Keywords
Anxiety, Balb/c mice, Elevated plus maze, Open field, Aqueous extract of Urtica urens, Morocco
Introduction
Anxiety affects one-eighth of the total population throughout the world, and it has become an important area of research in psychopharmacology during this decade [1]. In any given year, approximately 40 million adults are affected by anxiety disorder and can also precipitate or aggravate cardiovascular and behavioural disorders [2]. Benzodiazepines are the major class of compounds used in anxiety and they have remained the most commonly prescribed treatment for anxiety [3].
Current drug therapies for anxiety have many limitations, including the high financial expense (anxiety disorders incur substantial cost to both the individual and society), delay in onset, limited efficacy, unwanted side effects, dependence, and stigma associated with consuming and depending on pharmaceuticals [4,5].
Thus, a need to develop novel treatments to fulfill these shortcomings exists, and the study of medicinal plants could provide new therapeutic options [6].
In Morocco, four species of Urtica which belongs to the Urticaceae family are available [7]. Urtica urens commonly known as herbaceous annual plant species of the genus Urtica, and has long been known to have tranquillizing effects among the Moroccan people [8]. This Small nettle (Urtica urens) has antiinflammatory effect [9], nettle and their hybrids or mixtures are recommended for symptomatic treatment of rheumatoid arthritis or osteoarthritis and for increased dieresis [10]. Main constituents identified in the plant material are flavonoids, caffeoyl-esters, caffeic acid, scopoletin (cumarin), sitosterol (-3-O-glucoside), polysaccharides, fatty acids (e.g. 13-hydroxyoctadecatrienoic acid), minerals (herba: up to 20%; leaves: 1-5%) [11,12].
This work is aimed to evaluate a possible anxiolytic-like effect of aqueous extract of Urtica urens in the elevated plus maze (EPM) and open field (OF) models of anxiety.
Materials and Methods
Animals
Balb/c adult mice (20-30 g) of either sex were used for the study. The animals were acquired from the animal experimental centre of Mohammed V souissi University, Medicine and pharmacy Faculty, Rabat. The animals were maintained in a room with controlled temperature (25 ± 1°C) and lighting (light/dark 12:12 h in polypropylene cages, with food and water ad libitum. Animals were acclimatized to laboratory conditions at least 1 h prior to initiation of experiments. The animals were divided into four groups, each consisting of six mice, implemented in all sets of experiments.
Plant material
The aerial part of Urtica urens was collected from the north of Morocco near the town of Wazzan (Jaaouna el Basra), with assistance of a traditional medical practitioner. The plant was authenticated by botanists of scientific institute Pr. M. Ibn Tatou and Pr. Halim Khammar. A voucher specimen (N° RAB78983) was deposited in the Herbarium of Botany Department of the Scientific Institute of Rabat.
Preparation of the aqueous extract
The aerial part was dried at room temperature and crushed. 700 g of plant material was extracted with six liter of methanol by maceration at room temperature (25°C) over period of 48 hours. Water containing the extract was then filtered through Whatman paper and the solvent was vacuum-distilled at 60°C in a rotary evaporator. The remaining extract was finally dried by desiccator. Final extract was a dark green paste, with 11.92% dry weight. The residue was dissolved in water for final suitable concentrations.
Drugs
The aqueous extract of Urtica urens was suspended in distilled water. Diazepam (ampoule 10 mg/2 ml) was diluted with saline to the required concentration before use. It is well known that benzodiazepines act as anxiolytics at low doses and that they induce sedation and muscle relaxant effects at higher doses [13]. Therefore, we used diazepam (1 mg/kg) as a positive control for anxiolytic-like effects.
Treatment schedule
Experimental groups of mice were treated orally (p.o.) with aqueous extract of Urtica urens at graded doses of (100- 600 mg/ kg), whereas control groups received normal saline by the same routes. Diazepam (1 mg/kg) was administered intraperitoneally (i.p.). All drugs were freshly prepared before each experiment. The doses of extracts were calculated to administer 0.25 ml of the suspension of extracts to the mice of 20 g. The anxiolytic activity was examined by using the anxiolytic activity was examined by using the EPM and OF test.
Acute toxicity study
The procedure was followed as per OECD 423 guidelines [14] (OECD/OCDE. 2002). The extract was administered orally at a dose of 2000 mg/kg body weight. Mice were kept under observed for 14 days to register possible mortality; their weights were registered and study their behavioral neurological toxicity.
EPM test
The elevated plus maze (EPM) was first proposed as an animal model of anxiety by Handley and Mithani [15] Constructed of black colored wooden planks consisting of two close arms, 50 cm × 10 cm × 40 cm, and two open arm, 50 cm × 10 cm, connected to a central platform (10 cm × 10 cm). Covered with a removable lid, such that the open or closed arms were opposite to each other [16]. The maze was elevated to a height of 50 cm above the floor. During the experiment each mouse was placed in the central compartment facing one of the open arms. The number of entries and the time spent in open arms were recorded for 5 min. An entry was counted when all four paws of the mouse entered an open or closed arm. An increase in open arms entries and increase in time spent in open arms were interpreted as an index of potential anxiolytic activity. All test sessions were taped by using a video camera.
Open field test
Six experimental groups of 6 mice were treated with vehicle saline, methanolic extract of Urtica urens (100, 200, 400 and 600 mg/kg, p.o.) or diazepam 1 mg/kg, i.p). After the treatment -sixty minutes for methanolic extract and 30 min for Diazepam- the animals were placed individually in the center of the arena and were subjected to a 5 min period to the open field test. The open field apparatus was an opaque plexiglass cage (72 × 72 cm) with walls 35 cm in height where the floor was divided with white lines by 16 squares (18 × 18 cm) of identical dimension. A digital video camera was installed above the cage to record the activity of the mice. The entire room, except the OF was kept dark during the experiment, in this study was evaluated both the general motor activity of the mice to discard hypoactivity, hyperactivity, or no changes associated with treatments, which could interfere with the behavioral activity of the mice in the elevated plus maze also the observed parameters were as follows: (a) total number of crossings, (b) central area crossings, (c) time spent in central area (d) central area rearing [17,18].
After each test session, the elevated plus-maze apparatus and the open field cage were carefully cleaned with water and allowed to dry to remove the scent of the previously evaluated animal, which could otherwise modify the spontaneous behavior of the subsequent evaluated mice [19].
Also this test reflects the conflict between the innate fear that mice have of the central area of a novel open field versus their desire to explore new environments. When anxious, the natural tendency of mice is to prefer staying close to the walls.
Statistical analysis
All results are expressed as mean ± standard error of the mean. The data were analyzed statistically using one way analysis of variance ANOVA, followed by the Tukey post hoc test for multiple comparisons. P<0.05 was taken to be statistically significant. Results were presented as tables.
Results
Acute toxicity study
Following oral administration aqueous extract of Urtica urens at a dose of 2000 mg/kg, P.O., animals were observed for signs of toxicity such as convulsions, hypothermia, hyperactivity, and grooming continuously for 2 h and for mortality up to 24 h after administration of the doses. No toxicity and no significant changes in the body weight were observed between the treated and control group (Figure 1).
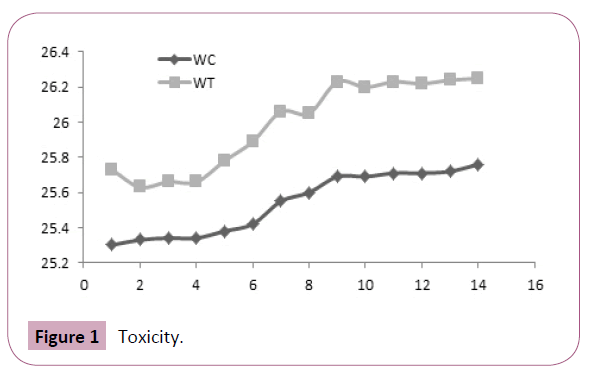
Figure 1: Toxicity.
Elevated plus maze
ANOVA of the times spent by mice in the open arms of elevated plus maze test showing significant (P?0.05) differences between the groups (Figure 2).
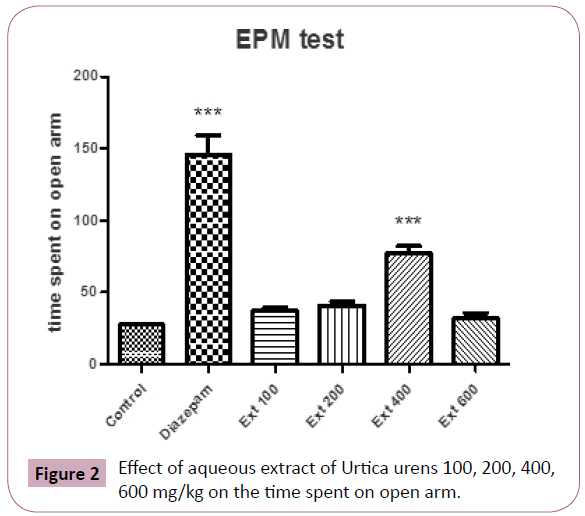
Figure 2: Effect of aqueous extract of Urtica urens 100, 200, 400, 600 mg/kg on the time spent on open arm.
Post hoc comparisons made with Tukey’s test revealed animals, which received aqueous extract of Urtica urens ( 100-400 mg/ kg, po.) and intraperitoneal diazepam 1 mg/kg increased the time spent on the open arms compared to the respective control groups (P?0.01 and P?0.001, respectively).
The values of the group treated with Urtica urens at 400 mg/kg body weight were higher than that of the treatment with lower doses (100,200 mg/kg).
Similarly, ANOVA of the number of entries into open arms of the elevated plus maze showing significant (P?0.05) differences between the groups. Further post hoc comparisons made with Tukey test revealed that animals receiving aqueous extract of Urtica urens (100-400 mg/kg) made statistically significantly more entries into open arms of the maze than control (P?0.05 and P?0.001, respectively) (Figure 3).
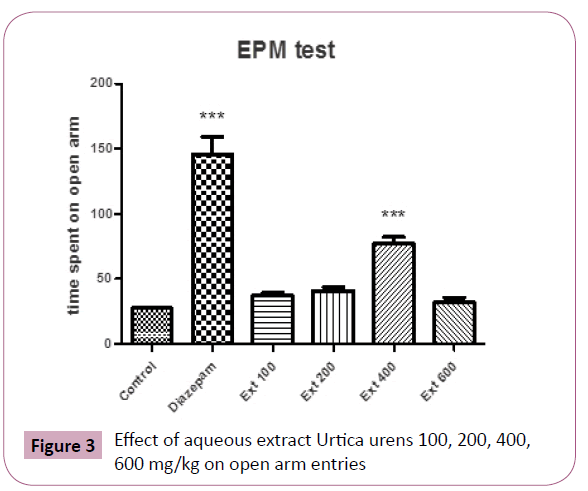
Figure 3: Effect of aqueous extract Urtica urens 100, 200, 400, 600 mg/kg on open arm entries
However, 400 mg/kg body weight treated groups showed higher number of entries and more time spent in open arms comparable to that of standard drug diazepam 1 mg/kg.
Open field
Total crossing
In the open field test, no difference was observed in locomotor activity (Total number of crossing) between groups (Figure 4).
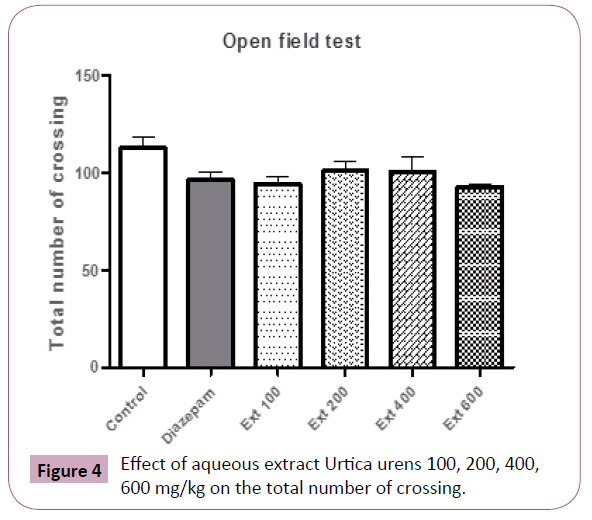
Figure 4: Effect of aqueous extract Urtica urens 100, 200, 400, 600 mg/kg on the total number of crossing.
Central area crossing
The post-hoc test revealed that central area crossing was only augmented in 200 and 400 mg/kg of Urtica urens and diazepam respectively (P<0.01, P<0.001, respectively) as compared with the control and remaining groups (Figure 5).
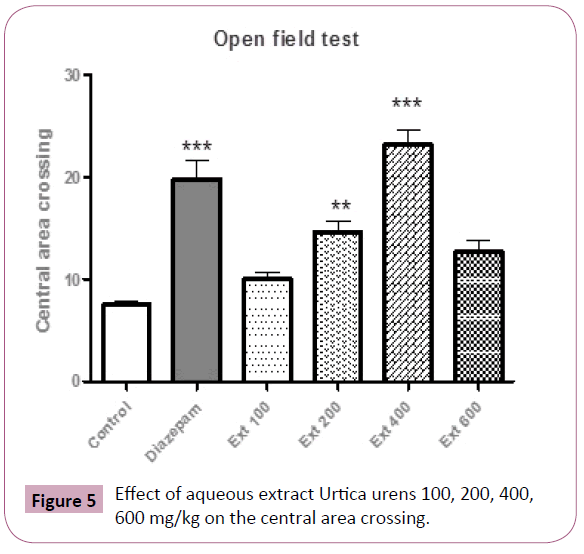
Figure 5: Effect of aqueous extract Urtica urens 100, 200, 400, 600 mg/kg on the central area crossing.
Time spent in central area
Mice treated with either 400 mg/kg or diazepam spent significantly more time in the center of mice respectively (P< 0.001, P< 0.001, respectively) (Figure 6).
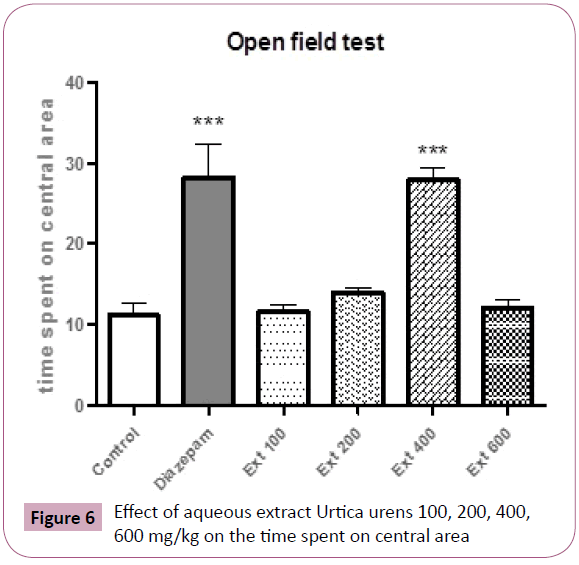
Figure 6: Effect of aqueous extract Urtica urens 100, 200, 400, 600 mg/kg on the time spent on central area
Central area rearing
Group received 400 mg/kg showed significant rearing in central area (P< 0.001) (Figure 7).
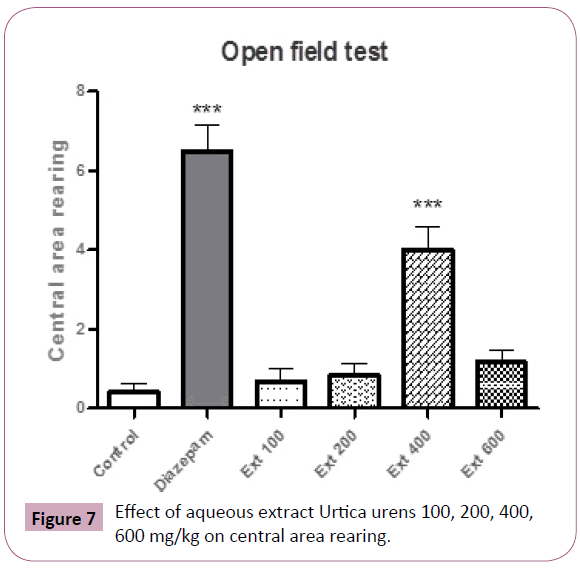
Figure 7: Effect of aqueous extract Urtica urens 100, 200, 400, 600 mg/kg on central area rearing.
Discussion
The aim of the present study was to analyse the therapeutic potential of aqueous extract of Urtica urens in experimental models of anxiety in mice. The U. urens extract showed promising anxiolytic effects. Various doses of the plant extract were tested on the EPM and the open field, they are usually the tests of choice when screening for new potential treatment for anxiety disorders.
EPM is based on the natural aversion of rodents for open spaces and uses an elevated plus-maze with two open and two closed arms [20]. An anxiolytic-like effect appears as decreased open arm avoidance because reduced anxiety removes the interference of fear with exploratory tendencies and hence drives greater exploration of the open arms.
An anxiolytic agent increases the frequency of entries into the open arms and increases the time spent in open arms of the EPM. The aerial part of extract of M. annua had similar effects on these parameters.
Only at 400 mg/kg, Animals increased the time spent on the open arms compared to the respective control groups (P?0.001) Figure 2, at doses lower than 400 mg/kg, there was no significant changes in this behavior parameter that was measured on the EPM. Doses higher than 400 mg/kg produce sedative effects.
Similarly, ANOVA of the number of entries into open arms of the elevated plus maze showing significant (P?0.05) differences between the groups. Further post hoc comparisons made with Tukey test revealed that animals receiving aqueous extract of Urtica urens (100-400 mg/kg) made statistically significantly more entries into open arms of the maze than control (P?0.001 and P?0.01, respectively) (Figure 2).
A graded dose study of aqueous extract reveals maximum anxiolytic activity of Urtica urens extract at 400 mg/kg, p.o. (P<0.001), and the higher doses of 600 mg/kg did not show significant activity compared with dose at 400 mg/kg. As expected, diazepam produced significant increases in open arm time and in number of entries into the open arms. Previous studies have shown that diazepam and other benzodiazepines produce significant anxiolytic effects in a variety of anxiolytic screening procedures, including elevated plus maze test procedures [21-25].
The behavioral responses of animals evaluated on the open field test were found to be modified. The OF is capable to induce anxiety in rodents and has been widely used to assess the behavioural effects of anxiety [26]. The OF allows rodents to explore in a novel environment and produces conflicting motivations between fear and exploration with regards to the threatening environment. More thigmotaxis and less locomotion activity is thought to indicate greater anxiety, whereas greater exploration and more ambulation to the centre arena of the OF reflect less emotion [27].
The most significant response was observed for the administration of the lower dose of Ma.
In the open field test, administration of U. urens induced no changes in the total number of crossing which supports the lack of alteration in motor coordination.
Aqueous extract of Urtica urens treatment with 400 mg/kg body weight increased the preference for the central area enhancing the crossing number, the time spent and the rearing in the central area of the apparatus, an increase in these parameters could be indicative of anxiolytic-like effects [28].
In addition to the fact that this reduction in anxiety like behavior was similar to the one observed following the conventional treatment with the diazepam 1 mg/kg, strengthen the assumption the U. urens has anxiolytic affects by its bioactive components and as so can serve as a potential candidate for treating anxiety disorders in humans, perhaps by targeting similar mechanisms as diazepam. Our data offer new prospects for investigating the action of these bioactive compounds present in the aqueous extract of Urtica urens responsible for the showed activity.
Conclusion
These results are promising since Urtica urens might be considered as an alternative for the treatment of anxiety disorders to other medications currently used. The phytoconstituent(s) responsible for the observed central effects has to be isolated and identified in future studies. Also the precise mechanism(s) underlying the effects of our treatment remain to be determined.
Author’s contributions
DZ, I carried out all the studies and drafted the manuscript with the help of the above authors, as regards TK participated in this work and drafted with me the manuscript. EHB helped us in the chemistry part and NM carried out the behavioral tests with me, and CY is the director of the laboratory he advises me and guides me always in my work, after my PhD supervisor KA she corrects the manuscript, guides me and advises me. All authors read and approved the final manuscript.
Acknowledgments
Authors are grateful to Dr. Hamid Khammar, botanist of scientific institute, Rabat.
8756
References
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, et al. (1998) Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 280: 1569-1575.
- Weissman MM, Markowitz JS, Ouellette R, Greenwald S, Kahn JP (1990) Panic disorder and cardiovascular/cerebrovascular problems: results from a community survey. American Journal of Psychiatry 147: 1504–1508.
- Lader M, Morton S (1991) Benzodiazepine problems. Br J Addict 86: 823-828.
- Davidson JR, Feltner DE, Dugar A (2010) Management of generalized anxiety disorder in primary care: identifying the challenges and unmet needs. Prim Care Companion J Clin Psychiatry 12.
- Huffman JC, Alpert JE (2010) An approach to the psychopharmacologic care of patients: antidepressants, antipsychotics, anxiolytics, mood stabilizers, and natural remedies. Med Clin North Am 94: 1141-1160, x.
- Faustino TT, Almeida RB, Andreatini R (2010) Plantasmedicinais no tratamento do transtorno de ansiedadegeneralizada: umarevisão dos estudosclínicoscontrolados (Medicinal plants for the treatment of generalized anxiety disorder: a review of controlled clinical studies). RevistaBrasileira de Psiquiatria 32: 429-436.
- Doukkali Z (2015) Practice flora of Morocco, Scientific Institute, University Mohammed V Agdal, Rabat 1: 126-128.
- Doukkali Z, Bouidida H, Srifi A, Taghzouti K, Cherrah Y, et al. (2014) Anxiolytic plants in Morocco: Ethnobotanical and ethno-pharmacological study. Phytothérapie 3.
- Sevki A, Gulsum T, Serkan E, Hasalettin D, Alaattin S (2014) Assessing of anti-inflammatory effect of Small nettle’ (Urticaurens) increasing polarity extracts. Jneuroim 8: 361.
- (2003) Anonymous. Urticae folium/herba. In: ESCOP Monographs ESCOP Monographs, editor. European scientific cooperative on phytotherapy. 521–527, New York: Stuttgart, USA.
- (1998) Anonymus. In: Blaschek W, Ha¨nsel R, Kelber K, Reichling J, Rimpler H, Schneider G (Eds.)HagersHandbuch Sequential, Berlin, Heidelberg: Springer-Publ. Comp 3: 710-723.
- Wichtl M (2002) Tee-Drogen und Phytopharmaka, Aufl, Wiss. VerlagsgesellschaftmbH. Stuttgart 617.
- Novas ML, Wolfman C, DeRobertis E (1988) Proconvulsantand ‘anxiogenic’ effects of n butyl ß carboline-3 carboxylate, an endogenous benzodiazepine binding inhibitor from brain. Pharmacology Biochemistry and Behavior 30: 331–336.
- (2002) OECD/OCDE. Guidelines for the testing of chemicals, revised draft guidelines 423; acute oral toxicity-acute toxic class method, OECD publishing.
- Sharma V, Gilhotra R, Dhingra D, Gilhotra N (2011) Possible underlying influence of p38MAPK and NF-B in the diminished antianxiety effect of diazepam in stressed mice. J Pharmacol Sci 116: 257-263.
- Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149-167.
- Archer J (1973) Tests for emotionality in rats and mice: a review. AnimBehav 21: 205-235.
- Siegel PS (1946) A simple electronic device for the measurement of gross bodily activity of small animals. Journal of Psychology 21: 227-236.
- Gutierrez-Garcia, Contreras AG, Mendoza-Lopez CM, Cruz-Sanchez MR, Garcia-Barradas S, et al. (2006) Asingle session of emotional stress produces anxiety inWistarrats. BehaviouralBrain Research 167: 30-35.
- Lister RG (1995) NG-monomethyl-L-arginine, an inhibitor of nitric oxide synthase, increases extracellular GABA in the striatum of the freely moving rat. Neuroreport 6: 1426-1428.
- Vogel JR, Beer B, Clody DE (1971) A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacologia 21: 1-7.
- Winslow JT, Insel TR (1991) Infant rat separation is a sensitive test for novel anxiolytics. ProgNeuropsychopharmacol Biol Psychiatry 15: 745-757.
- File SE (1980) The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods 2: 219-238.
- Andrews JS, Stephens DN (1990) Drug discrimination models in anxiety and depression. Pharmacol Ther 47: 267-280.
- Hall CS (1936) Emotional behavior in the rat. III. The relationship between emotionality and ambulatory activity. J Comp Psychol 22: 345-352.
- Choleris E, Thomas AW, Kavaliers M, Prato FS (2001) A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav 25: 235-260.
- Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463: 3-33.













