Olumide O Adenmosun1,2,4,*, Waseem Asghar3 and James Kumi-Diaka1
1Department of Biological Sciences, Florida Atlantic University, 3200 College Avenue, Davie FL 33314 USA
2Department of Biological Sciences, Bowen University, P.M.B. 284, Iwo, Osun State Nigeria
3Department of Computer and Electrical Engineering and Computer Science, Florida Atlantic University, 777 Glades Road, Boca Raton FL 33431 USA
4Eurekan Biotechnologies, Abeokuta, Ogun State, Nigeria
*Corresponding Author:
Olumide O Adenmosun
Department of Biological Sciences, Florida Atlantic University
3200 College Avenue, Davie FL 33314 USA
Tel: +19548640860
E-mail: olucyno@gmail.com
Received date: December 12, 2016; Accepted date: February 22, 2017; Published date: February 28, 2017
Citation: Adenmosun OO, Asghar W, Kumi-Diaka J. Sickle Cell Sperm Selection with Hb-S Mab: A Future Application for Intracytoplasmic Genotypically Selected Sperm Injection (IGSI). Arch Clin Microbiol. 2017, 8:1. doi: 10.4172/1989-8436.100064
Copyright: © 2017 Adenmosun O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Sickle cell sperm; IVF/PGD; Hb-S; Monoclonal antibodies; Hbb; Hemoglobin; IGSI
Introduction
Sickle cell disorder is a genetic disease that denotes all genotypes that includes the presence of at least one Hb-S gene in a homozygous state. Its distribution cuts across Africa, some parts of Asia, and as a result of the vagaries of war and slave trade in the mid twentieth century – the disease was further disseminated in diaspora including Europe and the Americas. Five genotypes have been indicated in its pathophysiology and they include – HbSS, HbSC, HbSβ0, HbSβ+, and HbS/HbE syndrome [1].
Sickle Cell Epidemiology
The epidemiology of sickle cell disorder as reported by the Centers for Disease Control (CDC) from a 2008 census indicates about 100,000 known cases of homozygous subjects in the USA – mostly African-Americans; and a current prevalence of 1 in 12 persons in that ethnic subgroup have the sickle cell trait in a heterozygous state [2]. In the African continent, the prevalence of heterozygous subjects is 1 in 4 persons [3]. Being a genetic predisposition, there is no known cure for sickle cell disorder except for the immunologically-invasive treatment by stem cell transplant from bone marrow or cord blood [4]. Sickle cell trait among African descents is associated with natural selective pressures for survival where protective adaptive mechanisms against malaria have been recorded for individuals with the sickle cell trait. However, the consequences of continued inbreeding among surviving heterozygotes further increases the global pool of sickle cell births [5]. A genetic outcross of couples with the sickle cell trait usually predisposes them to a twentyfive per cent chance of having a sickle cell progeny [3].
Preventing Sickle Cell Births
There are no medical alternatives known to prevent sickle cell births from heterozygous couples (each with the sickle cell trait), other than the bio-ethically controversial processes of IVF/PGD (Preimplantation Genetic Diagnosis following In-Vitro Fertilization) or prenatal testing-including amniocentesis, chorionic villus testing or percutaneous umbilical cord testing-all of which require that a difficult ethical decision be made on whether or not to terminate growing embryos or fetuses bearing sickle cell genes. To enable the preservation of growing embryos and eliminate genetic combinations that give rise to sickle cell embryos; a scientific method to select sickle cell sperm prior to the events of fertilization and eventual conception is proposed.
Myths Surrounding Hbb Gene Expression in Sperm Cells
Consistent with previous studies, sperm antigen characterization [4,5] may be achievable with known antigenic features that are attributable to specific genotypic characteristics [6]. There is however no study that has yet shown the characterization of hemoglobin or its associated proteins expressed on the sperm cell membrane. Though it is usually hypothetically asserted that specialized gametocytes like the sperm cell do not express the hemoglobin protein; no study has equally shown its total absence as well. The Hbb protein truly has no physiological use in gamete cells, hence it is downregulated by the GATA-1 transcription factor during pre-meiotic stages [6]. The concept of down-regulation does not however mean that protein expression is out-rightly excluded, but rather reduced. In a study by Ike et al. (2007) [7] on the DNA microarray analyses of gene expression in testes producing haploid germ cells, Hbb proteins were found to be well expressed in juvenile mice testes contrary to the expected pattern of deregulated expression in gamete tissues [7]. Bioinformatics data from studies pooled on GeneCards also show significant expression profiles for Hbb in human reproductive tissues such as the prostate and testes. For a wellknown gene such as Hbb (Alias – CD113t-C), its characterization – as a translated protein on/in a haploid germ cell may be achievable by immuno-assay studies with specific monoclonal antibodies.
Characterization of Sickle Cell Sperm with an Hb-S Mab
A blind immuno-assay study using a monoclonal antibody (Mab) for sickle cell hemoglobin (HuS 1&2 – described elsewhere) [8] was therefore conducted on a semen sample belonging to one of the authors who is a heterozygous sickle cell carrier (Hemoglobin Genotype - AS).
The methodology adopted included pre-treating a 96-well plate with Hb-S Mab for 48 hours, which were then washed with PBS to remove excess unattached Mabs in the wells. Incapacitated sperm cells disrupted by pre-treating with 30% alcohol were introduced into antibody-activated wells and incubated at room temperature on a plate-rocker at a gentle oscillating speed for an hour. Wells were gently washed with PBS after one hour incubation before observing under a dissecting microscope for any binding or agglutination activity. We observed more sperm cells immobilized in wells functionalized with Hb-S Mabs (Figure 2) compared to the negative control (well without Hb-S Mabs – Figure 1).
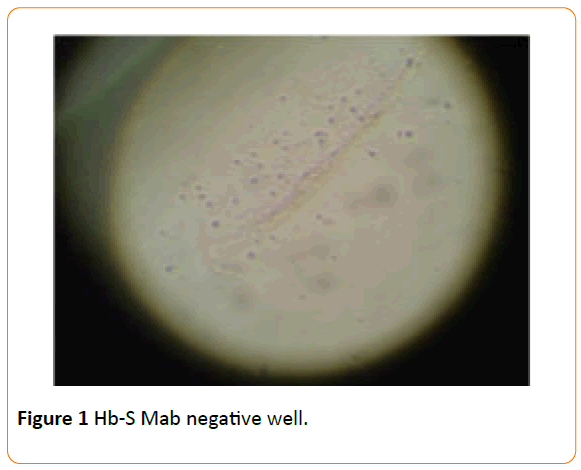
Figure 1 Hb-S Mab negative well.
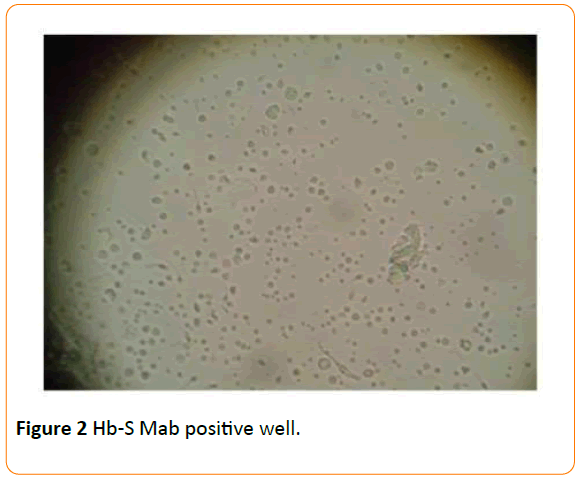
Figure 2 Hb-S Mab positive well.
Future Prospects
This preliminary experimental observations suggest a possible presence and antigenic activity of hemoglobin or its associated proteins on/in the sperm cells of a heterozygous sickle cell carrier. However, before a final scientific assertion can be made regarding the efficacy of this sperm selection method, extensive protein characterization studies are recommended to well delineate the Hb-S molecule on/in haploid germ cells of at-risk couples – who may consider seeking assisted conception alternatives such as IGSI (Intracytoplasmic Genotypically Selected Sperm Injection) to select genetically healthy sperm cells, in a bid to further reduce the chances of having a sickle cell birth. It may also be possible to selectively remove Hb-S positive sperm cells leaving behind normal non-sickle cell sperms that can be used for in vitro fertilization. And in such cases, selection of fertilized embryo may be further confirmed by genetic testing.
18397
References
- Hassell KL (2010) Population estimates of sickle cell disease in the U.S. Am J Prev Med 38: 512-521.
- Goldsmith JC (2012) Framing the research agenda for sickle cell trait: building on the current understanding of clinical events and their potential implications. Am J Hematol 87: 340-346.
- Thompso LM, Ceja ME, Yang SP (2012) Stem cell transplantation for treatment of sickle cell disease: bone marrow versus cord blood transplants. Am J Health Syst Pharm 69: 1295-1302.
- Cabrera G (2005) The sickle cell trait is associated with enhanced immunoglobulin G antibody responses to Plasmodium falciparum variant surface antigens. J Infect Dis 191: 1631-1638.
- Yomogida K, Ohtani H, Harigae H, Ito E, Nishimune Y, et al. (1994) Developmental stage- and spermatogenic cycle-specific expression of transcription factor GATA-1 in mouse sertoli cells. Development 120: 1759-1766.
- Ike A, Tokuhiro K, Hirose M, Nozaki M, Nishimune Y, et al. (2007) Comprehensive analyses of gene expression in testes producing haploid germ cells using DNA microarray analyses. Int J Androl 30: 462-475.
- Jensen RH, Vanderlaan M, Grabske RJ, Branscomb EW, Bigbee WL, et al. (1985) Monoclonal antibodies specific for sickle cell hemoglobin. Hemoglobin 9: 349-362.







