Keywords
|
| Epidural malignancy; Spinal cord; Laminectomy |
Discussion
|
| Spinal metastases are estimated to occur in between 5 and 20% of all patients with systemic cancer. The vertebral column is the most commonly affected region of the skeleton. More than 70% of patients who die from systemic cancer have vertebral metastases demonstrable at post-mortem examination [1]. The scale of the problem is huge: in the USA alone, over 18,000 new cases of spinal metastases are presented each year [2]. Furthermore, as better palliative care and advances in oncology prolong the survival of patients suffering from cancer, the number of those with spinal metastases is destined to further increase. |
| Thus, there may still be a role for laminectomy for lesions of the upper dorsal spine, probably combined with posterior spinal instrumentation, in the treatment of malignant spinal cord compression, especially when anterior surgical techniques are either inappropriate or not immediately available. |
| Metastatic spread to the spine can occur by several routes: direct extension, haematogenous, lymphatic or perineural. Traditionally, Batson's vertebral venous plexus was considered to be the main route for the spread of metastases to the epidural space [3]. Autopsy studies, however, have shown that, in humans, most epidural tumors arise from metastases growing in the bone marrow of the vertebral body. About 85% of epidural metastases lie anterior to the spinal cord arising from the vertebral body. Twenty percent show, in addition, circumferential involvement of the cord [4-6]. |
| Metastatic deposit of tumor can occur anywhere along the spinal canal, but almost 90% are either thoracic or lumbar. Between 20-30% of patients have tumor involvement at multiple levels of the spinal cord [7]. |
| The management of epidural spinal malignancy, the majority of which is metastatic, has been a source of controversy over several decades. The traditional treatment for metastatic cord compression is a decompressive laminectomy. Widely popular throughout the 1950s and 1960s, this treatment has more recently been criticized for an unacceptably high level of neurological deterioration in the ambulatory patient (around 52% being worse after the operation) [8], and, more specifically, has been reported as a potentially dangerous operation when performed in the presence of anterior vertebral collapse, resulting in major neurological deterioration, and a high incidence of spinal instability [9]. Despite these criticisms, laminectomy still has some advantages over other forms of treatment: it provides rapid and effective decompression of posterior lesions with an immediate histological diagnosis, is a relatively easy operative procedure, and may be the only realistic treatment option in patients who have had previous radiotherapy. |
| The purpose of this study was to audit the practice of laminectomy, specifically with reference to anterior vertebral collapse in patients with malignant spinal tumors, and to compare these results with the reported results with anterior decompression. |
Method
|
| This retrospective study consists of 135 consecutive patients presenting spinal cord compression in either the Department of Neurosurgery, Karolinska Institutet, Stockholm, Sweden (96 patients) or the Department of Neurosurgery, Newcastle General Hospital, Newcastle upon Tyne (39 patients) between 1986-1992. |
| All patients were treated by a standard decompressive laminectomy. In only three cases was the spine stabilized using Harrington rods. Patients whose clinical condition and tumour histology made it appropriate were treated with radiotherapy after the surgical wound had healed. |
| The neurological deficit at presentation was graded as either: ambulant, walking without human assistance but, if necessary, with aids; paraparetic, but non-ambulant; and paraplegic, with a complete motor and sensory deficit. |
| The same grading system was used after laminectomy to assess the best neurological grade attained, even if there was some neurological deterioration later. Improvement was only considered to exist if a patient changed to a better grade and if, similarly, deterioration indicated a decline to a lower grade. |
| Plain spine X-Rays were performed in all 132 cases. Sixty-five patients had evidence of metastatic spinal cord compression associated with anterior vertebral collapse, being defined as the loss of more than 50% of the height of the vertebral body. |
| In all other respects, the two groups were similar, showing no significant differences as regards age, sex distribution, tumor histology, or pretreatment neurological status. Myelography was performed in 127 patients and demonstrated a complete block in 91 cases (69%). |
| In total, the study consisted of 30 primary and 102 metastatic tumors. |
| The primary malignancies included multiple myeloma, lymphoma, chordoma, and rhabdomyosarcoma. The metastatic tumors were from primary lesions in the breast, lung, prostate, genitourinary, and gastrointestinal tracts. |
| The patients were 85 males and 47 females, ranging from 7 to 87 years, most of them being in their sixth and seventh decades (mean age 57.5 years). |
| The level of compression was thoracic in 116 cases, and lumbar in 16. There were no patients with cervical lesions. There were three or more levels decompressed in 111 patients and less than three in 21. |
| Back pain was the initial symptom in 77 patients, motor weakness in 75 patients, and sensory deficit in 7 patients. |
Results
|
| The neurological status of the patients at presentation is shown in Table 1. |
| There was no significant difference in the degree of neurological deficit prior to laminectomy. Table 2 shows the best neurological classification after laminectomy with radiotherapy, where appropriate. |
| Thus, in a group of 135 patients with malignant spinal cord compression, no significant differences could be demonstrated with regard to the outcome of patients with anterior vertebral collapse compared to those without it. After treatment, the percentage of walking patients in each group was very similar: 50.8% in the collapsed group and 52.2% in the group without anterior vertebral collapse. |
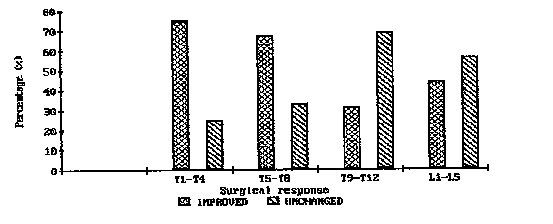
| The surgical responses to laminectomy at different levels of the spinal cord were also taken into consideration. |
| Significantly more patients improved with lesions between T1 and T8 than those with lesions between T9 and L5 (Figure 1). |
| The short-term ambulation rates of tumors located between T1-T4 were also compared with similar results from those between T5-T8 and T9-T12. The surgical response appeared to be significantly better for tumors located between Tl-T4 compared to those between T9-T12 (p<0.05). |
| Survival after surgery was limited. Only 43% of patients were alive at six months, and 28% survived a year. Long-term survival was, unfortunately, unusual, with only 23 patients (17%) alive at two years. The small group of patients who had survived long-term had primary tumors of the breast, prostate, lymphoma, and myeloma (Table 3). |
Discussion
|
| The management of spinal epidural metastases has been a source of much debate over the past twenty years. To date, the only attempt at a randomized controlled trial has been Young et al. in 1980 [10] which failed to show any significant differences in outcome between radiotherapy alone and laminectomy and radiotherapy. |
|
Part I. Laminectomy as a treatment for metastatic cord compression in patients with AVC
|
| Laminectomy was one of the earliest treatments for metastatic cord compression. LeCat is believed to have been the first to perform a laminectomy for a spinal epidural tumor in 1751 [11]. In recent years, controversy has centered on the relative efficacy of laminectomy (usually combined with radiotherapy) compared with radiotherapy alone. In general, several extensive literature reviews and many large retrospective studies have failed to demonstrate any significant difference in outcome between the two methods of treatment [2,8,10,12-16] although several authors have qualified this by advising that paraparetic patients may benefit from combined therapy [12,16]. |
| The review of recent papers which contain a total of 939 patients between 1986-1991 reveals similar results. |
| Laminectomy shows an operative mortality of between 3 and 14%, with an average of about 9%. The incidence of non-neurological complications e.g. wound infection, CSF leak, etc. ranges from 8% to 42%, with a mean of 11%. |
| Laminectomy has also been identified as a potentially hazardous procedure when performed in the presence of anterior vertebral collapse (defined as more than 50% compression of the vertebral body). In a study of 80 patients, the collapsed group did considerably worsen [9]. Of the 37 patients in the collapsed group who could not walk prior to surgery, only one could walk after laminectomy. Moreover, patients with anterior vertebral collapse (AVC) had almost a 50% risk of major neurological deterioration, and a high incidence of spinal instability (22%) compared to those without AVC (0%). The instability may occur because laminectomy removes support for the spine which is already compromised by an anterior destructive lesion. |
| To a large extent, the problem of painful spinal instability can be resolved by posterior stabilisation of the spine which may be combined with decompressive laminectomy. Although this requires more expertise than simple laminectomy alone, posterior stabilisation provides excellent pain relief [17-27]. The percentage of pain free patients has been consistently high in a number of series: 74% [28]; 81% [27]; 91% [29]; 100% [30]. Moreover, posterior fixation allows early mobilization of the patient, and is thought by some to be at least as effective in the control of spinal instability as an anterior approach [29] (Table 4). |
| The role of laminectomy in creating the major neurological deterioration seen in some studies is by no means clear [9]. |
| A review of the literature provides little information about this important issue. |
| Although it is still difficult to reconcile these varying results, the apparent discord may be due, in some part, to differences in ambulatory status before laminectomy, rather than to vertebral collapse alone. For example, in the present audit, about one-third of the patients in both groups (AVC and no AVC) could walk preoperatively, whereas in the only similar study [9], the proportion who were ambulant preoperatively was halved, to around 13% (Table 5) [9,31-33]. |
| The level of spinal cord involvement may be an important differentiating factor: in the cervical spine, the absence of supporting tissues may make laminectomy dangerous, but in the thoracic spine, further collapse may be prevented by an intact rib cage. In this audit, there were no patients with cervical compression. Laminectomy was associated with the best results in the upper thoracic spine. |
| Moreover, experimental evidence suggests that removing the lamina in a spine which is compressed by an anterior dural mass will not create posterior displacement of the dura nor spinal instability. In vitro, the dural sheaths of the nerve roots, the lateral dural fascia, and the anterior dural ligaments anchor the dura in place; the contribution of the lamina appears to be minimal [34]. |
| Over the past decade, much interest has been focused on anterior and anterolateral approaches to the spine as a treatment for metastatic cord compression. Indeed, the outcome for patients for all degrees of motor dysfunction is excellent (Tables 6-8). The improved efficacy is attributed to the pathological finding that 85% of metastatic tumors arise anterior to the spinal cord, and thus, anterior surgery is the only method, as yet tried, for directly relieving the compression. As well as improving motor function, anterior surgery is also beneficial for the relief of pain. Approximately 84% were pain-free in a series by Sundaresan, an improvement reproduced in many series: 73% [23], 71% [24], 62% [35] and 61% [6]. The surgical mortality from anterior procedures is between 4% [26] and 31% [24] with an average of 10%, which is comparable to laminectomy. The neurological deterioration is minimal, but the incidence of non-neurological postoperative complications can be high, ranging from 12% [5] to 54% [23] with a mean of 25%. Spinal reconstruction after anterior decompression can be achieved by a variety of methods: bone grafts, methyl methacrylate cement, Harrington rods and Zielke rods, amongst others. Anterior approaches to the spine are not without some difficulties, and may not be appropriate for all patients. The operations are longer, technically more complex, and usually result in more blood loss [35]. Also, anterior incisions tend to be more painful and debilitating than posterior incisions, and may result in longer hospital stays, and more perioperative morbidity. Mediastinal, pulmonary, or retroperitoneal disease may make an anterior approach to the spine risky or impossible. Posterior stabilization may still be required, resulting in another incision, or even another operation [34]. |
| Thus, there may still be a role for laminectomy for lesions of the upper dorsal spine, probably combined with posterior spinal instrumentation [35-38], in the treatment of malignant spinal cord compression, especially when anterior surgical techniques are either inappropriate or not immediately available. There may also be a place for a formal prospective randomized controlled trial of laminectomy compared with anterior decompression for patients with anterior vertebral collapse in the upper thoracic spine. Certainly, such a study has never been performed with matched patients in each group, and until completed, uncertainty will persist. |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
 |
 |
 |
 |
| Table 5 |
Table 6 |
Table 7 |
Table 8 |
|
Figures at a glance
|
 |
| Figure 1 |
|
| |
|
|






