with five days of treatment and was transferred to an acute rehabilitation facility (Hospital day 5). At discharge, the Patient’s bilateral Patellar and Achilles tendon reflexes remained absent. However, the Patient was able to stand and ambulate with a walker.
Five days after discharge to the rehab facility, the patient reported worsening of bilateral lower extremity pain (per Patient, this pain was worse than her prior admission). The patient was therefore readmitted to the hospital. On examination, the patient had 5/5 bilateral upper extremity strength, 5/5 bilateral strength on lower extremity dorsiflexion, normal bilateral sensation in upper and lower extremities, and 3/5 bilateral lower extremity hip flexion strength. This strength assessment was consistent with the Patient’s previous presentation. Furthermore, light touch sensation was intact in all four extremities. There was no apparent sensory loss. Bilateral Achilles and Patellar tendon reflexes remained absent. However, the Patient could no longer stand with and without assistance and therefore could no longer ambulate. This presentation significantly deviated from her prior discharge appearance.
Initially the patient described neuropathic pain and therefore, she was treated with Gabapentin and amitriptyline. However, the patient became hyponatremic (133 mmol/L on admission to 122 mmol/L after treatment) and had significant fluctuations in her blood pressure (ranging from 110/67 mmHg to 182/88 mmHg). Additionally, the Patient unfortunately feels due to weakness while attempting to walk with assistance. She suffered a nondisplaced spiral fracture through the distal fibular metaphysis and had a large ankle joint effusion. The Patient was seen by Orthopedic Surgery and was placed in a CAM boot. Plasma exchange was initiated because the Patient’s muscular strength continued to decline, and her lower extremity pain was increasing.
After undergoing three plasma exchange treatments, the Patient showed remarkable improvement with lower extremity strength and power. The Patient was then able to ambulate the hospital halls with the use of a walker and some assistance after five treatments of plasma exchange. Additionally, the Patient’s neuropathic pain improved. She was then discharged to an acute rehabilitation center with scheduled physical therapy (Figures 1 and 2).
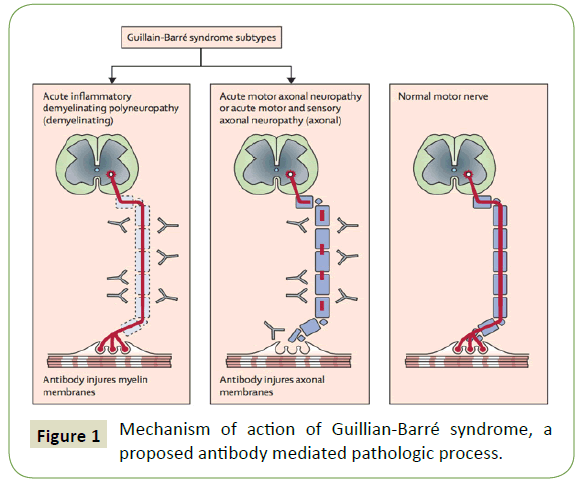
Figure 1: Mechanism of action of Guillian-Barré syndrome, a proposed antibody mediated pathologic process.
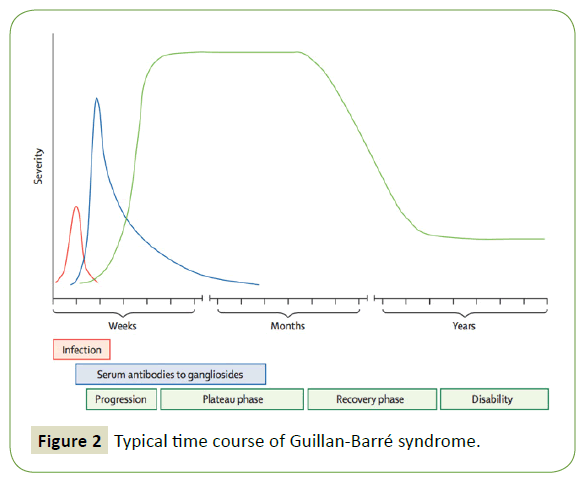
Figure 2: Typical time course of Guillan-Barré syndrome.
Discussion
We present a case of Guillian-Barré syndrome that occurred 10 days after Shingrix® vaccine administration. We identified no other possible etiology of the Patient’s symptoms. The Patient displayed several key features of GBS established by the Brighton Criteria of weakness; paresthesias, diminished Patellar and Achilles deep tendon reflexes, hyponatremia secondary to Syndrome of Inappropriate Antidiuretic Hormone secretion (SIADH), dysautonomia, and cytoalbuminological dissociation on cerebral spinal fluid analysis. We believe that this is an example of post-vaccine related Guillain-Barré Syndrome, given the timing of the presentation and when she received her vaccine [2-6].
While electrodiagnostic studies have been used to help diagnose GBS secondary to bacterial, viral, and vaccination origins, these results are not necessary. The diagnosis of GBS is confirmed with the elevated CSF protein level and absence of deep tendon reflexes (found in 90% of GBS affected patients) [2]. Additionally, it can take up to four weeks before electrodiagnostic studies show loss of motor and sensory response secondary to GBS [7]. GBS is a rapidly progressing disease and if not treated in a timely fashion can result in paralysis of the diaphragm and even death [7].
There have been numerous vaccines connected to GBS, including the H1N1 swine flu vaccine and the pneumococcal vaccine. However, the newly release Shingrix® vaccine has yet to be reported to be associated with this potentially life threatening polyradiculoneuropathy [3]. Shingrix® is a non-live recombinant vaccine containing the varicella-zoster virus glycoprotein E in combination with an adjuvant ASO1B suspension component [8]. This immunization was FDA approved and released to the public in 2017 to prevent latent activation of varicella zoster virus (Chickenpox) as herpes zoster virus (Shingles) in patients 50 plus years old [9]. This vaccination presents in a two-dose series and is administered intramuscularly 2-6 months apart (Table 1).
| H1N1 Swine Flu Vaccine |
Pneumococcal Vaccine |
Cytomegalovirus |
| Campylobacter jejuni bacteria |
Herpes Zoster Virus |
Zika Virus |
Table 1: Previously documented causes of GBS.
Before Shingrix® was released; the Zoster vaccine was used to inhibit varicella zoster virus reactivation [10]. However, Shingrix® has been found to be superior. Shingrix® not only prevents latent herpes zoster virus reactivation within the dorsal root ganglia of a dermatome after primary infection, but also prevents the subsequent post-herpetic neuralgia [9-11]. Post-herpetic neuralgia affects 10-13% of infected individuals. Following the resolution of shingles, post-herpetic neuralgia consists of persistent pain for at least 90 days [11]. Documented adverse reactions to the Shingrix® vaccination from eight high quality randomized control trials, include local injection site rash or irritation, disseminated rash, herpes zoster infection in immunocompetent patients, and life-threatening complications in immunocompromised recipients [9-13]. However, none of these studies demonstrated a relationship between Shingrix®, nor the previous Zoster vaccine, to GBS. As a result, GBS likely secondary to the Shingrix® vaccine could not have been expected in this patient after receiving this immunization.
Interestingly, there have been multiple cases of GBS secondary to herpes zoster infection. The first documented case was in 1924. Since then, there have been over 50 cases described [14]. It is thought that the mechanism by which GBS results from herpes zoster infection is similar to that of GBS secondary to Campylobacter jejuni [4]. It has been hypothesized that GBS results from C. jejuni infection because the bacteria’s lipopolysaccharide contains epitopes that resemble human peripheral nerve ganglioside epitopes [4,9]. Consequently, the human body generates antibodies against these bacterial lipopolysaccharide epitopes. These antibodies then also target the patient’s peripheral nerve gangliosides because they cannot distinguish between the ganglioside epitope and the bacterial epitope. In summary, the bacterial lipopolysaccharide epitope acts as a molecular mimicker [4]. The precise nature by which this occurs is still unclear. Therefore, the mechanism by which GBS may result from Shingrix® vaccine remains unknown. If further cases reveal a similar association, this should be further explored. Per this case report and prior documentation of GBS secondary to herpes zoster, there is now evidence to state that antibodies generated in a lab against herpes zoster and then given intramuscularly via vaccination (Shingrix®) may be a sufficient nidus for Guillain-Barré syndrome (Table 2).
| Brighton Diagnostic Criteria for GBS |
Level of Diagnostic Certainty |
| Symptoms |
1 |
2 |
3 |
4 |
| Bilateral and flaccid weakness of limbs |
+ |
+ |
+ |
+/- |
| Decreased or absent deep tendon reflexes in weak limbs |
+ |
+ |
+ |
+/- |
| Monophasic course and time between onset-nadir = 12 hours to 28 days |
+ |
+ |
+ |
+/- |
| Absence of alternative diagnosis for weakness |
+ |
+ |
+ |
+/- |
| CSF cell count <50/ml |
+ |
+/-a |
- |
+/- |
| CSF protein concentration > 60 mg/dL |
+ |
+/-a |
- |
+/- |
Nerve conduction study findings consistent with one of
the subtypes of GBS |
+ |
+/-a |
- |
+/- |
Table 2: Level of diagnostic certainty in brighton diagnostic criteria for GBS.
Conclusion
In summary, we present a case of GBS that occurred shortly after Shingrix® vaccination. Our case is consistent with other vaccine and herpes zoster related cases of GBS. Clinicians should be cognizant of the possible association between Guillain-Barré syndrome and the new Shingrix® vaccine.
24680
References
- Kang JH, Sheu JJ, Lin HC (2010) Increased risk of Guillain-Barré syndrome following recent Herpes Zoster: A population-based study across Taiwan. Clin Infect Dis 51: 525-530.
- Cresswell F, Eadie J, Longley N, Macallan D (2009) Severe Guillian-Barré Syndrome following primary infection with varicella zoster virus in an adult. Int J Infect Dis 14: e161-163.
- Fokke C, Van den Berg B, Drenthen J, Walgaard C, Van Doorn PA, et al. (2014) Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain 137: 33-43.
- Ogawara K, Kuwabara S, Mori M, Hattori T, Koga M, et al. (2000) Axonal Guillain-Barré syndrome: relation to anti-ganglioside antibodies and Campylobacter jejuni infection in Japan. Ann Neurol 48: 624-631.
- Hoffmann O, Reuter U, Schielke E, Weber JR (1999) SIADH as the first symptom of Guillain-Barré syndrome. Neurology 53: 1365.
- Saifudheen K, Jose J, Gafoor VA, Musthafa M (2011) Guillain-Barré syndrome and SIADH. Neurology 76: 701-704.
- Gordon PH, Wilbourn AJ (2001) Early electrodiagnostic findings with Guillain-Barré syndrome. Arch Neurology 58: 913-917.
- Chlibek R, Bayas JM, Collins H, De la Pinta ML, Ledent E, et al. (2013) Safety and immunogenicity of an ASO1-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥ 50 years of age. J Infect Dis 208: 1953-1961.
- Cunningham AL, Heineman TC, Lal H, Godeaux O, Chlibek R, et al. (2018) Immune responses to a recombinant glycoprotein E Herpes Zoster vaccine in adults aged 50 years or older. J Infect Dis 217: 1750-1760.
- Macaladad N, Marcano T, Guzman M, Moya J, Jurado F, et al. (2007) Safety and immunogenicity of a zoster vaccine in varicella-zoster virus seronegative and low-seronegative healthy adults. Vaccine 25: 2139-2144.
- Baxter R, Bartlett J, Fireman B, Morgan M, John H, et al. (2016) Effectiveness of live Zoster vaccine in preventing Postherpetic Neuralgia (PHN). Oral Abstract Session: Newer and older vaccines in older adults, ID Week presentation #128. New Orleans.
- Chlibek R, Pauksens K, Rombo L, Van Rijckevorsel G, Richardus JH, et al. (2016) Long-term immunogenicity and safety of an investigational herpes zoster subunit vaccine in older adults. Vaccine 34: 863-868.
- Chlibek R, Smetana J, Pauksens K, Rombo L, Van den Hoek JA, et al. (2014) Safety and immunogenicity of three different formulations of an adjuvant varicella-zoster virus subunit candidate vaccine in older adults: A phase II, randomized, controlled study. Vaccine 32: 1745-1753.
- Rabbani MU, Gupta D (1990) Guillian Barré Syndrome Following Herpes Zoster: A case report and review of literature. Jpn J Med 29: 397-398.







