Keywords
Type 2 diabetes mellitus, Total oxidative stress, Total antioxidant defense, Fasting blood glucose
Abbreviations
TOS: Total Oxidative Stress, TAD: Total Antioxidant Defense, mM/l: milimol/litre, FBG: Fasting Blood Glucose, H2O2: Hydrogen Peroxide, TRAP: Total Radical Trapping Antioxidant Parameter, ORAC: Oxygen Radical Absorbance Capacity, DCFH-DA: DiChloroFluorescein-DiAcetate, R-Pe: Phycoerythrin, TEAC: Trolox Equivalent Antioxidant Capacity, FRAP: Ferric Reducing Ability of Plasma, FORT: Free Oxygen Radical Test, FORD: Free Oxygen Radical Defense, r.p.m.: revolutions per minute, FeCl3: Ferric Chloride, PBS: Phosphate Buffered Saline, AGE: Advanced Glycated Endproducts, UV-VIS: Ultraviolet and Visible, EPR: Electron Paramagnetic Resonance, FOX: Ferrous Oxidation in Xylenol Orange, ROM: Reactive Oxygen Metabolites, TMB: Tetra Methyl Benzene, ABTS: 2,2'-azinobis3- ethylbenzthiazoline-6-sulfonic acid, FRA: Ferric Ion-Reducing Ability, GOD-POD: Glucose oxidase-peroxidase.
Introduction
In the recent years the measurement of oxidative stress and antioxidant status in plasma, other body fluids and extracts has been considered to be included in the methodology by many researchers in their studies. Monitoring oxidative stress in humans is achieved by assaying products of oxidative damage or by investigating the potential of an organism, tissue or body fluids to withstand further oxidation. Unfortunately, there is little consensus about the selection of parameters of oxidative stress or antioxidant state in defined patients or diseases. This is not only due to the uncertainty whether or not a certain parameter is playing a causative role. Either distinct antioxidant components are assayed or the total antioxidant potency is estimated by the combined reducing activities of a given body fluid, especially plasma [1]. The plasma total antioxidant capacity is modulated either by radical overload or by intake of dietary antioxidants and can therefore be regarded as more representative of the in vivo balance between oxidizing species and antioxidant compounds, known and unknown, measurable and not measurable, than the concentration of single, selected antioxidant. Moreover, the methods of determination described in literature represent very different levels of analytical practicability, costs, and quality. Generally accepted reference ranges and interpretations of pathological situations are lacking as well as control materials. So the availability of rapid and simple assays which measures the combined effect of the overall oxidative stress and antioxidant defenses in blood is of fundamental importance to provide an index of ability to resist oxidative damage.
Many analytical methods have been developed at present to determine the antioxidant activity such as the total radical trapping antioxidant parameter (TRAP) assay [2], the oxygen radical absorbance capacity (ORAC) assay [3] as well as dichlorofluorescein-diacetate (DCFH-DA) [4], phycoerythrin (R-Pe) [5] and crocin bleaching assays [6] are included in the first category of methods. The second approach to measure antioxidant capacity in plasma is to quench a stable and preformed radical that does not act as pro-oxidant. Trolox Equivalent Antioxidant Capacity (TEAC) assay [7] Ferric Reducing Ability of Plasma (FRAP) assay [8] cyclic voltammetry [9] procedure are included in this second category of methods.
However, the test kits for photometric determinations applicable to small laboratories or Points of Care are increasingly available. In the screening of such simplified procedures, we found the FORT (Free Oxygen Radical Test) [10,11] and FORD (Free Oxygen Radical Defense) method developed by Callegari, Parma, Italy, Catellani Group, has been used in some studies [10].
We found the non-lyophilized chromogen in solution procured and used in our laboratory (lyophilized form used in FORT assay-4-amino-N-isopropyl-aniline hydrochloride) is very unstable and even when stored at -20°C, the reagent degraded within 3-4days. Other researchers also observed the same and criticized the method [12]. The chromogen (N,N-dimethyl-p- phenylenediamine sulphate) used for FORD test was found to be more stable even at room temperature. The current study was aimed to estimate Total Oxidative Stress (TOS) and Total Antioxidant Defense (TAD) levels in plasma in patients with diabetes mellitus by two simple colorimetric tests developed and standardized in our laboratory and to find out their relationship with the fasting blood glucose (FBG) levels.
Methods
Human subjects, controls, selection criteria: Sixty-two diabetic patients (30 males and 32 females) were enrolled for the present study, with their ages ranging from 20 to 50 years, and similar number of healthy voluntary controls (37 males and 25 females) of the same age group was recruited for the study. The study was preapproved by the Institutional Ethics Committee of N.R.S. Medical College, Kolkata, India. Patients having other endocrine disorders like thyroid disorders, pregnant mothers, patients suffering from polycystic ovarian disease, renal failure, any malignant disease and patients receiving antioxidant agents are excluded from the study.
Sample Collection: 5ml of whole blood (after 8 to 10 hours calorie deprivation) was drawn aseptically from superficial vein, in heparin containing vials, centrifuged for 5 minutes at 2500 r.p.m., plasma was used for estimation of different biochemical parameters.
Assay of Total Oxidative Stress (TOS) And Total Antioxidant Defense (TAD) in plasma:
Reagent Preparation:
1. Chromogen Reagent solution: Freshly prepared 100 milimolar working chromogen solution (N,N-dimethyl- pphenylenediamine sulphate, Aldrich, Sigma), by dissolving 23.5 mg in 10 ml of 20 milimolar PBS (pH 7.4) and kept in 4-8 °C for use during assay.
2. Acetate Buffer (pH 5.2): prepared by mixing 0.1N acetic acid (570 μl to 500 ml deionized water) and 0.1N sodium acetate (2.72g in 200ml) in 42: 158 proportions.
3. 20 Molar PBS (pH 7.4): Prepared by mixing 80.4ml of Solution A (Solution A prepared by dissolving 11.47 gm of disodium hydrogen phosphate dissolved in 1000 ml of deionized water) to 19.6 ml of Solution B(Solution B prepared by dissolving 9.08 gm of potassium dihydrogen phosphate to 1000ml of deionized water), and pH adjusted to 7.4
4. 7.6 Molar (0.26 mg/l) H2O2 prepared by adding 88 microliter of 30% H2O2 to 100 ml of deionised water
5. 100 m Molar FeCl3 ( MW 162.21) solution; prepared by adding 1.622 gm FeCl3 in 100ml of deionized water.
6. 2.5 milimolar Trolox solution; prepared by dissolving 15.6 mg Trolox (MW 250.29) in 25 ml of 20 m Molar PBS and stored in 0-4°C in aliquots.
Estimation of Total Oxidative Stress (TOS) in plasma: [10, 11]
Principle: This test is based on iron catalysed breakdown of hydroperoxides into alkoxyl (RO?) and peroxyl (ROO?) radicals which reacts with the chromogen (N,N-dimethyl- p phenylenediamine sulphate) towards formation of a colored compound the absorbance of which is photometrically detectable. The intensity of the color correlates directly with the quantity of radical compounds, according to the Lambert-Beer’s law, and it can be related to the oxidative status of the sample.
Assay Procedure: 100 microliters of plasma diluted to 20 times in PBS, was dissolved in one ml of acetate buffer. 25microliters of working chromogen solution added, absorbance taken at 505nm by 6 min time-scan in UV-VIS spectrophotometer. The absorbance values obtained at 4 to 6 minutes for each sample against blank, were compared to the curve obtained using H2O2.
Standardization: Standard curve of TOS by modified FORT was prepared using different dilutions of hydrogen peroxide (H2O2) in milimolar concentrations per liter and difference in absorbance values taken at 505nm in a six-minute time-scan between 6th and 4th minute (increase in absorbance noted maximum in this period) as depicted in Figure 1. Each data is the mean of three different values by the same procedure performed in four different occasions. The assay was completed in 6 minutes.
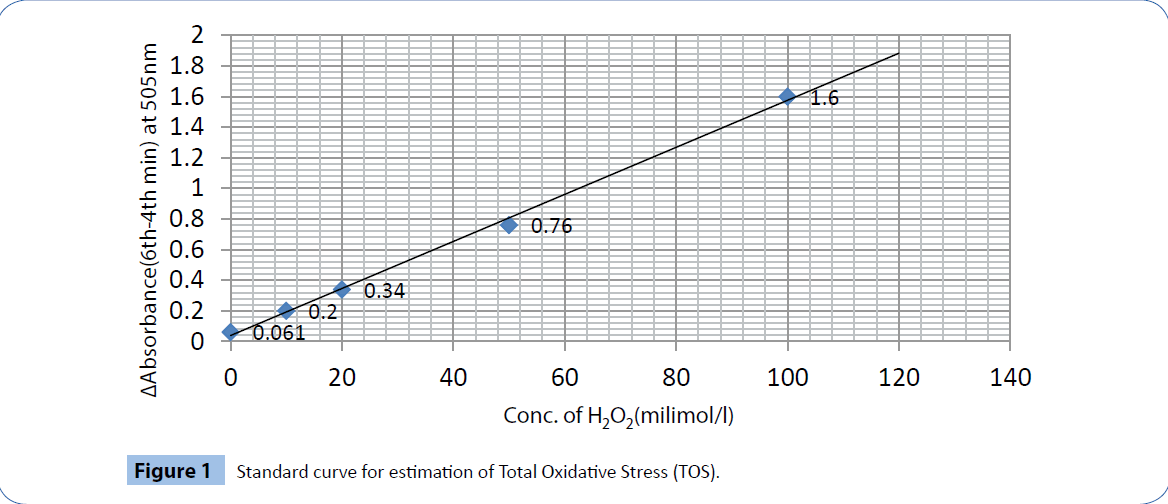
Figure 1: Standard curve for estimation of Total Oxidative Stress (TOS).
Intra assay and inter assay coefficients of variation: The maximum intra assay variation at 4 min was 4.762 and that at 6 min was 4.145 and inter assay variations at 4 and 6 minutes were 2.717 and 2.105 respectively for TOS. The maximum sensitivity of the assay was 1.22 milimol/l H2O2 and the linearity was up to 120milimol/l.
Estimation of Total Antioxidant Defense (TAD) in Plasma: [10]
Principle: In an acidic medium (pH=5.2) and a suitable oxidant (FeCl3), the chromogen (N, N-dimethyl- p- phenylenediamine sulphate) develops a stable and colored radical cation that is photometrically detectable at 505 nm at 37° C. Antioxidant compounds in the sample reduce the radical cation of the chromogen, quenching the color and producing a discoloration of the solution, which is proportional to their concentration. The absorbance values obtained for the samples are compared with a standard curve obtained using Trolox.
Assay procedure: One ml of acetate buffer (pH=5.2) taken in a test tube. 25 microliter chromogen reagent that contains N, N-dimethyl- p- phenylenediamine sulphate and 10 microliter FeCl3 solutions are added. 10 microliter of 20 times diluted plasma added to the mixture, the antioxidant compounds in the sample reduce the chromogen, quenching the color and producing a discoloration of the solution, which is proportional to their concentration.
Standardization: Standard curve of assay of TAD was prepared by using different dilutions of trolox, a potent antioxidant and it quenches the color of the chromogen maximally between 4th and 6th minute. With different concentrations of trolox, (6-hydroxy-2, 5,7,8-tetramethylchroman-2-carboxylic acid), a water soluble form of vitamin E, the difference in absorbance between the absorbance values at 505nm at 4th and 6th minute of the 6 minutes time scan was plot and a calibration curve was constructed (Figure 2).
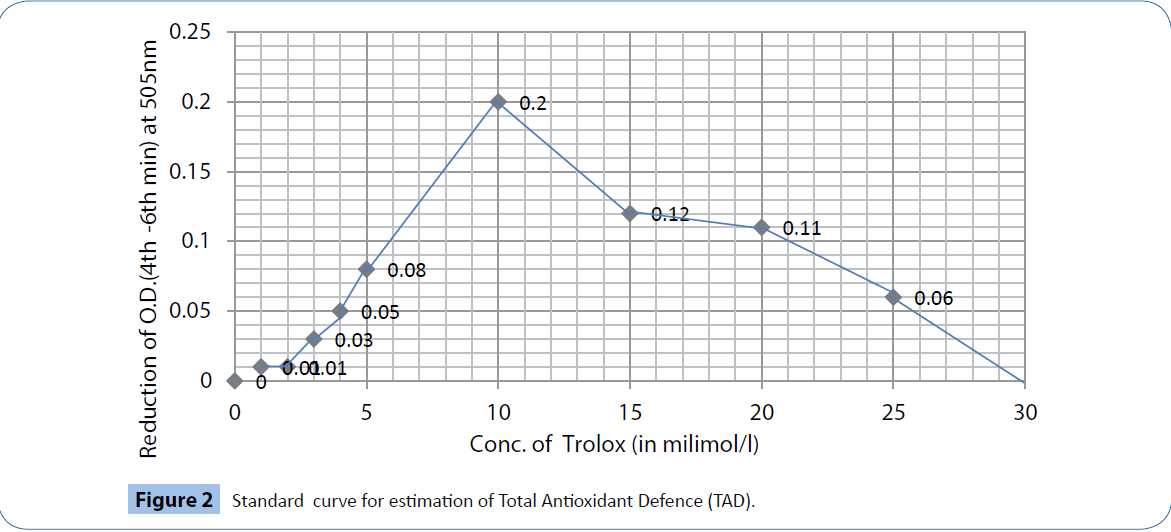
Figure 2: Standard curve for estimation of Total Antioxidant Defence (TAD).
Intra-assay and inter-assay coefficients of variation: The maximum intra-assay variations at 4 and 6 minutes were 7.914 and 9.009 respectively and inter-assay variation at 4minutes and 6minutes were 2.173 and 4.717 respectively, whereas the linearity ranged from 0.25 to 10.0 mmol/l equivalent of trolox.
Estimation of Fasting Blood Glucose: Estimation of fasting blood glucose (FBG) was done by GOD-POD method using standardized reagent kit.
Storage: All the above reagents in our method should be stored for future use in 6 to 8 degree for two weeks except the chromogen reagent (N, N-dimethyl- p- phenylenediamine sulphate) and FeCl3 which were required to be prepared freshly before the tests Plasma samples were stored in -40°C deep freezer. Tests were performed within a week after sample collection.
Statistical Analysis: Data were expressed as mean ± standard deviation (SD), comparison of data was done using unpaired two-tailed Students’ t-test and Pearson’s correlation, P<0.05 was considered as significant. Statistical analysis was done using Microsoft Office Excel-2007 and SPSS Statistics version 2020.
Results
The clinical and biochemical findings of the current study based on the diabetic patients are summarized in Table 1.
| Variables |
Patient mean ± SD |
Control mean ± SD |
P value |
| Age(years) |
44.03±5.27 |
43.63±5.56 |
|
| Sex(M/F) |
30/32 |
37/25 |
|
| Body mass index(BMI) |
24.15±4.09 |
24.81±2.63 |
NS |
| Fasting Blood Glucose(mg/dl) |
118.07±25.75 |
79.06±12.64 |
<0.001* |
| Total Oxidative Stress:TOS (milimol of H2O2/l) |
28.35±11.21 |
11.01 ±4.55 |
<0.001* |
| Total Antioxidant Defence: TAD(milimol/l equivalent of trolox) |
113.71±100.98 |
338.87 ±167.34 |
<0.001* |
Table 1: Clinico-biochemical parameters of the diabetic patients.
In our study, the fasting blood glucose level in patients (118.07 ± 25.75 mg/dl) are significantly (P<0.001) higher than that of the healthy controls (79.06 ± 12.64 mg/dl). We found that TOS values in patients (28.35 ± 11.21 milimol of H2O2/l) are also significantly higher (P<0.001) than that in the control group (11.01 ± 4.55 milimol of H2O2/l). In contrast, the TAD values of patients (113.71 ± 100.98 milimol/l equivalent of trolox) are significantly (Z<0.001) less than that of the controls (338.87 ± 167.34 milimol/l equivalent of trolox).There is a significant positive correlation of FBG levels with TOS (r=0.269, P=0.034, Figure 3) and a significant negative correlation with TAD values(r=-0.267, P=0.036, Figure 4).
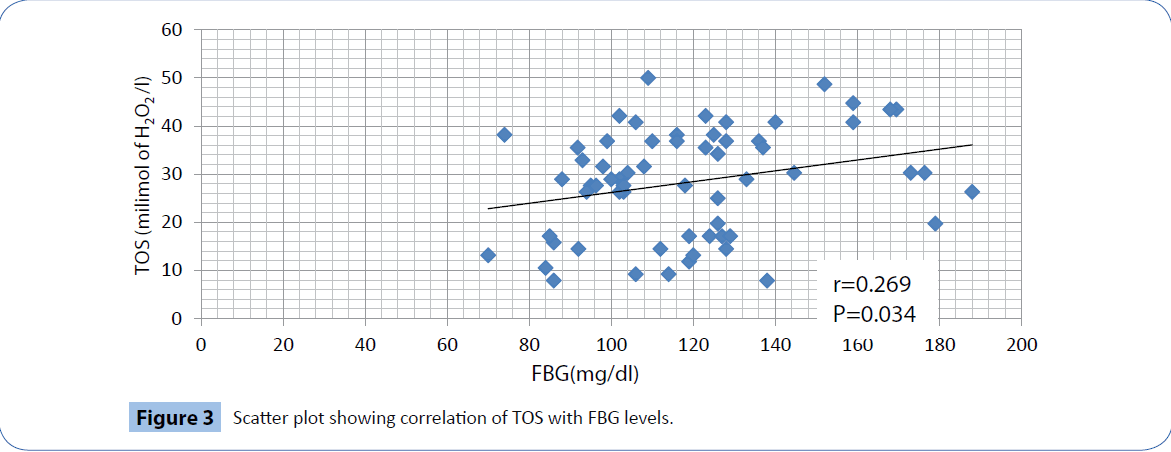
Figure 3: Scatter plot showing correlation of TOS with FBG levels.
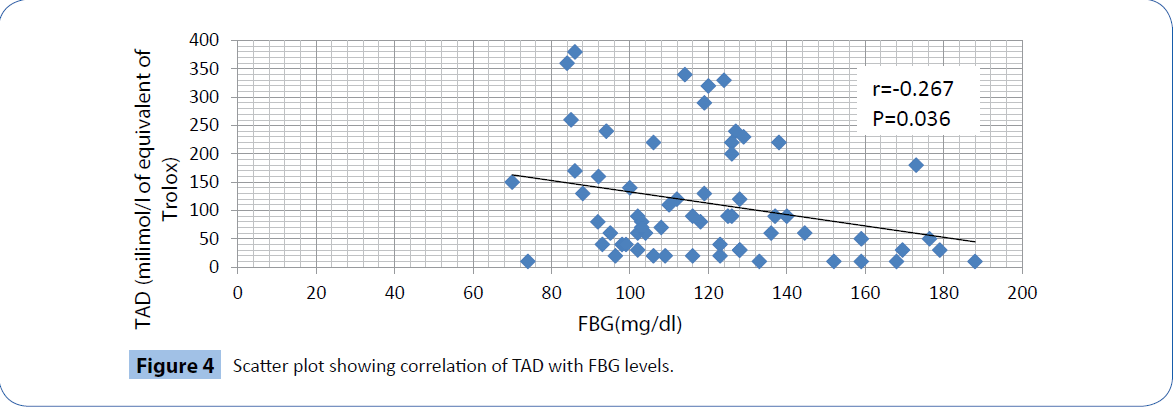
Figure 4: Scatter plot showing correlation of TAD with FBG levels.
Discussion
Diabetes mellitus has already being established as a disease associated with oxidative stress [13-15]. Several studies have reported that the disease causation and progression is due to increased oxidative stress along with deficient antioxidant defense mechanisms [16]. Free radicals play a major role in the etiopathogenesis of this multifactorial disease [17-19]. Glucose toxicity and generation of different advanced glycated end products (AGE), is associated with the generation of free radicals. It has been shown that elevated extra and intra-cellular glucose concentrations result in an oxidative stress, which is defined as an imbalance between prooxidants and antioxidants. Several mechanisms seem to be involved in the genesis of this oxidative stress, which has been reported both in experimental diabetes in animals and in type 1 and type 2 diabetic patients: glucose autooxidation, protein glycation and formation of advanced glycation end-products and the polyol pathway. Reciprocally, oxidative stress is involved in the origin of type 1 diabetes, especially via the apoptosis of pancreatic beta-cells, as well as insulin resistance in type 2 diabetes. Macromolecules such as molecules of extracellular matrix, lipoproteins and deoxyribonucleic acid are also damaged by free radicals in diabetes mellitus [20].
Comprehensive studies with total oxidative stress and total antioxidant defense status in this condition are limited. Direct measurement of free radical levels in biological fluids is extremely difficult, since their half life is short. Electron Paramagnetic Resonance (EPR) spectroscopy is the only direct method for detecting radicals as such with the help of ‘spin-trap’ reagent, but is still far from being a ‘routine procedure’ [21,22]. However, most of the studies have been conducted with different methods consisting of some of the oxidative stress parameters in the tissues or in plasma separately, but the total effects of both the oxidative stress as well as the antioxidant defense have been reported in very few literatures. The three most widely used colorimetric methods for measuring oxidative stress are i) TMB peroxidase assay (3,5,3',5'-tetra methyl benzene) ii) d-ROMs test (ROM-Reactive Oxygen Metabolites), sometimes referred to as the FORT test. iii) FOX (Ferrous Oxidation in Xylenol orange) assay. [23]. Some authors have reported that dROMs or FORT test does not measure the true oxidative stress, but only the ceruloplasmin oxidase activity [21,24,25]. Other authors have claimed that inspite the fact that dROMs test mainly measures ceruloplasmin oxidase activity but it is related to the oxidative stress [26]. Assesment of solitary effect of a deietary antioxidant say flavenoids may be measured in vitro, but inside the body its effects are influenced by other substances. Various assays have been described in which the putative antioxidant is added to a reaction mixture in which free radicals are generated such as 2,2'-azinobis3-ethylbenzthiazoline- 6-sulfonic acid (ABTS) assay, ferric ion-reducing ability (FRA) assay etc. [27]. Commercially marketed kits to measure TAD are very expensive and time consuming [28].
Because of the limitations of original FORT test (the nonlyophilized form of original chromogen in solution turned out unstable), we have used a different chromogen based on the above principle. The same chromogen was also useful for measurement of antioxidant defense. We have further modified and standardised to develop these two modified simple colorimetic methods in our laboratory. As both these tests do not require any sophisticated instruments and are cost effective, may be used by the researchers intending to study oxidative stress parameters working even in a small sized clinical laboratory and in a rural set-up.
Conclusions
We have developed two simple modified colorimetric tests for study of Total Oxidative Stress (TOS) and Total Antioxidant Defense (TAD) in our laboratory using a single chromogen, N,N-dimethylp- phenylenediamine sulphate. The present study further reveals that plasma total oxidative stress and antioxidant defense is well correlated with FBG levels in diabetes. Future study containing measurement of TOS and TAD values in plasma or on other body fluids can be conducted using these simple colorimetric and costeffective methods even in a small clinical laboratory.
Strength and weakness of the study: As both these tests do not require any sophisticated instruments and are cost effective, may be used by the researchers intending to study oxidative stress parameters working even in a small sized clinical laboratory and in a rural set-up which may not have a spectrophotometer but a simple colorimeter which can measure FBG, TOS and TAD. But the nonlyophilized form of chromogen is not very stable in the solution and practically everyday the chromogen and FeCl3 have to be prepared before the two tests were done. The sample size for the current study was small as it was a pilot study. Further studies are required to validate the results of the current study with large sample size.
7035
References
- Halliwell B (2001) Free Radicals and other reactive species in Disease. Encyclopedia of life sciences.
- GreiderCW, Blackburn EH (1985) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43: 405-413.
- Davalos A, Gomez-Cordoves C, Bartolome B (2004) Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J Agric Food Chem 52: 48-54.
- Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free RadicBiol Med 27: 612-616.
- Glazer AN (1990) Phycoerythrin fluorescence-based assay for reactive oxygen species. Methods Enzymol 186: 161-168.
- BathaieSZ, Shams A, MoghadasZadehKermani F (2011) Crocin Bleaching Assay Using Purified Di-gentiobiosylCrocin (-crocin) from Iranian Saffron. Iran J Basic Med Sci 14: 399-406.
- Arts MJ, Haenen GR, Voss HP, BastA (2004) Antioxidant capacity of reaction products limits the applicability of the Trolox Equivalent Antioxidant Capacity (TEAC) assay. Food ChemToxicol 42: 45-49.
- Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem 239: 70-76.
- Psotova J,Zahalkova J, Hrbac J, Simanek V, Bartek J (2001) Determination of total antioxidant capacity in plasma by cyclic voltammetry. Two case reports. Biomed Pap Med FacUnivPalacky Olomouc Czech Repub 145: 81-83.
- Pavlatou MG, Papastamataki M, Apostolakou F, Papassotiriou I, Tentolouris N (2009) FORT and FORD: two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metabolism 58: 1657-1662.
- Palmieri B, Sblendorio V (2007) Oxidative stress tests: overview on reliability and use. Part I. Eur Rev Med PharmacolSci 11: 309-342.
- Jackson MJ (1999) An overview of methods for assessment of free radical activity in biology. ProcNutrSoc 58: 1001-1006.
- ShindeSN, DhadkeVN, Suryakar AN (2011) Evaluation of Oxidative Stress in Type 2 Diabetes Mellitus and Follow-up Along with Vitamin E Supplementation. Indian J ClinBiochem 26: 74-77.
- Ramakrishna V, Jailkhani R (2007) Evaluation of oxidative stress in Insulin Dependent Diabetes Mellitus (IDDM) patients. DiagnPathol 2: 22.
- Wolff SP (1993) Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull 49: 642-652.
- Maitra A, Abbas (2004). Pathologic basis of disease Cotran. Robbins: 1191-1192
- Ceriello A (2000) Oxidative stress and glycemic regulation. Metabolism 49: 27-29.
- BaynesJW (1991) Role of oxidative stress in development of complications in diabetes. Diabetes 40: 405-412.
- BaynesJW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48: 1-9.
- Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J (2000) Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab 26: 163-176.
- Harma MI, Harma M, Erel O (2006) The FORT test--a novel oxidative stress marker or a well-known measure of ceruloplasmin oxidase activity? Atherosclerosis 187: 441-442.
- Kopani M, CelecP, Danisovic L, Michalka P, Biro C (2006) Oxidative stress and electron spin resonance. ClinChimActa 364: 61-66.
- Buico A, Cassino C, Ravera M, Betta PG, Osella D (2009) Oxidative stress and total antioxidant capacity in human plasma. Redox Rep 14: 125-131.
- Harma MI, Harma M, Erel O (2006) d-ROMs test detects ceruloplasmin, not oxidative stress. Chest 130: 1276.
- Harma MI, Harma M, Erel O (2006) Are d-ROMs and FRAP tests suitable assays for detecting the oxidative status? Eur J ObstetGynecolReprodBiol 127: 271-272.
- Abramson JL, Jones DP (2006) The FORT test: reply to Dr. Harma and colleagues. Atherosclerosis 187: 443-444.
- Collins AR (2005) Assays for oxidative stress and antioxidant status: applications to research into the biological effectiveness of polyphenols. Am J ClinNutr 81: 261S-267S.
- Gupta R, Sharma M, Lakshmy R, Prabhakaran D, Reddy KS (2009) Improved method of total antioxidant assay. Indian J BiochemBiophys 46: 126-129.










