Keywords
SOX4; Adenomyosis; Gene expression; Gynecological disorders
Introduction
Adenomyosis is a typical gynecological disorders; it is a myometrial lesion portrayed by the nearness of ectopic endometrial organs and stroma found inside the encompassing myometrium with contiguous myometrial hyperplasia and hypertrophy [1]. Indications are generally announced as menorrhagia, dysmenorrhea, pelvic paien, abnormal uterine bleeding and mass related side effects. In spite of its normal event, the etiology and pathogenesis of adenomyosis stays misty. Past epidemiologic examinations researched chance elements for adenomyosis. Hazard factors include: age between 40 and 50 years, early menarche, short menstrual cycles, a first birth at an early age, multiparity, sharp curettage amid early pregnancy, heftiness and tamoxifen utilize [2]. Adenomyosis, is pseudotumors of several organs, including the uterus and additionally the bile channels and gallbladder [3].
There are many genes that have an important role in induction of adenomyosis including CXCL10, calbindin D-28K, Prostaglandin related factors such as prostaglandin E receptor 3, COX-2, HLA-G, Interleukin-10, Beclin-1 and Histondeastylase [4- 9]. These data suggest that the genetic and epigenetic abnormalities contribute to the pathogenesis of adenomyosis.
SRY-related high-motility group box 4 gene SOX4 is overexpressed in a variety of cancers and disease, It has been shown to be involved in the determination of cell fate and the regulation of embryonic development of many organ systems including heart, pancreas, brain and endometrial cancers reported the important role of SOX4 gene in progression of endometrial hyperplasia to endometrial adenocarcinoma [10- 14].
SOX4 gene expression is up-regulated in many tumor types, with experimental evidence suggesting that this contributes to cellular transformation, control of apoptosis and or a metastatic phenotype [15-17]. Because of its role in much cellular transformation, this study aimed to evaluate the expression of SOX4 gene and its role in induction of uterine adenomyosis.
Materials and Methods
Subjects
All cases were obtained from Al-Zahra Teaching Hospital in Wasit province/ Iraq
1. Thirty Patients suffering from abnormal uterine bleeding followed by hysterectomy.
2. Ten healthy individuals as a Control group.
Histological study
Histological technique of all tissues was carried out to observe the changes in tissues. Thirty biopsies of hysterectomy and curettage were concluded in this study [18].
Gene expression
Total Ribonucleic Acid (RNA) of all samples was extracted using the TRIzol® LS Reagent according to the manufacturer's instructions. Total Ribonucleic acid (RNA) was reversely transcribed to complementary Deoxyribonucleic acid (DNA) using WizScript™ RT FDmix Kit. The procedure was carried out in a reaction volume of 20 μl according to the manufacturer's instructions. The expression levels of SOX4 gene were estimated by qRT-PCR. To confirm the expression of target gene, quantitative real time qRT-PCR SYBR Green assay was used. The mRNA levels of endogenous control gene GAPDH were amplified and used to normalize the mRNA levels of the SOX4 gene. SOX4 primers sequences are (Forward: 5′-AGGATTCAAA CGCAACTCAAAT-3, Reverse 5′-AAAGAAATACGAGGATGGAGCA-3) and sequence of GAPDH (Forward, 5- AACTTTGGCATTGTGGAAGG-3 and Reverse, 5- ACACATTGGGGGTAG GAACA-3).
Statistical analysis
?CT and ??CT were calculated according to their equation [19]. This was conducted according to Statistical Analysis System-SAS to measure the effect of different factors in studying the parameters. Least Significant Difference (LSD) test was used to compare between means and Chi-square test between percentages [20]. The means and standard deviations were recorded for each sample (test and control) variables included Ct values and gene expression levels. This included values of housekeeping gene and test gene. P value for all tests was considered significant if <0.05.
Results and Discussion
Distribution of adenomyosis according to age
This study involved 30 uterine adenomyosis fresh biopsies Iraqi patients women with age ranged between 35 and 60 years. The highest percentage was recorded in age ranged (40-50) years with percentage (53.33%) followed by age group (50-60) years with percentage 26.67%. Table 1 shows the age and uterine abnormalities.
| Age range |
Number of patients |
Percentage (100%) |
| 30-40 |
6 |
20 |
| 40-50 |
16 |
53.33 |
| 50-60 |
8 |
26.67 |
| Total |
30 |
100% |
Table 1: Weight change comparison of wistar rats at initial and final day of treatment.
Reported adenomyosis to be found in 16.59% of hysterectomy specimen commonly in multiparous women of 31-50 years. Recurrence of adenomyosis increments with age, peaks at 40-50 years and levels-off after menopause. Increment in recurrence is related with multiparty [22]. Menstrual disturbance, smoking, dilatation, and curettage aid to increase the risk of adenomyosis [23].
Histopathological findings
Adenomyosis refers to endometrial gland and stroma located randomly deep within the myometrium. Adenomyosis may be triggered by either a weakness of the smooth muscle or by increased intrauterine pressure or both [24]. Increased levels of Esteadiol-2 (E2) and impaired immune-related growth control in ectopic endometrium are important for maintaince of Adenomyosis [25]. Figure 1 showed the presence of endometrial gland and some of the endometrial stroma infiltrated into layers of myometrium.
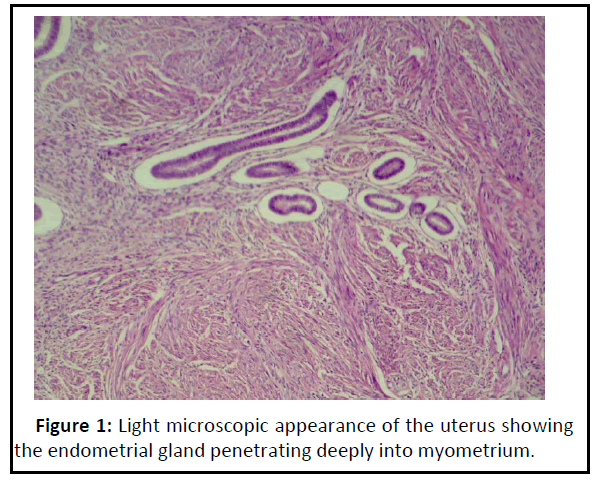
Figure 1: Light microscopic appearance of the uterus showing the endometrial gland penetrating deeply into myometrium..
Molecular study
Three essential steps to determine the gene expression of SOX4 in patients with adenomyosis:
Total RNA extraction: Total Ribonucleic acid (RNA) was successfully extracted from all samples because the tissues were immediately processed and completely homogenized. The concentration of total Ribonucleic acid (RNA) ranged from 63-96 ng/μl and the purity ranged from 1.69-1.97 ng/μl. A high concentration of total Ribonucleic acid (RNA) depends on the extraction conditions whereby strict aseptic techniques must be used
CDNA synthesis: Complementary Deoxyribonucleic Acid (cDNA) reverse transcription was carried out directly after the Ribonucleic acid (RNA) extraction processes. The kit was provided with random hexamer primers which coverage to all regions of Ribonucleic acid (RNA) to generate a Complementary Deoxyribonucleic Acid (CDNA). The random primers are unable to distinguished between Messenger Ribonucleic acid (mRNA) and other Ribonucleic acid (RNA) species including micro Ribonucleic acid (RNA) molecules in the reaction.
Quantitative real-time PCR results: Real time Polymerase Chain Reaction (PCR) quantification was applied in the present experiment utilizing the EVA green. The fluorescent dye recognized any double stranded Deoxyribonucleic Acid (DNA) including Complementary Deoxyribonucleic Acid (CDNA) and the amplification was recorded as a Ct value (cycle threshold).
The lower Ct value indicates the presence of higher copies of the target and vice versa. In terms of gene expression, high Ct values indicate a low gene expression and a low Ct value indicates a high gene expression [19,26].
GAPDH gene expression: There were no significant differences of Ct value of GAPDH in subjects and healthy control group (1 ± 0.00). The housekeeping gene used in the present study is shown in Table 2.
| Group |
Means Ct of GAPDH |
2-Ct |
Experimental group/ control group |
Mean fold of GAPDH gene expression |
| Adenomyosis |
29.977 |
9.5 E10 |
9.5 E10/9.7 E10 |
0.98 ± 0.08 |
| Control group |
29.946 |
9.7 E10 |
9.7 E10/9.7 E10 |
1 ± 0.00 |
| LSD value |
|
|
|
0.217 NS |
Note: NS -Non-significant.
Table 2: Comparison of GAPDH fold expression between study groups.
The mean fold of GAPDH gene expression in the patients groups was (0.98 ± 0.08) and Figures 2 and 3 show the amplification plots and melting curves for Glyceraldehyde 3- phosphate dehydrogenase (GAPDH) gene.
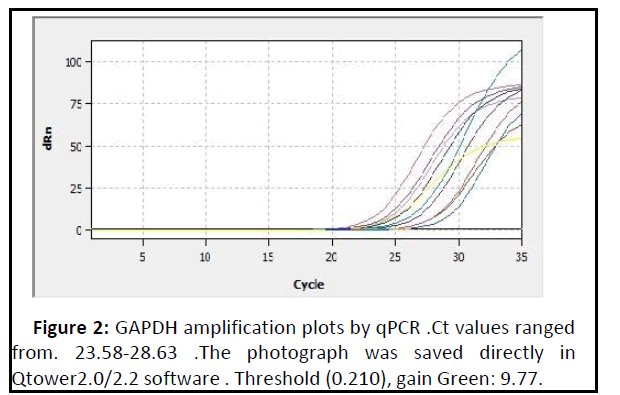
Figure 2: GAPDH amplification plots by qPCR .Ct values ranged from. 23.58-28.63 .The photograph was saved directly in Qtower2.0/2.2 software . Threshold (0.210), gain Green: 9.77..
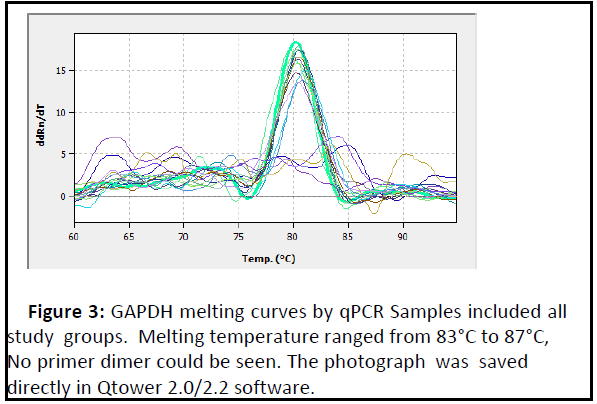
Figure 3: GAPDH melting curves by qPCR Samples included all study groups. Melting temperature ranged from 83°C to 87°C, No primer dimer could be seen. The photograph was saved directly in Qtower 2.0/2.2 software..
The inherent assumption in the use of housekeeping genes in molecular studies is that their expression remains constant in the cells [27]. One of the most commonly used housekeeping genes in comparison with the gene expression data is GAPDH [28].
Studied the expression of 1,718 genes using qRT-PCR [29]. They applied the GAPDH as a reference gene in 72 kinds of normal human tissues. They found that using GAPDH is quite a reliable strategy for the normalization in qRT-PCR when applied clinical studies.
SOX4 gene expression: Expression of the SOX4 gene was highly significant increased (p<0.01) in patients with adenomyosis when compared to the healthy control group as shown in Table 3 and Figures 4 and 5 show the amplification plots and melting curves for SOX4 gene.
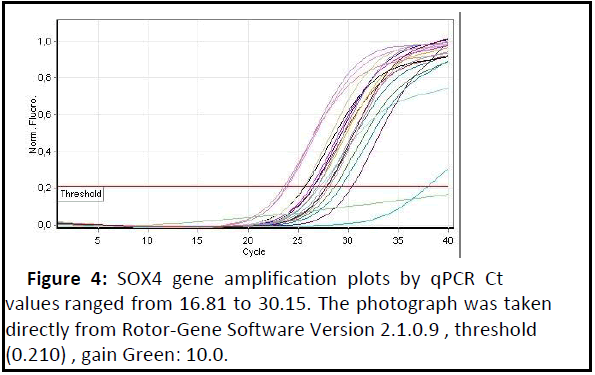
Figure 4: SOX4 gene amplification plots by qPCR Ct values ranged from 16.81 to 30.15. The photograph was taken directly from Rotor-Gene Software Version 2.1.0.9 , threshold (0.210) , gain Green: 10.0..
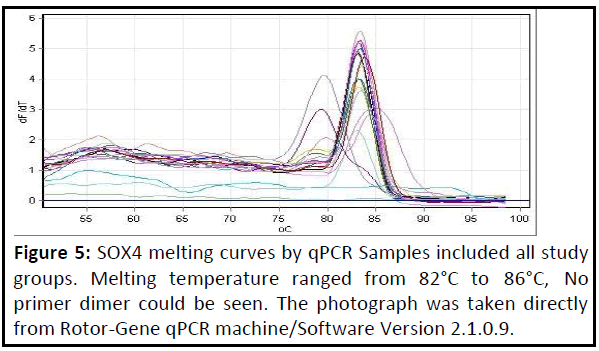
Figure 5: SOX4 melting curves by qPCR Samples included all study groups. Melting temperature ranged from 82°C to 86°C, No primer dimer could be seen. The photograph was taken directly from Rotor-Gene qPCR machine/Software Version 2.1.0.9..
| Groups |
Means ct of SOX4 |
Means ct of GAPDH |
ΔCt (means ct of SOX4- means ct of GAPDH |
2-ΔCt |
Experimental group/ control group |
Fold of SOX4 gene expression |
| Adenomyosis |
21.08 |
29.977 |
-8.897 |
476.72 |
476.72/68.40 |
6.96 ± 0.27 |
| Control |
23.85 |
29.946 |
-6.096 |
68.4 |
68.40/68.40 |
1 ± 0.00 d |
| LSD value |
--- |
--- |
--- |
--- |
--- |
2.073 ** |
Note: **(P<0.01).
Table 3: Fold of SOX4 gene expression. Depending on 2-ΔΔCt method.
Although the functional contributions of smooth muscle cells to adenomyosis development are poorly understood, reported that epithelial and stromal cells in endometriosis develop from persistent coelomic epithelial cells by metaplasia [30].
In pelvic endometriosis, eutopic endometrial cells arriving through retrograde transplantation at ectopic sites could induce the surrounding tissue to undergo smooth muscle metaplasia. Similar to endometriosis, adenomyosis associated smooth muscle cells may be of metaplastic origin. Myofibroblastic metaplasia with formation of an abnormal myometrial hypertrophy may contribute to an impaired uterine function.
Immunohistochemical study in adenomyosis tissue revealed expression of specific uterine marker molecules, including the essential components characterizing uterine myometrial cells such as Oxytocin Receptor (OTR), Vasopressin Receptors (VPR), Estrogen Receptors (ER) and Progesterone Receptors (PR) [31]. Oxytocin Receptor (OTR), Estrogen Receptors (ER) and Progesterone Receptors (PR) are also expressed in peritoneal endometrial smooth muscle cells [32].
On the other hand, reported that the presence of adenomyotic nodules in deeply infiltrating endometriosis lesions is accounted for by a reaction of the local environment to the presence of ectopic endometrium [33]. Myofibroblasts play an important role in the development of adenomyosis by their expression of Extracellular Matrix (ECM) proteins. These data suggest that myometrial hypertrophy is a response/reaction of the ectopic endometrial cells to the surrounding tissue, which shares characteristics with physiological and pathological mechanisms of wound healing (tissue injury and repair) [32-35].
Adenomyosis is a benign pathology represented by the presence of endometrial gland and stroma within the myometrium and it has been associated with endometrial cancer [36]. A high expression of SOX4 gene was reported with endometrial cancer incidence by epigenetic manner [13]. Confirm that the adenomyosis can be a precursor of some carcinoma but the exact molecular mechanisms that lead to malignancy are poorly understood. Therefore, this study suggested there are relationships between high expression of SOX4 gene and adenomyosis incidence [37,38].
Conclusion
MiRs play an important role in carcinogenesis by targeting tumor suppressor gene or by act as Oncogenes with elevated expression. MiR-203, miR-129-2, miR-596, and miR-618 identified to bound to the 3-UTR of SOX4 gene insilico analysis and these miRs keep the levels of SOX4 by the degradation of its mRNA. Reported that the hypermethylated promoters of miR- 129-2 and miR-203 lead to SOX4 overexpression. In conclusions the SOX4 gene participate in induction of uterine adenomyosis as well as in many histological changes especially when in overexpression status.
41949
References
- KoikeN, Shigemitsu A, Kobayashi H (2013) Pathogenesis and malignant of adenomyosis(Review). Onco Rep 29:861-867.
- Templeman C, Marshall SF, Ursin G, Horn-Ross PL, Clarke CA, et al. (2008) Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril 90:415-424.
- Kayahara M, Ohta T, Kitagawa H, Miwa K, Urabe T, et al. (2001) Adenomyomatosis of the papilla of Vater: a case illustrating diagnostic difficulties. Dig Surg 18:139-142.
- Hever A, Roth RB, Hevezi PA, Lee J, Willhite D, et al. (2006) Molecular characterization of human adenomyosis. Mol Hum Reprod 12:737-748.
- Wang F, Li H, Yang Z, Du X, Cui M, et al. (2009) Expression of interleukin-10 in patients with adenomyosis. Fertil Steril 91:1681-1685.
- Choi KC, Leung PC, Jeung EB (2005) Biology and physiology of Calbindin-D9k in female reproductive tissues: involvement of steroids and endocrine disruptors. Reprod Biol Endocrinol 3:66-72.
- Wang F, Wen Z, Li H, Yang Z, Zhao X, et al. (2008) Human leukocyte antigen-G is expressed by the eutopic and ectopic endometrium of adenomyosis. Fertil Steril 90:1599-1604.
- Ren Y, Mu L, Ding X, Zheng W (2010) Decreased expression of Beclin 1 in eutopic endometrium of women with adenomyosis. Arch Gynecol Obstet 282:401-406
- Liu X, Nie J, Guo SW (2012) Elevated immunoreactivity against class I histone deacetylases in adenomyosis. Gynecol Obstet Invest 74:50-55.
- Restivo A, Piacentini G, Placidi S, Saffirio C, Marino B (2006) Cardiac outflow tract: a review of some embryogenetic aspects of the conotruncal region of the heart. Anat Rec A Discov Mol Cell Evol Biol 288:936-943.
- Wilson ME, Yang KY, Kalousova A, Lau J, Kosaka Y, et al.(2005) The HMG box transcription factor Sox4 contributes to the development of the endocrine pancreas. Diabetes 54:3402-3409.
- Hong CS, Saint-Jeannet JP (2005) Sox proteins and neural crest development. Semin Cell Dev Biol 16:694-703.
- Huang YW, Liu JC, Deatherage DE, Luo J, Mutch DG, et al.(2009) Epigenetic repression of microRNA-129-2 leads to overexpression of SOX4 oncogene in endometrial cancer. Cancer Res 69:9038-9046.
- Al-Deresawi, MS Al-Faisal AH, Al-obaidi SR (2018) Role of SOX4 gene in progression the endometrial hyperplasia to endometrial adenocarcinoma. Iraqi Journal of Biotechnology 17:82-90.
- Liu P, Ramachandran S, Al- Seyed M, Scharer CD, Laycock N, et al.(2006) Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res 15:4011-4019.
- Pramoonjago P, Baras AS, Moskaluk CA (2006) Knockdown of Sox4 expression by RNAi induces apoptosis in ACC3 cells. Oncogene 25:5626-5639.
- Liao YL, SunYM, Chau GY, Chau YP, et al.(2008) Identification of SOX4 target genes using phylogenetic footprinting-based prediction from expression microarrays suggests that Overexpression of SOX4 potentiates metastasis in hepatocellular carcinoma. Oncogene 17:122-131.
- Bancroft J D, Stevens A (1999) Theory and Practice of Histological Techniques. (4th Edn) Churchill Livingstone Pp:127-129.
- Livak KJ, Schmittgen TD ( 2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔ CT Method. Methods 25:402-408.
- SAS (2012) Statistical Analysis System, User's Guide. Statistical ersion 9 ( 1th Edn) SAS Inst Inc Cary NC USA.
- Siddegowda MS, Manjunath MS, Shivakumar S (2015) Occurrence of Adenomyosis in Hysterectomy Specimen and its Clinical Correlation in a Tertiary Care Hospital in Mandya, Karnataka India. Inter J Sci Stud 3:61-64.
- Arunachalam B, Manivasakan J (2012) A clinic-Pathological study of adenomyosis. J Clin Diagn Res 6:428-430.
- Brucker SY, Huebner M, Wallwiener M, Stewart EA, Ebersoll, et al. (2014) Clinical characteristics indicating adenomyosis coexisting with leiomyomas: A retrospective, questionnaire-based study. Fertil Steril 101:237-241.
- Bergeron C, Amant F, Ferenczy A (2006) Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol 20:511-521.
- Struble J, Reid S, Bedaiwy MA (2016) Adenomyosis: a Clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol 23:164-185.
- Nolan T, Hands R, Bustin A (2006) Quantification of mRNA using real-time RT-PCR. Nat Protoc 1:1559-1582 .
- Reboucas E, Costa J, Passos M, Passos J, Hurk R, et al. (2013) Real Time PCR and Importance of Housekeepings Genes for Normalization and Quantification of mRNA Expression in Different Tissues. Brazil Arch Biol Technol 56:143-154.
- Barber D (2005) GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiological Genomics 21:389-395.
- Robert B, Harmer W, Coleman A, Clark B (2005) GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genom 21:389-395.
- Mechsner S, Bartley J, Infanger M, Loddenkemper C, Herbel, J, et al. (2009) Clinical management and immunohistochemical analysis of umbilical endometriosis. Arch Gynecol Obs tet 280:235-242.
- Barcena de Arellano, ML Gericke, J Reichelt, U Okuducu, AF Ebert, et al. (2011) Immunohistochemical characterization of endometriosis-associated smooth muscle cells in human peritoneal endometriotic lesions. Hum Reprod 26:2721-2730.
- Mechsner S, Grum B, Gericke C, Loddenkemper C, Dudenhausen JW, et al.(2010) Possible roles of oxytocin receptor and vasopressin-1α receptor in the pathomechanism of dysperistalsis and dysmenorrhea in patients with adenomyosis uteri. Fertil Steril 94:2541-2546.
- Van Kaam, KJ Schouten, JP Nap, AW Dunselman G, Groothuis, et al. (2008) Fibromuscular differentiation in deeply infiltrating endometriosis is a reaction of resident fibroblasts to the presence of ectopic endometrium. Hum Reprod 23:2692-2700.
- Ferenczy A (1998) Pathophysiology of adenomyosis. Hum Reprod Update 4:312-322.
- Leyendecker G, Wildt L, Mall G (1998) The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet 280:529-538.
- Musa F, Frey MK, Im HB (2012) Does the presence of adenomyosis and lymphovascular space invasion affect lymph node status in patients with andometrioid adenocarcinoma? Am j Obstret Gynecol 207:417-422.
- Devor EJ, Hovey AM, Goodheart MJ, Ramachandran S, Leslie KK (2011) MicroRNA expression profiling of endometrial endometrioid adenocarcinomas and serous adenocarcinomas reveals profiles containing shared, unique and differentiating groups of microRNAs. Oncol Rep 26:995-1002.
- HuangY, Kuob C, Chenb J, Paul J, Huangd T, et al. (2014) Hypermethylation of miR-203 in endometrial carcinomas. Gynecol Oncol 133:340-345.










