Keywords
Cholera, Sleep-wake cycle alterations, Stress, Immune responses, Dehydration
Introduction
Cholera is a worldwide devastating infectious disease [1]. First described in south-east Asia in the 16th century, successive epidemics spread throughout the world, reaching Africa in 1971 during the on going 7th pandemic. Today, the large majority of cases are observed on the African continent, especially in sub- Saharan countries, where it represents a major public health problem in large city suburbs and refugee camps [2].
The pathogen is a Gram-negative bacterium of the 0:1 or 0:139 Vibrio cholera serotypes. The disease severity is due to the toxin produced by the bacteria present in the small intestine lumen and brush border of enterocytes, provoking profuse cataclysmic diarrhoea and vomiting that lead rapidly to severe dehydration and potential shock. If untreated, 25-30% of cases may die in 2-3 days from cardiovascular collapse. Emergency rehydration (1-5 % of body weight) reduces the mortality rate to less than 1%. Complementary antibiotic therapy serves to eliminate the vibrio from the digestive tract, thus decreasing portage. Healing is completed in 2 to 3 days, with rapid recovery and without any complication or sequel. Hopefully, an estimated 90% of infected people are either symptom-free or present a non-febrile acute gastroenteritis.
When one speaks about sleep disturbances in cholera, it is in reference to lack of sleep in care givers rather than to patient’s sleep. We found allusions on patient sleep in a narration about Hahnemann’s proposals to treat cholera with Camphor in 1846. It was stated that patients suffer from “sleep loss” and that “sleep returns” when healing [3]. The first attempt to observe such alterations was, to our knowledge, undertaken by our team during the recent cholera epidemics that hit the Congo city of Pointe-Noire (November 2012-April 2013). During the epidemics, 650 patients were officially reported of whom 15 died [4]. Preliminary work on one patient was presented by Moukassa et al. [2], showing sleep diary data revealing large alterations in the sleep and wake alternation that disappeared rapidly after massive rehydration.
We therefore undertook a larger investigation using a sleep diary to confirm such alterations in the sleep-wake cycle.
Patients and Methods
The investigation took place in 2013 in Pointe-Noire, the second largest city of the Republic of Congo with an estimated 1.1 million inhabitants, between January 18 and February 9 (dates of hospital admission: January 18 to February 5). The 33 patients were hospitalized under tents in the dedicated isolation facility at the Loandjili General Hospital. Patients came from different areas of Pointe-Noire. They reported either directly to the cholera facility (15/33 patients), or were transferred from another hospital (Base Hospital of Tie-tie, 12 cases; Military Hospital, 5 cases; Adolphe Sice General Hospital, 1 case). In all patients, the disease started briskly with a profuse diarrhoea accompanied by vomiting, fever and muscle cramps. Dehydration occurred rapidly accompanied with sunken eyeballs, headaches and persisting skin fold after pinching (loss of skin elasticity). One third of patients presented mental clouding. Blood pressure was low. Three patients arrived at the ward in a state of shock.
The patients rested on specialized “beds” made of a rectangular wood table with a hole to evacuated diarrheic flows into a bucket disposed below. The patients received massive rehydration and antibiotherapy. Rehydration consisted of intravenous infusions of Ringer lactate combined with isotonic saline (2:3) and serum bicarbonate 14% (1:3). An adult who had lost 10% of his body weight was infused with the same amount of liquid during the first four hours. As an example, a 60 kg adult would receive 6 litres of liquid infused during the first 4 hours. When vomiting ceased, the patient pursued oral rehydration. Antibiotherapy consisted of intravenous administration of ciprofloxacin (200 mg or 400 mg twice a day). Some patients received the antibiotic per os (500 mg twice a day), especially when they were authorized to leave the facility to go home. In patients who did not vomit, tablets combining sulfamethoxazole and trimethoprime (400 mg) were administered per os.
Sleep-wake observation was conducted with a conventional sleep diary [5], as polysomnography monitoring had been discarded because of the septic conditions. Following the Loandjili General Hospital ethical requirements, the patients or their family consented for the procedure of sleep observation (Trial registration number: 2013-002-MSP-HGL-DAM). The sleep diary (Figure 1) presented 24 columns corresponding to the 24 hours of the day, and 15 rows corresponding to days eventually spent at the isolation facility. Sleep episodes were shaded, wake episodes being left blank. Taking drinks (full circle) and meals (full triangle), as well as nursing (downwards arrows), were also indicated. The sleep diary was completed every 15 minutes by one of the three nurses or assistant nurses who volunteered to make observations. Due to the epidemic, the observers worked on 12-hour shifts.
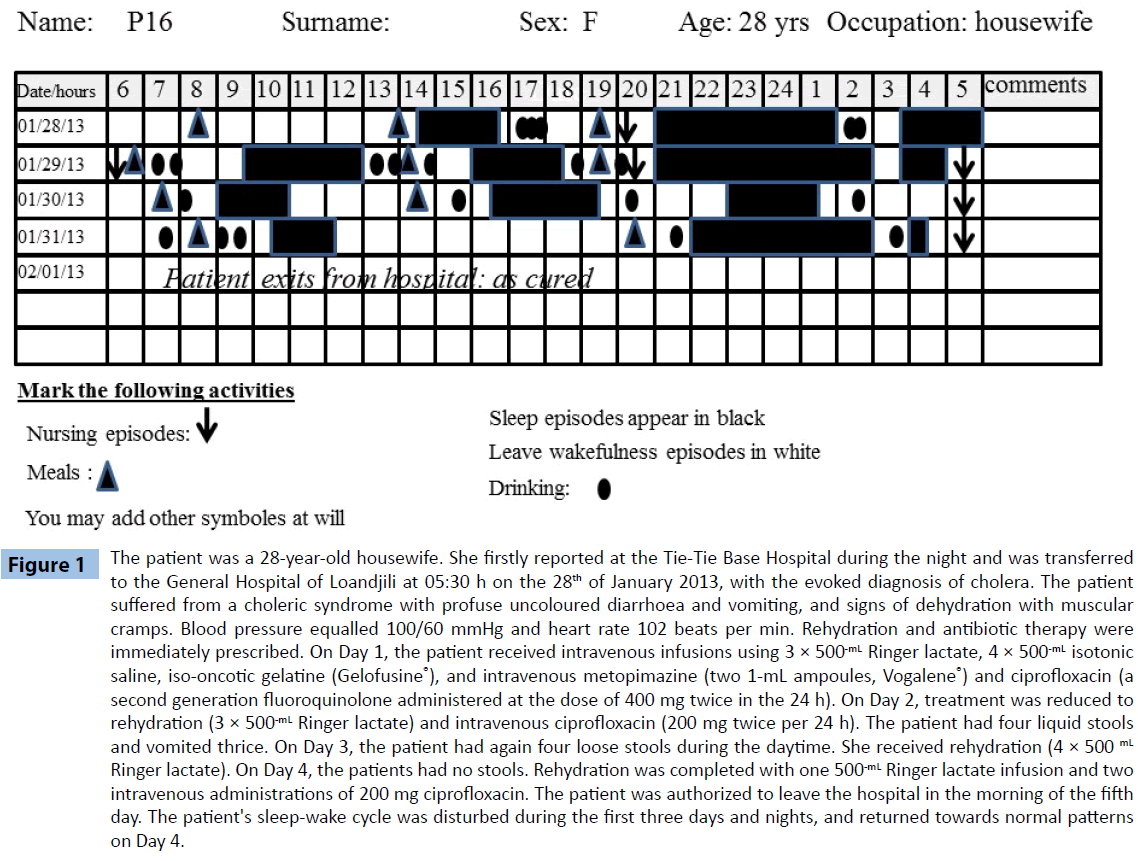
Figure 1: The patient was a 28-year-old housewife. She firstly reported at the Tie-Tie Base Hospital during the night and was transferred to the General Hospital of Loandjili at 05:30 h on the 28th of January 2013, with the evoked diagnosis of cholera. The patient suffered from a choleric syndrome with profuse uncoloured diarrhoea and vomiting, and signs of dehydration with muscular cramps. Blood pressure equalled 100/60 mmHg and heart rate 102 beats per min. Rehydration and antibiotic therapy were immediately prescribed. On Day 1, the patient received intravenous infusions using 3 × 500-mL Ringer lactate, 4 × 500-mL isotonic saline, iso-oncotic gelatine (Gelofusine®), and intravenous metopimazine (two 1-mL ampoules, Vogalene®) and ciprofloxacin (a second generation fluoroquinolone administered at the dose of 400 mg twice in the 24 h). On Day 2, treatment was reduced to rehydration (3 × 500-mL Ringer lactate) and intravenous ciprofloxacin (200 mg twice per 24 h). The patient had four liquid stools and vomited thrice. On Day 3, the patient had again four loose stools during the daytime. She received rehydration (4 × 500 mL Ringer lactate). On Day 4, the patients had no stools. Rehydration was completed with one 500-mL Ringer lactate infusion and two intravenous administrations of 200 mg ciprofloxacin. The patient was authorized to leave the hospital in the morning of the fifth day. The patient's sleep-wake cycle was disturbed during the first three days and nights, and returned towards normal patterns on Day 4.
To accomplish statistical analyses, sleep data were classed into three categories: daytime, night-time and 24-h sleep-wake patterns (daytime+night-time). In each category, the number of sleep episodes, the total duration of sleep and the mean duration of sleep episodes were analysed. Drinks and meals were also analysed in a similar manner. Because of the limited number of patients remaining at the ward on Days 5,6 and 7, statistics were conducted on the first four days (Day 1 to Day 4) spent at the ward. Statistical analysis was performed with one way ANOVA for time effects on each variable, with post-hoc Fisher's PLSD, using the Statview 5 software. The Pearson product-moment correlation coefficient (r) was used to measure linear correlation between the number of meals and drinks vs the number of sleep episodes. Statistical significance was set at P<0.05.
Results
The mean duration of sojourn in the cholera facility equalled 3.4 ± 0.2 days, with a minimum of one day and a maximum of 6.5 days. The 33 patients exited the ward having been rehydrated, treated with antibiotics and cured. All patients were able to drink except on the first day for patient P1, who was maintained at the ward for 6.5 days. Only three patients had difficulties to eat on the first day (patients P1, P12 and P22). These patients spent 4 days at the ward.
Table 1 presents the data obtained on sleep observations. Daytime sleep values showed an interaction with time (Day 1 to Day 4) spent at the ward for total sleep duration (P=0.0203), with a tendency for the number of sleep episodes (P<0.0546), but no time effect for the mean duration of sleep episodes. Total sleep duration on Day 1 was shorter than on Day 2 (P= 0.0351) and higher on Day 2 vs Day 3 (P=0.0042). Compared to Day 1, the number of sleep episodes increased on Day 2 (P=0.0055) and Day 4 (P=0.0399). Compared to Day 3, it was higher on Day 2 (P=0.0007) and Day 4 (p=0.0084). Mean sleep episode duration did not differ from one day to another.
| Sleep characteristics |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
| Daytime sleep |
| Number of sleep episodes |
1.07 ± 0.14 |
1.31 ± 0.11** |
1.00 ± 0.12$$$ |
1.28 ± 0.18*££ |
| Total sleep duration (h) |
3.13 ± 0.36 |
3.95 ± 0.42* |
2.77 ± 0.34$$ |
3.23 ± 0.42 |
| Mean sleep duration (h) |
2.56 ± 0.26 |
2.77 ± 0.21 |
2.34 ± 0.25 |
2.49 ± 0.19 |
| Night-time sleep |
| Number of sleep episodes |
1.73 ± 0.09 |
1.53 ± 0.11 |
1.68 ± 0.12 |
1.36 ± 0.20* |
| Total sleep duration (h) |
5.58 ± 0.31 |
5.23 ± 0.30 |
5.05 ± 0.35 |
4.23 ± 0.52**$ |
| Mean sleep duration (h) |
3.61 ± 0.33 |
3.72 ± 0.27 |
3.36 ± 0.40 |
3.27 ± 0.20 |
| 24-h sleep pattern |
| Number of sleep episodes |
2.8 ± 0.16 |
2.73 ± 0.19 |
2.73 ± 0.20 |
2.91 ± 0.37 |
| Total sleep duration (h) |
8.72 ± 0.50 |
9.2 ± 0.53 |
7.74 ± 0.43$ |
7.89 ± 0.80 |
| Mean sleep duration (h) |
3.27 ± 0.21 |
3.38 ± 0.20 |
3.02 ± 0.24 |
2.81 ± 0.15 |
Table 1: Daytime, night-time and 24-h sleep characteristics during Days 1 to 4 (± standard deviation).
At night-time, a time effect was also found for total sleep duration (P=0.0052), with a tendency for the number of sleep episodes (P=0.0671), but no time effect was observed for mean duration of sleep episodes. Differences were found for total sleep duration on Day 1 vs Day 4 (P=0.0088), and on Day 2 compared to Day 4 (0.0495). The tendency for a time effect on the number of sleep episodes was due to higher values on Day 1 than Day 4 (P=0.0487). Again, mean sleep episode duration was not different between days spent at the ward.
The overall 24-h sleep pattern exhibited a time effect only for total sleep duration (P<0.0001). This effect was related to a significant decrease on Day 3 compared to Day 2 (P=0.0450). No differences were observed between days at the ward for the number of sleep episodes and their mean duration.
Table 2 presents the data on oral rehydration through drinking and eating. During the daytime, there were no differences in the number of drinks between days spent at the ward. Similar data were collected at night-time. However, when the latter data were considered over the 24-hour period, the patients took more drinks on Day 1 (P=0.0014), Day 2 (P<0.0001) and Day 3 (P=0.0031) compared to Day 4. No differences in the number of meals taken during the day were observed throughout the sojourn at the ward. But, at night-time, the number of meals consumed by the patients was low on Day 1 compared to Day 2 (P=0.0048), Day 3 (P=0.0295) and Day 4 (P=0.0106). Consequently, the number of meals taken throughout the 24-hour epoch was highest on Day 2 compared to Day 1 (P=0.0026) and Day 4 (P=0.0057).
| Time period |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
| Number of drinks |
| Daytime |
2.81 ± 0.25 |
1.31 ± 0.31 |
1.00 ± 0.48 |
1.28 ± 0.26 |
| Night-time |
3.13 ± 0.25 |
3.95 ± 0.17 |
2.77 ± 0.21 |
3.23 ± 0.30 |
| 24 hours |
2.56 ± 0.39 |
2.77 ± 0.37 |
2.34 ± 0.50 |
2.49 ± 0.35*$ |
| Number of meals |
| Daytime |
1.52 ± 0.14 |
1.63 ± 0.09 |
1.62 ± 0.13 |
1.42 ± 0.15 |
| Night-time |
0.27 ± 0.08 |
0.59 ± 0.09** |
0.55 ± 0.14* |
0.67 ± 0.19* |
| 24 hours |
1.81 ± 0.16 |
2.22 ± 0.10** |
2.04 ± 0.17 |
1.78 ± 0.17$$ |
*differences with Day 1 with P<0.05, and ** with P<0.01; $differences with Day 2 with P<0.05, and $$ with P<0.01.
Table 2: Daytime, night-time and 24-h drinks and meals during Days 1 to 4 ( ± SEM).
A correlation was found within the 24-hour epoch between the number of meals and the number of sleep episodes (P=0.0013; y=1.256+0.271 x; R2=0.106), and total sleep duration (P=0.0002; y =1.171+0.104 x; R2=0.143). No relationship was found with mean sleep duration of sleep episodes. Similarly, no relationship was found between the number of fluid intakes and sleep patterns.
Discussion
The sleep patterns of patients admitted in the cholera nursing and treatment facility at the Pointe-Noire Loandjili General Hospital were highly disturbed. The patients slept during the day and night in short episodes alternating with wakefulness episodes. In similar climatic conditions, healthy subjects sleep mainly at night with occasional afternoon naps, for a total of 7.5 hours of sleep daily [6]. The patients drunk and ate while awake. The question is whether the observed disruption of the nychthemeral (24-hour) alternation of sleep and wake episodes was due to the intricacy of several environmental and/or endogenous factors.
An elevation of the number of sleep episodes with an increase in total sleep duration occurred on Day 2, especially in the daytime. Such a day-effect is similar to the sleep rebound phenomenon observed after disturbed sleep or loss of sleep [7], or after the so-called first night effect due to sleeping in new environmental or uncomfortable conditions [8]. The bedding and environmental conditions of the cholera facility are uncomfortable and stressful. Lying down on a wood bed with a hole in the middle is highly uncomfortable. The facility environment was noisy, with busy nurses and assistants from the hospital and from the Congo Red Cross having to provide care to neighbouring patients. Patient unrest was also influenced by psychological and emotional factors. It was obviously difficult for the individuals to be exposed to others and, of course, the fear of dying was overwhelming in the patient’s mind. Fluid replacement through an indwelling venous catheter will also disturb sleep and wake patterns [9,10]. For all these reasons, patients at the cholera facility lived in a stressful situation. Stress or exposure to stressful conditions or environment is susceptible to lead to decrements in sleep quality [11,12], with sleep instability due to numerous awakenings, a decrease in slow-wave sleep or deep sleep and/or a decrease in rapid eye movement sleep (REM sleep).
Infection-dependent immune reactions may also be related to the particular aspects of this infectious disease. Von Economo’s report [13] on lethargic encephalitis in patients who suffered from pharyngitis was the first description of the impact of an infectious disease on sleep. In the last 30 years, the influence of bacterial products on sleep has been revealed when pyrogenic and somnogenic activities of muramyl peptides and endotoxin (bacterial lipopolysaccharides, LPS) were described [14,15]. This action is mediated by host’s immune reactions. Several proinflammatory cytokines are pyrogenic and somnogenic, such as tumor necrosis factor-α(TNF-α), interferon-β (IFN-β) and interleukin-1 (IL-1). Cholera Vibrio is susceptible to release pyrogenic bacterial factors, such as muramyl dipeptides, which are known to promote the release of pro inflammatory cytokines. Furthermore, the cholera toxin acts as endotoxin, itself promoting sleep [16]. The intervention of such products is likely to have concurred to the elevation of sleep duration on the first two days and nights at the ward. Thereafter, their treatmentdependent decrement may have allowed sleep time to return below 8 hours per 24 hours, which is considered as the average human sleep duration under normal conditions in intertropical Africa [6,12,17,18].
Cholera toxin provokes water loss and dehydration that is the major cause for fatal outcome. Adequate hydration is essential for maintaining brain function, and dehydration impairs performance and mood, promotes fatigue and provokes confusion, depression and tension proportionally to body weight loss [19]. Increased slow-wave sleep after exercise in Africa has been reported to be a consequence of body temperature elevation [20]. However, such an effect on sleep is hampered by rehydration after exercise, which limits exercise-induced hyperthermia [21].
In conclusion, sleep changes observed in cholera patients during the acute phase of the infectious disease may be related to environmental strains and host’s reactions. Sleep-wake disturbances constitute an integrated response to psychological and physical stress, immune system alterations due to the release of bacterial products especially endotoxin, and to dehydration and rehydration. The simple sleep diary technique may prove to be useful as a non-invasive tool for following cholera patient recovery.
7723
References
- Sack DA, Sack RB, Nair GB, Siddique AK (2004) Cholera. Lancet 363: 223-233.
- Moukassa D, Obengui, Ibara JR (2015) Cholera. Sleep medicine: a comprehensive guide to its development, clinical milestones and advances in treatment. Springer, USA.
- Clarke JH (1999) Cholera, diarrhoea, and dysentery: homeopathic prevention and cure.
- Bastuji H, Jouvet M (1985) [Value of the sleep diary in the study of vigilance dis]. ElectroencephalogrClinNeurophysiol 60: 299-305.
- Bogui P, Keita M, Dah C, Fidier N, Buguet-Brown ML, et al. (2002) [The sleep of Africans and Europeans in the Ivory Coast: questionnaire study]. Sante 12: 263-270.
- Buguet A,Montmayeur A, Pigeau R, Naitoh P (1995) Modafinil, d-amphetamine and placebo during 64 hours of sustained mental work. II. Effects on two nights of recovery sleep. J Sleep Res 4: 229-241.
- Agnew HWJr, Webb WB, Williams RL (1966) The first night effect: an EEG study of sleep. Psychophysiology 2: 263-266.
- Adam K (1982) Sleep is changed by blood sampling through an indwelling venous catheter. Sleep 5: 154-158.
- Buguet A,Tapie P, Bert J (1999) Reversal of the sleep/wake cycle disorder of sleeping sickness after trypanosomicide treatment. J Sleep Res 8: 225-235.
- Buguet A,Cespuglio R, Radomski MW (1998) Sleep and stress in man: an approach through exercise and exposure to extreme environments. Can J PhysiolPharmacol 76: 553-561.
- Buguet A(2007) Sleep under extreme environments: effects of heat and cold exposure, altitude, hyperbaric pressure and microgravity in space. J NeurolSci 262: 145-152.
- Von Economo K (1917) Encephalitis lethargica. Wien KlinWochenschr 30: 581-585.
- Krueger JM,Majde JA (2003) Humoral links between sleep and the immune system: research issues. Ann N Y AcadSci 992: 9-20.
- OppMR,Toth LA (2003) Neural-immune interactions in the regulation of sleep. Front Biosci 8: d768-779.
- Mullington J,Korth C, Hermann DM, Orth A, Galanos C, et al. (2000) Dose-dependent effects of endotoxin on human sleep. Am J PhysiolRegulIntegr Comp Physiol 278: R947-955.
- Gati R, Pétieu R, Wamba B, Buguet A (1990) Human sleep in dry tropical Africa. Pontenagel Press, Germany.
- Tapie P,Buguet A, Tabaraud F, Bogui P, Doua F, et al. (1996) Electroencephalographic and polygraphic features of 24-hour recordings in sleeping sickness and healthy African subjects. J ClinNeurophysiol 13: 339-344.
- Lieberman HR,Bathalon GP, Falco CM, Kramer FM, Morgan CA 3rd, et al. (2005) Severe decrements in cognition function and mood induced by sleep loss, heat, dehydration, and undernutrition during simulated combat. Biol Psychiatry 57: 422-429.
- Montmayeur A,Buguet A (1992) Sleep patterns of European expatriates in a dry tropical climate. J Sleep Res 1: 191-196.
- Montmayeur A,Buguet A, Sollin H, Lacour JR (1994) Exercise and sleep in four African sportsmen living in the Sahel. A pilot study. Int J Sports Med 15: 42-45.







