Keywords
|
| Angiomatoid Fibrous Histiocytoma, EWSR1, Angiomatoid Malignant Fibrous Histiocytoma, Florescence In Situ Hybridization |
Introduction
|
| Angiomatoid fibrous histiocytoma was first described by Enzinger in 1979, and is a rare tumor mainly affecting children and young adults [1]. It was initially called angiomatoid malignant fibrous histiocytoma, however as patients with this tumor were followed the malignant modifier was dropped, since the majority of these tumors generally behave in a benign fashion [2]. Nevertheless, metastatic rates have been reported as high as 5% [3,4]. It most commonly presents on the extremities as a painful or tender mass [5]. Occasionally systemic symptoms, such as anemia, weight loss and fever, accompany the lesion [6]. |
| Most cases of AFH show some form of cystic/hemorrhagic spaces, hence the name “angiomatoid”. However, these tumors are histologically heterogeneous and may lack any of the three characteristic features: lymphoid tissue, cystic spaces or a capsule, which can lead to diagnostic difficulty. Furthermore, Immunohistochemistry can be equivocal. We have encountered several cases of AFH which did not show the typical histologic features and subsequently reviewed our files for similar cases. Herein, we review the characteristic features of AFH, highlight the solid form of the tumor, and study the utility of fluorescent in situ hybridization (FISH) for the EWSR1 gene in confirming the diagnosis. |
|
Methods
|
| Eleven total cases of Angiomatoid Fibrous Histiocytoma” or “Angiomatoid Malignant Fibrous Histiocytoma” were identified in the files at our institution. Three cases were consults and the original slides were not available for evaluation. Hematoxylin and eosin stained slides were available for eight cases and paraffin-embedded tissue was available for seven of them. Hematoxylin and eosin stained slides were reviewed and evaluated for encapsulation, hemorrhagic/cystic spaces and lymphoid aggregates. Atypia was determined on a scale of 0-3 corresponding to absent, mild, moderate or severe atypia. The number of mitotic figures was counted per 10 high power fields, and the presence of necrosis and hemosiderin were noted. |
| Immunohistochemical (IHC) stains for cytokeratin (AE1/3), S100 protein, smooth muscle actin, desmin, CD99 and CD68 were performed. FISH studies for EWSR1 gene rearrangement were performed using a break-apart probe. Additional information including patient’s demographics, symptoms at presentation, duration, location of the tumor and the clinical impression were obtained via electronic medical records. |
|
Immunohistochemistry procedure
|
| Immunohistochemistry was performed on the automated Leica Microsystem Bond III platform using their Polymer Refine DAB kit. The pretreatments Enzyme 1 and ER2 (epitope retrieval solution-2) were supplied by Leica (Leica Biosystems Newcastle Ltd, Newcastle Upon Tyne, United Kingdom) (Table 1). |
|
FISH procedure
|
| Sections were cut to 4-6 μm thickness then deparaffinized. The slides were baked overnight at 56°C, then immersed in Hemo-D for 3 times at 10 minutes each, dehydrated in 100% ethanol twice for 5 minutes and air dried on 45-50°C slide warmer for 2-5 minutes. Slides were pretreated by immersing them in 0.2 N Hydrochloric acid (HCl) for 21 minutes, followed by purified water for 3 minutes with a pretreatment wash buffer (2 X SSC) for 3 minutes. Slides were then immersed in a second pretreatment solution (Sodium Thiocyanate/NaSCN) at 80°C for 30 minutes, rinsed in purified water for 1 minute and placed in a pretreatment Wash Buffer (2 XSSC) for 5 minutes. Slides were then immersed in a protease solution (25 mg. Pepsin/50 mLs. 0.1 M HCl) at 37°C for >20 minutes until adequately digested, then in a in Wash Buffer (2X SSC) for 5 minutes and dried. |
| The probe, Vysis LSI EWSR1 [22q12] (Abbott Molecular, Abbott Park, IL) was pipetted (10 ul) onto the slide, coverslipped and placed in a VYSIS Hybrite at 73°C for overnight. Slides were post washed in 2X SSC/0.3% NP-40 to remove the coverslip then immersed in 2.0 X SSC/0.3%NP-40 at 73°C for 2 minutes. Slides were then air dried and DAPI was applied as a counterstain. FISH results were scored independently by two technologists and the laboratory cytogenetic director. |
Results
|
|
Clinical
|
| Table 2 summarizes the clinical features. The patients included 3 females and 4 males with a female to male ratio of 0.75:1. Ages ranged from 9 months to 32 years, with an average age of 13.2 |
| years. The duration ranged from 1 week to 4 years, and 5 out of seven cases were increasing in size up to the time of resection. Two of the patients noted the lesion to be painful; however none reported systemic symptoms that are known to accompany AFH. Sites of involvement included upper extremity (3), back (3), neck (1) and abdomen (1). The clinical impression prior to operation ranged from benign entities such as lipoma and sebaceous cyst to malignant neoplasms such as sarcoma and lymphoma. Follow up was available on 7 cases and ranged from 1 to 96 months (average 16.57 months) with no evidence of recurrence or metastasis in any of the patients. |
|
Histology
|
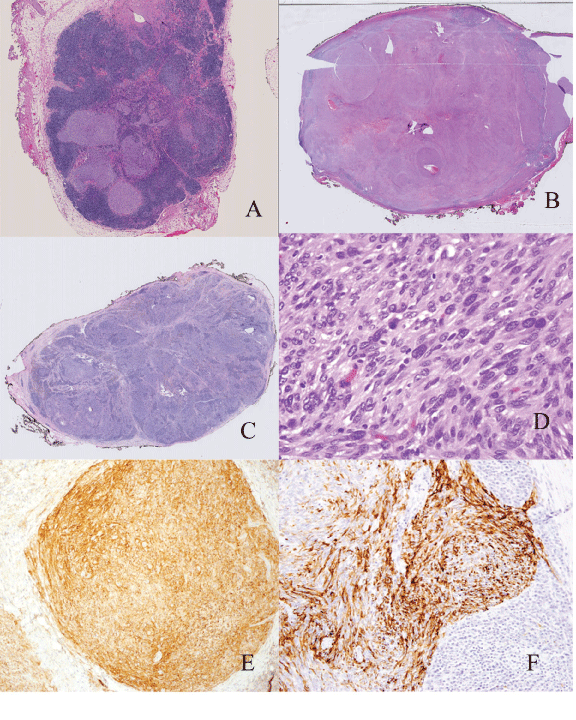
| Grossly the tumors ranged from 2.2 cm to 4.0 cm with an average size of 2.94 cm. One case showed classic histology and all the major criteria for AFH. Seven cases lacked one major histologic feature (88%). Six cases were at least partially encapsulated (75%) and four exhibited lymphoid aggregates (50%). The presence of hemorrhagic/cystic spaces was identified in 5 cases (63%) which often corresponded to the presence of hemosiderin. Three cases (38%) were architecturally solid. Four cases exhibited mild cytologic atypia and four were moderately atypical. Mitotic figures were present in three cases and ranged from 1- 3 per 10 high power fields. None of the tumors showed necrosis (Figure 1). |
|
IHC and FISH
|
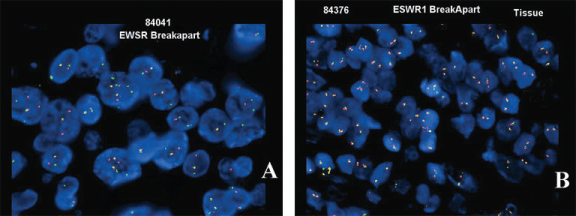
| Of the IHC, CD99 and CD68 showed the highest rates of positivity within the tumors at 86% and 100%, respectively. Three cases (43%) were reactive for desmin (Figure 2). All of the cases were negative for smooth muscle actin, S100 protein and cytokeratin (AE1/3). FISH studies revealed that three out of 7 cases (43%) had rearrangement of the EWSR1 gene (Table 3). |
|
Discussion
|
| The classic morphology of AFH has been described as being well encapsulated and composed of a solid cellular proliferation of histiocyte-like cells with vesicular nuclei and eosinophilic cytoplasm [5]. Fanburg-Smith described four common histologic features: fibrous pseudocapsule, round or spindled fibrohistiocytic proliferation, pseudo-angiomatous pattern and a plasma-lymphocytic response [2]. The differential diagnosis for AFH includes vascular and myofibroblastic tumors such as nodular Kaposi sarcoma, aneurysmal benign fibrous histiocytoma, palisaded myofibroblastoma, inflammatory pseudotumor and metastatic tumors to lymph nodes [2,7]. |
| Not infrequently, however, these tumors do not show the classic histology and/or present in unusual locations. Several authors described pleomorphic features and/or round cell morphology [8-10]. Chen et al., described cases with unusual morphologic patterns including clear cells, rhabdomyoblasts-like, pulmonary edema-like and cell cords. Unusual locations reported include brain, mediastinum, lung, bone, omentum, retroperitoneum, ovary, vulva and most recently endobronchial [11-13]. |
| In our series, the majorities of the cases lacked one key histologic feature but were recognizable as AFH. On the other hand, three cases had solid architecture and two cases in particular (cases 1 and 4) lacked 2 key histologic features (both capsule and cystic hemorrhagic spaces), and proved to be diagnostically challenging. We will refer to these cases as the solid form of AFH in this paper. This form can be mistaken for metastatic tumor to a lymph node (e.g. sarcoma, spindle cell melanoma or spindle cell carcinoma) and care should be taken not to inadvertently call it malignant. |
| By immunohistochemistry these tumors show variable positivity for EMA, desmin, CD68 and CD99, whereas S100 protein and cytokeratin are negative [2,8,11,14,15]. Our cases showed high percentage of positivity for CD99 and CD68. Desmin was positive in less than half of the cases. Although none of these stains is specific for AFH, they are helpful in excluding other entities in the differential. One of the solid forms of AFH in our series was positive for both desmin and CD99, whereas the two other cases were only positive for CD99. Although CD68 is positive in the majority of cases, it is a much less specific marker. |
| Cytogenetic studies show that many instances of AFH have a Ewing sarcoma breakpoint region 1 (EWSR1) gene rearrangement. The rearrangement usually involves the N-terminal of the EWSR1 gene being fused to a C- terminal DNA binding domain of another gene, this encoding a transcription factor. In AFH the most common fusions with EWSR1 involve the CREB1 or the ATF1 genes [15,16]. Other gene rearrangements such as FUS/ATF1 gene fusion have also been reported, but are far less common [6]. |
| Some studies have reported more than 90% sensitivity for the EWS FISH [8,11,15]. In their series, Tanas et al., found that 74% of the cases were positive for EWS break-apart probe [14]. However, in our patients only three out of seven cases (43%) showed an abnormal result by FISH. This low percentage of positivity may be explained by poor preservation of archival tissue. Only one of the solid forms of AFH in our series was positive by FISH. |
| Most large series with prolonged follow up confirm the indolent behavior of these tumors with variable risk for local recurrence (2-12%), but only rare metastasis and death [2,3,4]. Chen et al., showed that visceral/extrasomatic tumors tend to be larger, present at an older age, have associated systemic symptoms and carry higher risk for local recurrence [11] In general, the degree of atypia and mitosis in AFH do not correlate with clinical behavior; however, irregular tumor borders and head and neck location may be associated with increased local recurrence [3]. The mainstay of therapy is wide local excision and careful follow up [2,7]. All cases with clinical follow up in our series, including the solid forms, behaved in a benign fashion with no recurrences or metastases |
Summary
|
| AFH is a soft tissue tumor with variable morphology. The fact that this tumor has the potential for metastasis and that it may be confused with other soft tissue tumors with worse prognosis makes it important that an accurate diagnosis be made. Often times one or more of the characteristic histologic features will be absent, leading to further diagnostic difficulty. The solid form, in particular, can be diagnostically challenging and may be mistaken for metastatic malignancy. The constellation of morphologic features, immunostains and FISH testing, when necessary, should lead to the correct diagnosis. EWSR1 FISH probes are readily available in many institutions and, if positive, can aid in the diagnosis of equivocal cases of AFH. |
Acknowledgement
|
| This study was presented as a poster presentation at the 2011 American Society of Clinical Pathology Annual Meeting in Las Vegas Nevada. |
| This study had been approved by Wake Forest Internal Review Board (IRB) |
Tables at a glance
|
|
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |
| |
| |







