Keywords
Sex characteristics; Long-term potentiation; Long-term synaptic depression; Hippocampus; Learning and memory; Gene expression
Introduction
The hippocampus is a medial temporal lobe structure implicated in the consolidation of declarative memory in humans and spatial memory in rodents and shows high degree of synaptic plasticity. Although there is general consensus that males outperformed females in spatial tasks [1], limited number of studies concerned with sex difference in the longterm potentiation (LTP) and the long-term depression (LTD), two general forms of synaptic plasticity thought to underlie memory acquisition. A robust sex difference in the magnitude of LTP induced was reported in the dentate gyrus of pentobarbital-anesthetized rats [2] and the CA1 region of hippocampal slices [3,4], whereas others did not observe any sex difference in LTP at the perforant pathway (PP)–the dentate gyrus (DG) synapses [5]. Only one study appears to have addressed sexual dimorphism in LTD and found that female animals seem to exhibit LTD in vivo in much more easily than their male counterparts [6]. We have thought that these findings need to be repeated because there is no consistency in the available data on synaptic plasticity forms.
The results of previous studies suggest that both the direction and the magnitude of synaptic changes are determined as a function of the calcium current mediated by the N-methyl-D-aspartate (NMDA) receptors [7]. NMDA receptors are tetrameric ligand-gated ion channels comprised of GluN1, GluN2A-D, and GluN3A-B subunits. The majority of native NMDA receptors are tetrameric assemblies of two GluN1 subunits and two GluN2 subunits. The two predominant GluN2 subunits in the hippocampus are GluN2A and GluN2B, although GluN2C and GluN2D are also present, in particular early in development, but also in low quantities in adulthood [8]. Co-expression of GluN1 with two different GluN2 subunits (e.g. GluN2A and GluN2B) in heterologous systems generates three populations of functional NMDA receptors consisting of two different diheteromeric receptors (e.g. GluN1/GluN2A and GluN1/GluN2B) as well as triheteromeric receptors (e.g. GluN1/GluN2A/GluN2B) [9,10]. The GluN1 subunit is an obligate part of all NMDA receptors and is widely expressed in the central nervous system, whereas the different GluN2 subunits endow NMDA receptors with markedly different biophysical and pharmacological properties [10].
Many forms of LTP and LTD require the activation of NMDA receptors and the consequent rise in the postsynaptic Ca2+ concentration. Strong depolarization of the post-synaptic cell completely displaces the magnesium ions that block NMDA ion channels and allows calcium ions to enter a cell causing LTP [11], while weaker depolarization only partially displaces the Mg2+ ions, resulting in less Ca2+ entering the post-synaptic neuron and lower intracellular Ca2+ concentrations and induces LTD [12]. Previous studies have suggested that the balance between GluN2A subunit and GluN2B subunit may alter the critical level for postsynaptic responses. It is likely that an increase in the GluN2A/GluN2B ratio would raise the LTD induction threshold and a decrease in the GluN2A/GluN2B ratio would lower the LTD induction threshold [13]. Herein, we associated sex differences in the LTP and LTD with NMDA receptor subunits, given their well-known roles in the establishment of long-term memory.
Materials and Methods
Animals
Totally 30 male and 30 female Wistar rats between the ages of 2 and 4 months were used in this study, in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) regarding the protection of animals used for experimental purposes and with the guiding principles for the care and use of laboratory animals approved by Erciyes University. Microscopic evaluation of vaginal smear was used to document the stages of the estrous cycle, and female rats in proestrus phase were included to study.
Electrophysiology
Details of the protocols used for electrophysiological experiments are described elsewhere [14]. Briefly, after rats were anesthetized with intraperitoneally injected urethane (1.2 g/kg), a glass micropipette (Borosilicate, outer diameter 1.5 mm, length 10 cm length; World Precision Instruments) was inserted into the granule cell layer of the dentate gyrus (DG) in the right hemisphere (in mm, from bregma: anteroposterior: −3.5; mediolateral: 2.15; dorsoventral: 2.5-3 mm below the dura) to record the field potential. A bipolar tungsten electrode (stainless steel, Teflon-coated, 127 μm in diameter, insulated except at its tips) was used to stimulate the medial perforant path (PP, from bregma, in mm: anteroposterior: −8.0; mediolateral: 4.2; dorsoventral: 2-2.5 below the dura) of the right hemisphere. The depth of recording and stimulating electrodes (dorsoventral coordinate) was adjusted to obtain a large positive excitatory postsynaptic potential (EPSP) followed by a negative-going population spike (PS) in response to perforant path stimulation. These positions of both electrodes were previously verified to be in the granule cell layer of the dentate gyrus and in the PP [14].
After a stable EPSP was obtained, the PP was stimulated by pulses at an intensity that ranged from 0.1 to 1.5 mA at 0.05 Hz three times and by increasing the intensities from a 0.1 mA to a 1.5 mA step by 0.2 mA per step to create an input-output curve, which was stored for off-line analysis. The stimulus intensity produced by half of the maximum PS amplitude was determined (test stimulus) and then used throughout the experiment. LTP was induced using high-frequency stimulation (HFS; 100 Hz, 1 sec, 4 times). LTD was evoked using lowfrequency stimulation (LFS) that consisted of 900 stimuli at 1 Hz, with the test stimulus occurring after 15 min of baseline recording. Following the delivery of HFS/LFS, the test stimulus was repeated every 30 s for up to 60 min.
Molecular experiments
Isolation of total RNA and cDNA synthesis: For the gene expression studies, randomly selected 6 rats of each sex were sacrificed 60 min after the end of HFS and LFS by decapitation. To determine rapid effects of induction protocols on the expression of NMDA receptor subunits, additional groups of 6 rats of each sex underwent similar surgery for hippocampal recording but were sacrificed 5 min after the end of HFS and LFS, or the sham session consisting of pulses at a frequency of 0.033 Hz. By this way, five groups were composed as the HFS5 group, the LFS5 group, the HFS60 groups, the LFS60 groups, and sham treatment group for each sex. The left hippocampi of these rats were separately dissected from the surrounding area and weighed. Total RNA was isolated from the hippocampi of rats using an ExiPrepTM plus Tissue Total RNA Kit and an automated nucleic acid extraction instrument from ExiPrepTM 16 Plus, following the manufacturer’s protocols (Bioneer, South Korea). The quantity and quality of the isolated total RNA were determined with a DS-11 spectrophotometer (Denovix, USA). cDNA was synthesized from 2 μg of total RNA, using AccuPower RT PreMix (Bioneer, South Korea) in a final volume of 20 μl.
Real-time Quantitative Polymerase Chain Reaction (RTqPCR) Analysis: RT-qPCR was performed using an Exicycler TM 96 (Bioneer, South Korea) with AccuPower GreenStarTM qPCR PreMix (Bioneer, South Korea). Primer sets used were validated by and purchased from Bioneer (Bioneer, South Korea). The primer sequences used were as follows: NMDA-1 (forward: 5'- TAAGTATGCGGACGGAGTGA -3' and reverse: 5'- CTCATTGTTGATGGTCAGTGG -3'), NMDA-2A (forward: 5'- CTGTCTCCCCTTCTGCTTTC -3' and reverse: 5'- CAGGTTGGCTGTGTAACTGG -3'), NMDA-2B (forward: 5'- TGAACGAAACTGACCCAAAG -3' and reverse: 5'- TAGGTGGTGACGATGGAAAA -3') and glyceraldehyde 3- phosphate dehydrogenase (GAPDH) (forward: 5'- AATGTATCCGTTGTGGATCT -3' and reverse: 5'- CAAGAAGGTGGTGAAGCAGG -3'). The cycle threshold (Ct) was determined for each target gene in duplicate. ΔCt was calculated by the difference between the Ct of each target gene and the Ct of GAPDH (reference gene).
Data Analysis and Statistics
Two components of each field potential, the EPSP and PS, were used for statistical analysis of LTP and LTD data. The slope of the EPSP was calculated as the amplitude change at 20-80% of the voltage difference between the start and the peak of the waveform, and the amplitude of the PS was the difference between the first positive peak and the negative peak for each current value. Raw values for the EPSP slopes and PS amplitudes obtained during the I/O experiment were analyzed separately using repeated measures ANOVA, with sex and experimental (LTP vs. LTD) conditions used as betweensubjects variables and stimulus intensity (8 levels of intensity) used as a within-subjects factor.
The mean value of the EPSP slope and the PS amplitude during baseline recording was chosen to represent 100 percent, and each EPSP and PS was expressed as a percentage of this value. Each HFS was able to induce a short-term potentiation. In contrast to this, LFS was able to induce a shortterm depression, as shown by a sharp decrease in PS amplitude within the first 5 min of the post-LFS period. The magnitudes of initial and persistent potentiation (or depression) of LTP (or LTD) were calculated from the averages in the interval 15-20 min and 70-75 min after induction protocol, respectively. These data were compared Student t test.
The effect of sex on baseline gene expression was tested by comparison of mRNA levels for hippocampus that was removed 5 min after the sham session consisting of pulses using Student t test. To determine the effect of sex on gene expression measured after 5-min and 60-min post induction (HFS or LFS), ΔCt values (=Ct target gene–Ct Gapdh) were compared using a Repeated Measures of ANOVA. When significant interaction was found between sex and treatment, pairwise comparisons were made using independent samples t-tests. An α-level of ≤ 0.05 (two-tailed) was applied for all comparisons to determine statistical significance. All statistical analyses were performed using SPSS software (SPSS, Chicago, IL).
Results
Input-Output function
Figure 1 shows the PS amplitude and the EPSP slope as a function of perforant pathway stimulus intensity, namely the I/O curves, before the LTP and the LTD were induced. Separate 8 (intensity levels) × 2 (pre-LTP vs. pre-LTD) × 2 (sex) repeated measures of ANOVAs did not find any significant betweensubjects effect (Sex: F1, 56=0.35 and 0.03; Experiment: F1, 56=0.42 and 1.93; P’s >0.05) or any significant interaction related to intensity (with sex: F7, 392=0.32 and 1.15; with Experiment: F7, 392=0.85 and 0.36; with Group x Experiment: F7, 392=0.13 and 0.33; P’s >0.05 for PS amplitude and EPSP slope, respectively). As expected, the PS amplitude (F7, 392=239.7; p<0.001) and the EPSP slope (F7, 392=177.3; p<0.001) increased systematically as a function of perforant pathway stimulus intensity. These data indicate that there appear to be no gross differences in baseline function between sexes and between experiments.
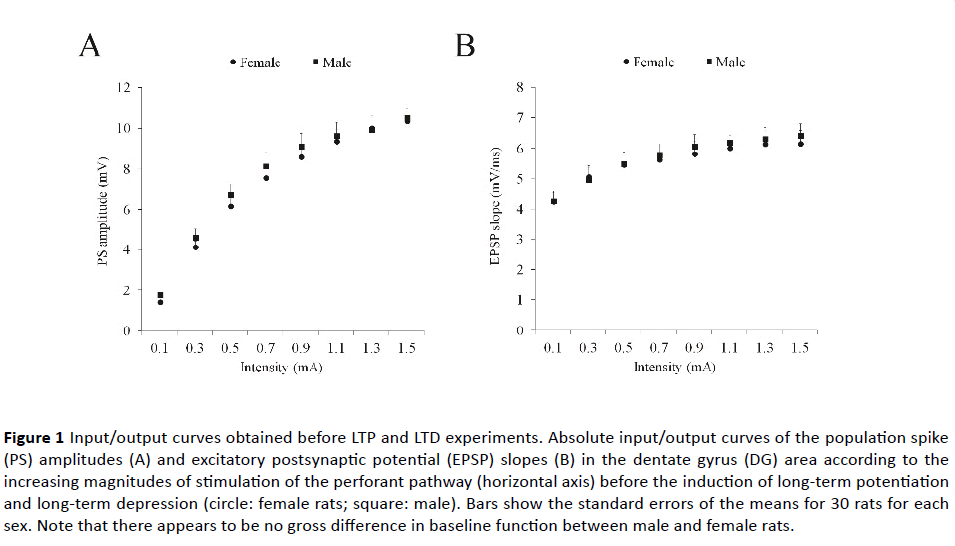
Figure 1 Input/output curves obtained before LTP and LTD experiments. Absolute input/output curves of the population spike (PS) amplitudes (A) and excitatory postsynaptic potential (EPSP) slopes (B) in the dentate gyrus (DG) area according to the increasing magnitudes of stimulation of the perforant pathway (horizontal axis) before the induction of long-term potentiation and long-term depression (circle: female rats; square: male). Bars show the standard errors of the means for 30 rats for each sex. Note that there appears to be no gross difference in baseline function between male and female rats.
Initial and long-term potentiation
Figures 2A and 2B show the time course of PS amplitude and EPSP slope. Independent samples T test failed to show a significant difference between male and female rats for the initial (341.3 ± 18.0% vs. 315.3 ± 23.2% of baseline; p>0.10 ) and long-term potentiation of PS amplitude (255.8% ± 13.9 vs. 239.9 ± 5.1% of baseline; p>0.10), and for the initial (153.4 ± 5.5% vs. 158.9 ± 4.8% of baseline; p>0.10) and long-term potentiation of the EPSP slope (120.6 ± 6.7% vs. 130.5 ± 4.2% of baseline; p>0.10).
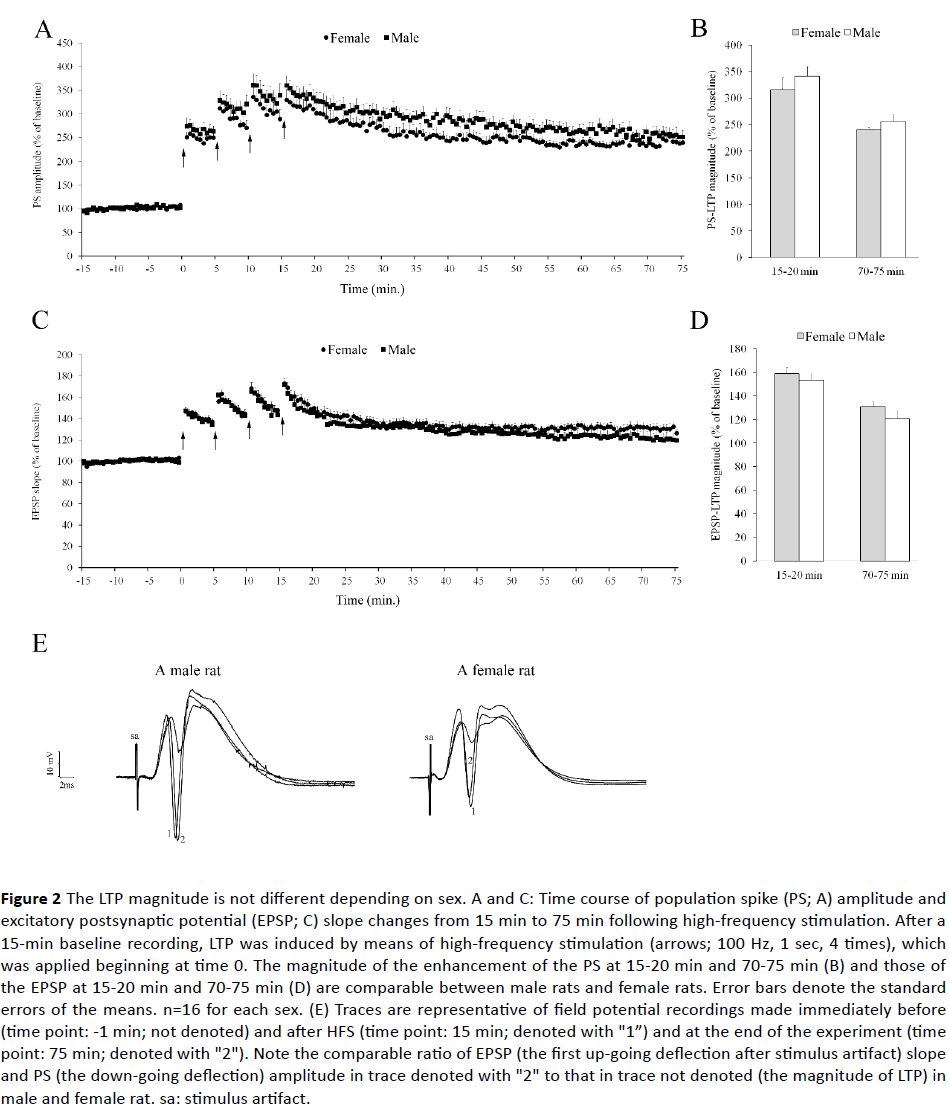
Figure 2 The LTP magnitude is not different depending on sex. A and C: Time course of population spike (PS; A) amplitude and excitatory postsynaptic potential (EPSP; C) slope changes from 15 min to 75 min following high-frequency stimulation. After a 15-min baseline recording, LTP was induced by means of high-frequency stimulation (arrows; 100 Hz, 1 sec, 4 times), which was applied beginning at time 0. The magnitude of the enhancement of the PS at 15-20 min and 70-75 min (B) and those of the EPSP at 15-20 min and 70-75 min (D) are comparable between male rats and female rats. Error bars denote the standard errors of the means. n=16 for each sex. (E) Traces are representative of field potential recordings made immediately before (time point: -1 min; not denoted) and after HFS (time point: 15 min; denoted with "1”) and at the end of the experiment (time point: 75 min; denoted with "2"). Note the comparable ratio of EPSP (the first up-going deflection after stimulus artifact) slope and PS (the down-going deflection) amplitude in trace denoted with "2" to that in trace not denoted (the magnitude of LTP) in male and female rat. sa: stimulus artifact.
Long-term depression
Figures 3A and 3B show the time course of PS amplitude and EPSP slope. Independent samples T test failed to show a significant difference between male and female rats for the initial potentiation of PS amplitude (66.2 ± 8.9% vs. 51.1 ± 5.9% of baseline; p>0.10) and the EPSP slope (82.5 ± 4.5% vs. 71.7 ± 4.9% of baseline; p>0.10). Nevertheless, both PS amplitude and EPSP slope were more depressed in female rats (49.5 ± 9.2% and 64.1 ± 6.0% of baseline, respectively) than male rats (98.6 ± 15.8% and 85.9 ± 4.8% of baseline; Ps=0.012 and 0.009) at 70 - 75 min.
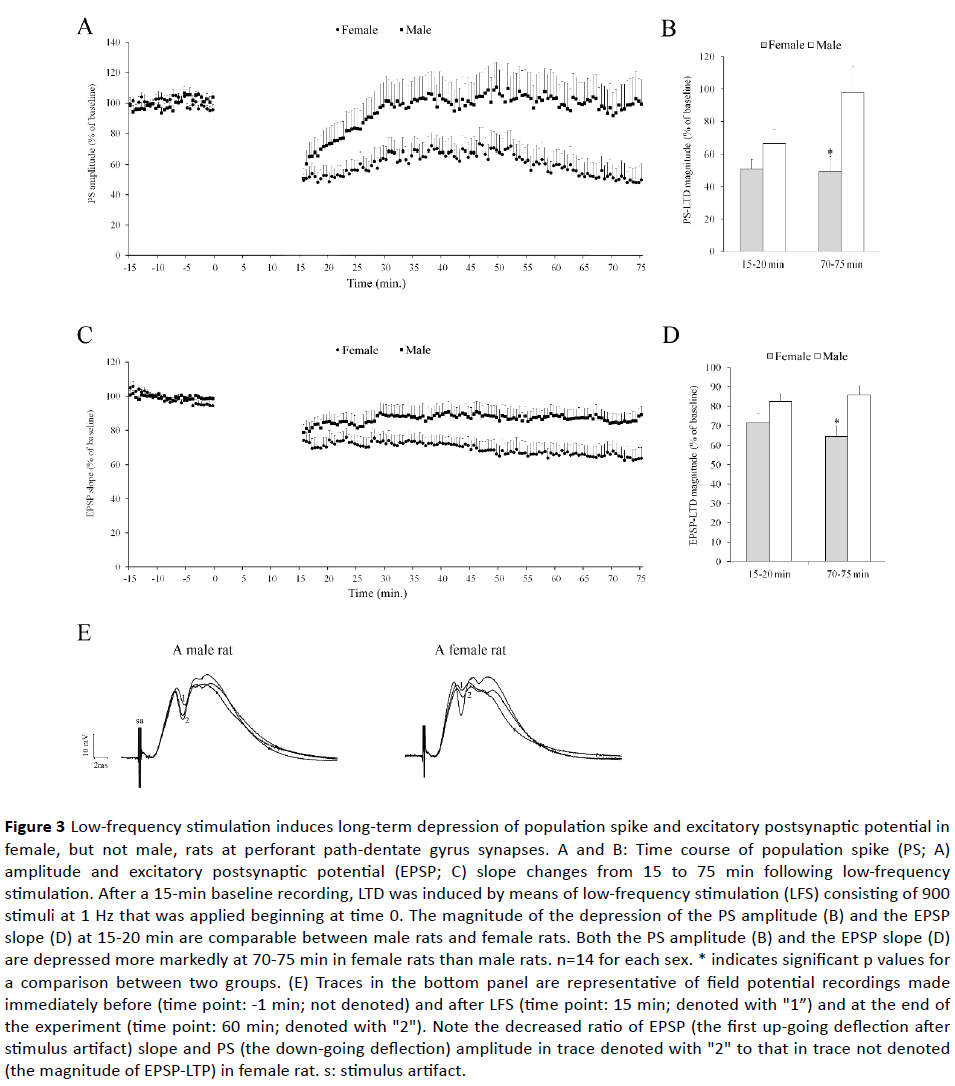
Figure 3 Low-frequency stimulation induces long-term depression of population spike and excitatory postsynaptic potential in female, but not male, rats at perforant path-dentate gyrus synapses. A and B: Time course of population spike (PS; A) amplitude and excitatory postsynaptic potential (EPSP; C) slope changes from 15 to 75 min following low-frequency stimulation. After a 15-min baseline recording, LTD was induced by means of low-frequency stimulation (LFS) consisting of 900 stimuli at 1 Hz that was applied beginning at time 0. The magnitude of the depression of the PS amplitude (B) and the EPSP slope (D) at 15-20 min are comparable between male rats and female rats. Both the PS amplitude (B) and the EPSP slope (D) are depressed more markedly at 70-75 min in female rats than male rats. n=14 for each sex. * indicates significant p values for a comparison between two groups. (E) Traces in the bottom panel are representative of field potential recordings made immediately before (time point: -1 min; not denoted) and after LFS (time point: 15 min; denoted with "1”) and at the end of the experiment (time point: 60 min; denoted with "2"). Note the decreased ratio of EPSP (the first up-going deflection after stimulus artifact) slope and PS (the down-going deflection) amplitude in trace denoted with "2" to that in trace not denoted (the magnitude of EPSP-LTP) in female rat. s: stimulus artifact.
qRT-PCR gene expression
Figure 4 shows ΔCt as a measure of baseline and plasticitydependent expression levels of the target genes after the sham session and after LTP or LTD induction.
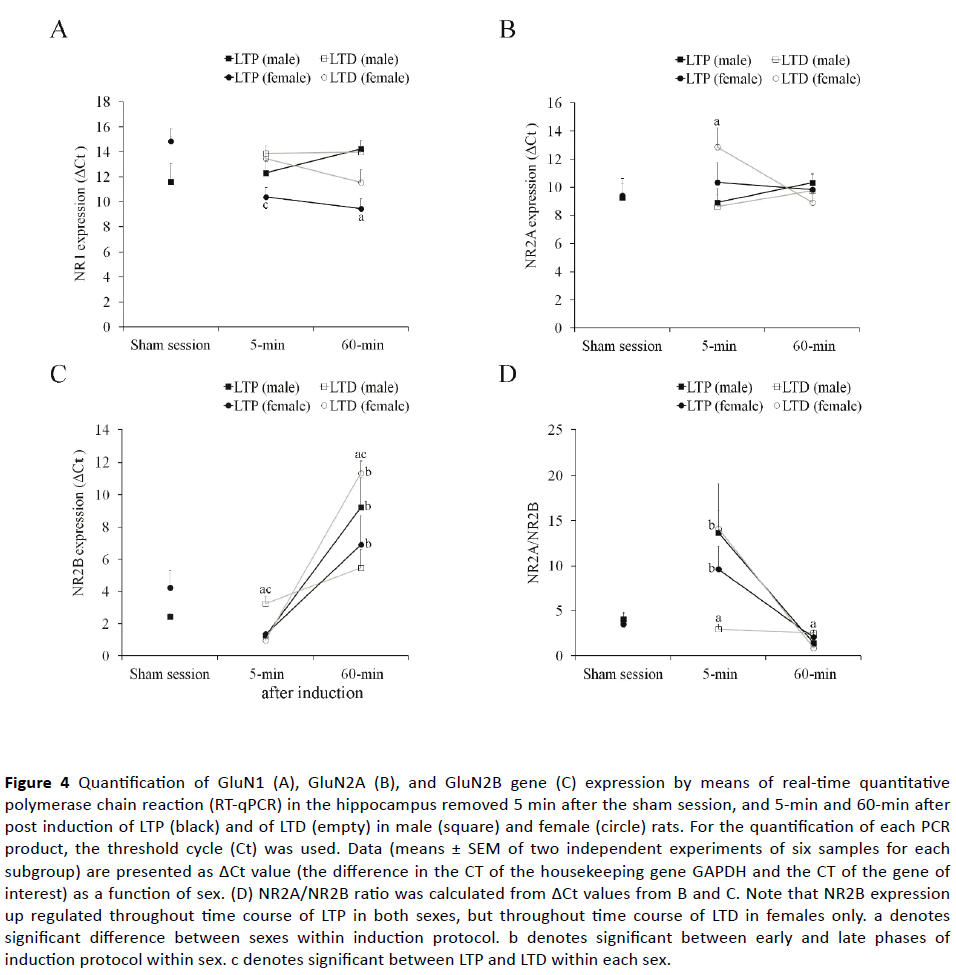
Figure 4 Quantification of GluN1 (A), GluN2A (B), and GluN2B gene (C) expression by means of real-time quantitative polymerase chain reaction (RT-qPCR) in the hippocampus removed 5 min after the sham session, and 5-min and 60-min after post induction of LTP (black) and of LTD (empty) in male (square) and female (circle) rats. For the quantification of each PCR product, the threshold cycle (Ct) was used. Data (means ± SEM of two independent experiments of six samples for each subgroup) are presented as ΔCt value (the difference in the CT of the housekeeping gene GAPDH and the CT of the gene of interest) as a function of sex. (D) NR2A/NR2B ratio was calculated from ΔCt values from B and C. Note that NR2B expression up regulated throughout time course of LTP in both sexes, but throughout time course of LTD in females only. a denotes significant difference between sexes within induction protocol. b denotes significant between early and late phases of induction protocol within sex. c denotes significant between LTP and LTD within each sex.
Baseline gene expression: Based on transcript abundance, GluN1 was highly expressed (Ct<20), GluN2A was expressed at a moderate level (Ct=20–25), and GluN2B showed less expression levels (Ct>25) in the sham-treated hippocampus of both sexes. None of transcript significantly differed between sexes, but a trend to increase was observed in female rats compared to male rats for ΔCt values of NR1 transcript (t10=2.14; p=0.058) after sham session.
Plasticity-dependent gene expression: A repeated measures of ANOVA on the ΔCt values of NR1 transcript revealed that male and female rats responded differently to induction protocols in early and late phases, as indicated by significant interaction between sex and protocol (F1, 10=6.40; p=0.003), between sex and phase (F1, 10=7.12; p=0.024), as well as a significant main effect for sex (F1, 10=11.30; p=0.007) and for phase (F1, 10=19.55; p=0.001) but not for induction protocol (p>0.05). A further analysis of significant interaction effects revealed that NR1 mRNA expression in early and late phases of both form plasticity was similar both between and within sexes, except which demonstrated higher expression in males than in females at 60-min after LTP induction (t10=4.71; p =0.001). In addition, NMDAR subunit NR1 demonstrated higher expression in early phase of LTD than LTP in female rats (t10=2.98; p=0.014) (Figure 4A).
A repeated measure of ANOVA on the ΔCt values of NR2A transcript revealed a significant sex x protocol interaction (F1, 10=5.79; p=0.037), in the absence of any significant main effect. A further analysis of significant interaction effect revealed that GluN2A mRNA expression in early and late phases was similar both between induction protocols and between and within sexes, except which demonstrated higher expression in females than in males at 5-min after LTD induction (t10=2.24; p =0.049; Figure 4B).
A repeated measure of ANOVA on the ΔCt values of NR2B transcript yielded a main effect for induction protocol (F1, 10=67.60; p<0.001), such that the average ΔCt was significantly higher for LTD than for LTP. The main effect of sex and the main effect of phase were non-significant (p>0.05). However, most of the interaction effects were significant (sex x phase x protocol interaction: F1, 10=19.38; p=0.001; sex x phase: F1, 10=5.19; p=0.046; but sex and protocol: p=0.099), indicating that the effect of induction protocol on NR2B transcript differs between sexes and between phases. A further analysis of significant interaction effect revealed that, although GluN2B mRNA expression in early and late phases was similar between sexes in LTP-induced hippocampus of male rats, it was lower in females than in male rats at 5-min (t10=5.26; p=0.001), but higher 60-min after LTD induction (t10=4.27; p=0.003; Figure 4C). GluN2B mRNA expression was increased from early to late phase of both LTP (t10=3.06; p=0.012) and LTD (t10=14.67; p<0.001) in females, whereas, such an increase was only observed after LTP induction (t10=4.23; p=0.002), but not after LTD induction (t10=2.66; p=0.073) in male rats (Figure 4C). In addition, subunit GluN2B mRNA demonstrated higher expression in early LTP phase than early LTD phase in male rats (t10=3.87; p=0.004) and in late LTD phase than late LTP phase in female rats (t10=2.29; p=0.045).
When NR2A/NR2B ratio was calculated for each animal, a repeated measure of ANOVA yielded a main effect for induction protocol (F1, 10=27.70; p<0.001), such that the average NR2A:NR2B ratio was significantly higher for LTP than for LTD. The main effect of sex and the main effect of phase were non-significant (p>0.05). However, sex x phase x protocol interaction was significant (F1, 10=7.60; p=0.020), indicating that the effect of induction protocol on NR2A/NR2B ratio differs between sexes and between phases. A further analysis of significant interaction effects revealed that, although NR2A/ NR2B ratio was unchanged throughout LTP and LTD time courses within male rats, it decreased from 5-min to 60-min after both LTP (t10=2.85; p =0.032) and more obviously LTD time courses (t10=6.66; p<0.001) in female rats (Figure 4D). In addition, this ratio was higher at early phase (t10=5.38; p=0.012) but lower at late phase of LTD time course (t10=3.73; p=0.011) in female rats, whereas comparisons of the time course of LTP at 5 and 60 min after induction revealed no significant difference between sexes (Figure 4D).
Discussion
This study demonstrates that: (1) there appear to be no gross differences in baseline function and glutamatergic receptor subunit expression in the hippocampus between female and male rats. (2) The LTP magnitude is not different depending on sex. (3) The capacity for LTD expression in vivo is different in male and female animals in young adult ages. (4) NR2B expression up regulated throughout time course of LTP in both sexes, but throughout time course of LTD in females only.
According to our study, the LTP magnitude is not different depending on the sex, confirming our previous study [15] and a study that failed to show sex dependency for the LTP of population spike [2]. In contrast, it has been previously reported that the magnitude of the EPSP potentiation in the CA1 region of hippocampus [3,16] and the dentate gyrus [2,17] is significantly lower in female rats than their male counterparts. In these studies, LTP has been induced by means of theta-burst stimulation (TBS) mimicking endogenous theta frequency EEG activity (4–8 Hz) recorded in the rat hippocampus during behavioral activities [18]. In contrast to TBS, HFS protocols do not possess a theta-like pattern and produces less potentiation than TBS in the early phase of LTP [19]. Sex difference in LTP can be depend on stimulus protocol because HFS protocol evoked similar LTP in female and male rats, whereas LTP induced by a single TBS was significantly larger in male rats than that induced in female rats [3].
The present study found that an LFS protocol (1 Hz for 15 min), which failed to induce LTD at PP-DG synapses in male rats, leads to subsequently induced LTD in female rats. We did not find any difference in pre-LFS synaptic strength between male and female young-adult rats in the DG, confirming previous studies [20,21]. Therefore, sex difference in the LTD magnitude is not due to differences in baseline function between groups. In agreement with the present study, an earlier study found significant LTD in naive female, not male offspring [6]. Male rats in that study showed LTD after LFS when they were exposed stress [6]. On the other hand, several recent studies used male rats aged 3 to 9 mo old have reported the same stimulation protocol does not induce a persistent LTD at CA3-CA1 synapses in vitro [22,23], but rather induces a slow onset long-term potentiation at perforant pathdentate gyrus synapses in vivo [24]. In contrast to the present study, little or no LTD was induced by LFS in hippocampal slices from female rats aged between 40 days and 16 weeks [25]. In that study, field EPSPs were recorded from CA1 region in response to the Schaffer collateral-commissural pathway. Therefore, the dentate gyrus in female rats appears to be more susceptible to LTD than area CA1.
The present data suggest the idea that NMDA receptor subunit composition is differentially regulated in part in male and female rats. Subunit composition can determine the direction of synaptic plasticity because the GluN2A subunit is involved in the induction of LTP, whereas the GluN2B subunit contributes to the formation of LTD in the hippocampus and cortex [26,27]. The observed (non-significant) slight differences which were noted in the mRNA levels of GluN2A subunit and GluN2B between sham-treated hippocampus of male and female rats suggest capacity of LTP and LTD induction is comparable between female and male rats. In the present study, this was confirmed by no significant differences in the initial potentiation/depression of PS amplitude and EPSP slope. Moreover, no significant difference in mRNAs for GluN2A and GluN2B subunits supports comparable LTP magnitudes between females and males.
In this study, we describe two important findings pertaining to sex-based differences in NMDAR subunits. The first finding is that, during the time course of LTD, increasing GluN2B subunit expression and decreasing GluN2A/GluN2B is observed in female, but not male rats. The second finding is that LFS-induced expression of NR2B subunit is more pronounced compared to HFS-induced expression at early phase in male rats but late phase in female rats. Nevertheless, induction of LTP do not show sex-based differences in expression pattern of GluN2A and GluN2B subunits during LTP time course. Because current evidence does suggest that the GluN2B subunit might be particularly important for LTD, these findings confirm the ability of female rats for express a durable LTD. These findings have also extended our knowledge about sex-specific regulation NMDA receptors reported by a study using quantitative in vitro receptor autoradiography technique and showing more NMDA receptors in the hippocampus of male rats than that of female rats [28] and a study showing estrogen-regulated GluN1 and GluN2A expressions in female rats [29]. However, the present findings do not mean that female synapses contained more functional GluN2B receptors but rather suggest that they are in the process of expressing more functional GluN2B after undergoing LFS-induced LTD, because we measured mRNA levels.
This sex specific modulation of synaptic plasticity by estrogens could be responsible for the increased LTD magnitude in young female rats. Nevertheless, how the estrous cycle affects LTP and LTD is not well understood. Although maximal induction of LTP has been occurred in female rats during the afternoon of proestrus, when endogenous estrogen concentrations are highest [20], no study investigated the relationship between LTP and estrous cycle. In present study, we selected females on proestrus phase. It has been reported that high estrogen levels prevent the inhibitory-avoidance task-induced delivery of AMPARs into hippocampal CA3-CA1 synapses [30], and that NMDAR activation during HFS results in the delivery of AMPARs to spines, while NMDAR activation during LFS results in AMPAR removal [31]. If this is the case, it would suggest that an inhibition of delivery of AMPARs into synapses might further enhance the capacity for LTD in female rats. The present study has focused on sex difference in the LTP/LTD, but not on changes in LTP/LTD across menstrual cycle. Nevertheless, investigating the relation between expression levels of genes related with synaptic plasticity forms and estrogen levels in female rats will provide us invaluable information in this area.
Generally, male rats out-performed female rats in tests of spatial ability [1,4,32-34], although female superiority [35] or equal performance [36] was also noted. In that case, how can be any prediction regarding spatial memory made based on female's LTD ability? LTP contributes to the formation of new synapses [37], while LTD may facilitate the loss of inappropriate synaptic contacts by causing synapse weakening [38] and/or prevent synaptic saturation in neural networks [39]. It has been reported that hippocampal LTD is related to memory processes requiring behavioral flexibility in spatial learning [40], and that hippocampal lesions impair behavioral flexibility [41]. Moreover, LTD-like mechanisms have been demonstrated to involve reversal learning in the Morris water maze [42]. Results from those experimental studies suggest that females are behaviorally more flexible, whereas males show a greater degree of behavioral persistence [43]. Therefore, more depressed LTD observed in female rats suggests greater female behavioral flexibility. Moreover, females can be expected to forget learned information and to learn a new one more easily than males.
Conclusion
In conclusion, the capacity for LTD expression in vivo is higher in female rats compared to male rats in young adult ages, and this sex difference was paralleled by a sex difference in the expression of GluN2B subunit generated by perforant path LFS. These alterations could be related to the modulation of NMDA receptors function and of synaptic plasticity by estrogens. The present study suggests that sex differences in hippocampus-dependant learning tasks may be result of sexually dimorphic hippocampal LTD, but not LTP.
Acknowledgements
This research was financially supported by TUBITAK grant number 113S345 to C.S. This work was further supported by the Erciyes University Research Found grant number TOA-2013-4555. The authors have no conflicts of interest to declare.
23356
References
- Jonasson Z (2005) Meta-analysis of sex differences in rodent models of learning and memory: A review of behavioral and biological data. Neurosci Biobehav Rev 28: 811-825.
- Maren S, Deoca B, Fanselow MS (1994) Sex-differences in hippocampal long-term potentiation (Ltp) and Pavlovian fear conditioning in rats - Positive correlation between Ltp and contextual learning. Brain Research 661: 25-34.
- Yang DW, Pan B, Han TZ, Xie W (2004) Sexual dimorphism in the induction of LTP: critical role of tetanizing stimulation. Life Sci 75: 119-127.
- Monfort P, Gomez-Gimenez B, Llansola M, Felipo V (2015) Gender differences in spatial learning, synaptic activity, and long-term potentiation in the hippocampus in rats: molecular mechanisms. ACS Chemical Neuroscience 6: 1420-1427.
- Helfer JL, White ER, Christie BR (2012) Enhanced deficits in long-term potentiation in the adult dentate gyrus with 2nd trimester ethanol consumption. PLoS One 7: e51344.
- Titterness AK, Christie BR (2008) Long-term depression in vivo: Effects of sex, stress, diet, and prenatal ethanol exposure. Hippocampus 18: 481-491.
- Cummings JA, Mulkey RM, Nicoll RA, Malenka RC (1996) Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron 16: 825-833.
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529-540.
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, et al. (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62: 405-496.
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, et al. (1992) Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science 256: 1217-1221.
- Kessels HW, Malinow R (2009) Synaptic AMPA receptor plasticity and behavior. Neuron 61: 340-350.
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, et al. (2000) Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nature Neuroscience 3: 1291-1300.
- Yashiro K, Philpot BD (2008) Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 55: 1081-1094.
- Artis AS, Bitiktas S, Taşkın E, Dolu N, Liman N, et al. (2012) Experimental hypothyroidism delays field excitatory post-synaptic potentials and disrupts hippocampal long-term potentiation in the dentate gyrus of hippocampal formation and Y-maze performance in adult rats. J Neuroendocrinol 24: 422-433.
- Elmarzouki H, Aboussaleh Y, Bitiktas S, Suer C, Artis AS, et al. (2014) Effects of cold exposure on behavioral and electrophysiological parameters related with hippocampal function in rats. Front Cell Neurosci 8: 253.
- Monfort P, Felipo V (2007) Hippocampal long-term potentiation is reduced in mature compared to young male rats but not in female rats. Neuroscience 146: 504-508.
- Bronzino JD, Kehoe P, Austin-LaFrance RJ, Rushmore RJ, Kurdian J (1996) Neonatal isolation alters LTP in freely moving juvenile rats: Sex differences. Brain Research Bulletin 41: 175-183.
- Mizuseki K, Buzsaki G (2014) Theta oscillations decrease spike synchrony in the hippocampus and entorhinal cortex. Philos Trans R Soc Lond B Biol Sci 369: 20120530.
- Hernandez RV, Navarro MM, Rodriguez WA, Martinez JL Jr, LeBaron RG (2005) Differences in the magnitude of long-term potentiation produced by theta burst and high frequency stimulation protocols matched in stimulus number. Brain Res Brain Res Protoc 15: 6-13.
- Warren SG, Humphreys AG, Juraska JM, Greenough WT (1995) LTP varies across the estrous cycle: Enhanced synaptic plasticity in proestrus rats. Brain Research 703: 26-30.
- Maren S (1995) Sexually dimorphic perforant path long-term potentiation (LTP) in urethane-anesthetized rats. Neurosci Lett 196: 177-180.
- Norris CM, Korol DL, Foster TC (1996) Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci 16: 5382-5392.
- Wong TP, Howland JG, Robillard JM, Ge Y, Yu W, et al. (2007) Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc Natl Acad Sci USA 104: 11471-11476.
- Gonzalez J, Morales IS, Villarreal DM, Derrick BE (2014) Low-frequency stimulation induces long-term depression and slow onset long-term potentiation at perforant path-dentate gyrus synapses in vivo. Journal of Neurophysiology 111: 1259-1273.
- Kemp N, McQueen J, Faulkes S, Bashir ZI (2000) Different forms of LTD in the CA1 region of the hippocampus: Role of age and stimulus protocol. Eur J Neurosci 12: 360-366.
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, et al., (2004) Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304: 1021-1024.
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, et al. (2007) Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology 52: 60-70.
- Palomero-Gallagher N, Bidmon HJ, Zilles K (2003) AMPA, kainate, and NMDA receptor densities in the hippocampus of untreated male rats and females in estrus and diestrus. J Comp Neurol 459: 468-474.
- Adams MM, Morrison JH, Gore AC (2001) N-methyl-D-aspartate receptor mRNA levels change during reproductive senescence in the hippocampus of female rats. Experimental Neurology 170: 171-179.
- Tada H, Koide M, Ara W, Shibata Y, Funabashi T, et al. (2015) Estrous cycle-dependent phasic changes in the stoichiometry of hippocampal synaptic AMPA receptors in rats. PLoS One 10: e0131359.
- Heynen AJ, Quinlan EM, Bae DC, Bear MF (2000) Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron 28: 527-536.
- Williams CL, Meck WH (1991) The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology 16: 155-176.
- Roof RL, Havens MD (1992) Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res 572: 310-313.
- Jones CM, Braithwaite VA, Healy SD (2003) The evolution of sex differences in spatial ability. Behav Neurosci 117: 403-411.
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH (2000) In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiology & Behavior 70: 311-317.
- Healy SD, Braham SR, Braithwaite VA (1999) Spatial working memory in rats: no differences between the sexes. Proceedings of the Royal Society B-Biological Sciences 266: 2303-2308.
- Engert F, Bonhoeffer T (1999) Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399: 66-70.
- Heynen AJ, Yoon BJ, Liu CH, Chung HJ, Huganir RL, et al. (2003) Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci 6: 854-862.
- Rolls ET, Treves A (1998) Neural networks and brain function. Oxford university press Oxford, UK.
- Nicholls RE, Alarcon JM, Malleret G, Carroll RC, Grody M, et al. (2008) Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron 58: 104-117.
- Ramos JMJ, Vaquero JMM (2000) The hippocampus and flexible spatial knowledge in rats. Journal of Physiology and Biochemistry 56: 313-320.
- Dong ZF, Bai Y, Wu X, Li H, Gong B, et al. (2013) Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology 64: 65-73.
- Lucon-Xiccato T, Bisazza A (2014) Discrimination reversal learning reveals greater female behavioural flexibility in guppies. Biology Letters 10: 20140206.









