Keywords
Ultra High Pressure Liquid Chromatography, Maraviroc ICH guidelines, Validation
Introduction
Maraviroc (UK-427,857) is an orally-available selective chemokine receptor CCR5 antagonist with potent anti–HIV-1 activity. It has exhibited activity against all CCR5-tropic HIV-1 viruses tested, including 43 primary isolates from various diverse sources. Maraviroc was also active against 200 clinically derived HIV-1 envelope-recombinant pseudoviruses, 100 of which were derived from viruses resistant to existing classes.[1] Maraviroc prevents the binding of the viral envelope, gp120, to CCR5 and, thus, prevents the subsequent membrane fusion events that are necessary for viral entry into the CD41 cells.[2-3] Maraviroc is not active against CCR2-, CXCR4-and dual-tropic viruses, nor is it cytotoxic.3 Maraviroc binds to the host cells rather than the virus envelope; thus, its mechanism of action differs from enfuvirtide, a fusion inhibitor.[4] Additive or synergistic activity has been observed when maraviroc has been assessed in combination with other antiretroviral agents, including abacavir, amprenavir, atazanavir, delavirdine, didanosine, efavirenz, emtricitabine, enfuvirtide, indinavir, lamivudine, lopinavir, nelfinavir, nevirapine, ritonavir, saquinavir, stavudine, tenofovir, zalcitabine, and zidovudine.[1]
Maraviroc, Chemically 4,4-difluoro-N-[(1S)-3- [(1R,5S)-3- [3-methyl-5-(propan-2-yl)-4H-1,2,4- triazol-4-yl]-8-azabicyclo[3.2.1]octan-8-yl]-1- phenylpropyl]cyclohexane-1-carboxamide is a white to yellowish or brownish powder with a molecular formula of C29H41F2N5O and a molecular weight of 513.67. Maraviroc is practically insoluble in water, slightly soluble in ethanol, soluble in methanol, dimethyl sulfoxide and PEG 400.[4- 5]
There are only few liquid chromatographic[6-8] available for the determination of maraviroc in bulk and formulations. The aim of the present work is to develop a stability indicating cost effective method for estimation of maraviroc in bulk and in formulations.
Materials and Methods
Reagents and Chemicals
HPLC gradient grade acetonitrile and methanol from Merck (Mumbai, India) has been used. Potassium dihydrogen phosphate (AR grade), ortho phosphoric acid and triethylamine Solution from Merck have been used. Demineralized water was further purified in the laboratory by filtering through an ultrapure Milli-Q (Millipore, Milford, MA, USA), Maraviroc working standard from Dr. Reddys Laboratories.
Instrumentation and liquid chromatographic conditions
LC was carried out on a Agilent 1290 series UHPLC. The output signal was monitored and processed using chemstation software. The chromatographic column used Waters X bridge C18 20 x 4.6 mm, 2.5μ
Standard preparation
Accurately weighed and transferred 100 mg of working standard in to a 100 mL volumetric flask, dissolved and diluted volume with diluent (50:50 water, methanol), filtered through 0.22 μm filter and sonicated for 10 min. Further 5 ml of solution diluted to 25mL in a 25 mL volumetric flask.
Sample preparation
Accurately weighed and transferred 100 mg of sample in to a 100 ml volumetric flask, dissolved and diluted volume with diluent (50:50 water, methanol), filtered through 0.22 μm filter and sonicated for 10 min. Further 5 ml of solution diluted to 25mL in a 25 mL volumetric flask.
Mobile phase preparation
To 1000mL of 0.01M KH2PO4, 1mL triethylamine added, pH adjusted to 7.0.with H3PO4.
Mixed 600mL of above buffer and 400mL of acetonitrile (v/v) and sonicated for 10 mins.
Optimized Chromatographic Conditions
Column : X Bridge C18 20 x 4.6 mm, 2.5μ
Column temperature : 30°C
Wavelength : 210 nm
Flow rate : 0.5 mL/min
Injection volume : 5 μl
Diluent : Water and methanol in the
ratio of 50:50 v/v
System Suitability
Standard solution was injected six times and % relative standard deviation (%RSD) for peak area (PA) and retention times (RT) calculated. Average of tailing factor (T.F) for individual peak and average theoretical plates (T.P) were calculated. Resolution (RS) between peaks was evaluated.
Specificity
To demonstrate the specificity of the method blank, standard solution and sample solutions were injected.
Degradation studies
To check the performance of the optimized LC method for the separation of degradation products, the drug was subjected to various stress conditions.
Acidic hydrolysis
Maraviroc sample was refluxed with 5N HCl at 60°C for 1hour and then neutralized by adjusting pH to 7.0 with 5N NaOH. The Solution was further diluted to required concentration with diluent.
Note: Sample was not degraded in 0.1N HCL and 1N HCL. So high stress conditions 5N HCL was used.
Alkaline hydrolysis
Maraviroc sample was refluxed with 2N NaOH at 60°C for 1hour and then neutralized by adjusting pH to 7.0 with 2N HCl. The Solution was further diluted to required concentration with diluent.
Note: Sample was not degraded in 0.1N NaOH and 1N NaOH. So high stress conditions 2N NaOH was used.
Oxidative stress
Maraviroc sample was refluxed with 30% 10% H2O2 by heating on water bath at 60°C for 1hour. The solution was further diluted to required concentration with diluent.
Photolytic stress
Maraviroc sample was exposed to UV (200watt hour/m2) and Visible (1.2million Lux hours) as per ICH Guidelines. The Sample was prepared as per sample preparation.
Thermal stress
Maraviroc sample was exposed to Temperatures at 105°C for 3days. The Sample was prepared as per sample preparation.
For Water Degradation
Maraviroc sample was refluxed with water by heating on water bath at 100°C for 1hour. The Sample was prepared as per sample preparation.
For Humidity Degradation
Maraviroc sample was exposed to 85% Humidity (Prepared potassium nitrate saturated solution) at 3days. The Sample was prepared as per sample preparation.
Precision
Prepared the standard solution, sample solution prepared at same level at in six replicates, injected into system, chromatograms were recorded, assay of sample solutions was performed and %RSD was calculated.
Intermediate Precision (Ruggedness)
Ruggedness of the method was evaluated by determining the precision of method by analyzing same sample on different system, by different analyst and on different column.
Accuracy
Prepared in triplicate standard solutions at different concentration levels (50-150%), and amount added was calculated in terms of % recovery.
Linearity
Linearity was demonstrated by injecting standard solutions of 10 - 150% with respect to the specification level. Plotted the calibration curve by taking concentration on X-axis and peak area on Y-axis, calculated the correlation coefficient and % y-intercept.
Robustness
Robustness of the method was demonstrated by doing the small variations in mobile phase flow rate, column temperature, organic variation in mobile phase and buffer pH.
Results and Discussion
Method development
The goal of the method is to detect the impurities and quantify Maraviroc present in bulk drug and in formulations. Based on the pKa value of the drug the pH of the buffer was selected as 7.0. KH2PO4 (0.01M), (1mL triethylamine added, pH adjusted to 7.0.with H3PO4) was used as buffer. Initially methanol was used as organic phase; the maraviroc peak shape was not good and eluted very lately. Acetonitrile was used as organic phase, initially in 80: 20 buffer: acetonitrile used, peak tailing was observed, acetonitrile content increased to 40% the peak symmetry was good. Zorbax SB C8 (4.6x50 mm, 1.8μ), Kinetex XB-C-18 (20 x 2.1 mm, 2.5μ), BEH C18 (20X2.1mm, 2.5μ) and X Bridge C18 (20 x 4.6 mm, 2.5μ) columns were tried, good separation with good peak symmetries was observed with X bridge C18 (20 x 4.6 mm, 2.5μ). At 30oC the peaks symmetry is good and eluted in time. Flow rate was varied between 0.3 mL/min – 0.5 mL/min and 0.5 mL/min flow rate found to be suitable. L injection volumes were tried, at 5 μL the response was adequate at 5 μL with good peak shape and size.. On observation 3D spectra of sample in diode array detector response at 210 nm, it has shown good response, hence 210 nm optimized as the detection wavelength.
Method Validation
System suitability
%RSD of retention times, peak areas were less than 1 for maraviroc, average of tailing factor found to be less than 1. Theoretical plates were found to be more than 5000 for, hence method passes system suitability tests. The results were shown in table no 1.
| Parameter |
Result |
| % RSD of RT |
0.32 |
| % RSD of PA |
0.39 |
| T. Factor |
0.89 |
| T. Plated |
9690 |
Table 1: Results of system suitability
Specificity
Blank and Placebo interference
Chromatograms of placebo solutions showed no peaks at the retention time of Maraviroc. This indicates that the excipients used in the formulation do not interfere in the estimation of Maraviroc. The standard and sample chromatograms were identical, peak purity angle, peak purity threshold were good that proves methods specificity.
Fig 1: Blank Chromatogram

Fig 2: Standard Chromatogram.

Fig 3: Placebo chromatogram.

Fig 4: Sample chromatogram.
Interference from degradation products
A study was conducted to demonstrate the effective separation of degradants from Maraviroc and its related known impurities. Separate portions of drug product, drug substance and placebo were exposed to the stress conditions to induce degradation. Stressed samples were injected into the HPLC system with photo diode array detector as per following test method conditions. All degradant peaks were resolved from Maraviroc peak and its related impurities in the chromatograms of all samples. The Chromatograms of the stressed samples were evaluated for peak purity of Maraviroc using chemstation software. In all forced degradation samples, peak purity was passed for Maraviroc Purity factor is within the purity threshold. The method can be used for determining Maraviroc bulk and pharmaceutical formulations. In all the conditions Maraviroc peak purity angle is less than the purity threshold. Results are shown in table no 2.
| S. No |
Stress condition |
%Net degradation |
Peak Purity |
| 1 |
2 hrs in Water @60°C |
4.0 |
999 |
| 2 |
2 hrs in 2N HCl @ 60°C |
3.2 |
999 |
| 3 |
2 hrs in 3N NaOH @ 60°C |
15 |
999 |
| 4 |
2 hrs in 10%H2O2 @ 25°C |
3 |
999 |
| 5 |
Thermal(105°C for 7 days |
Nil |
999 |
| 6 |
UV and Visible |
Nil |
999 |
| 7 |
Humidity |
Nil |
999 |
Table 2: Results of degradation studies

Fig 5: Water degraded sample.
Fig 6: Acid degraded sample.

Fig 7: Base degraded sample.

Fig 8: Peroxide degraded sample.

Fig 9: Photolytic sample.

Fig 10: Thermally degraded sample.

Fig 11: Humidity sample.
Precision and Intermediate Precision
The average amount total impurities found to be 100.4% and % RSD was less 1, when analysis was performed by second analyst on second system the results were well under limits, method is precise.
| S. No |
Precision |
Int. Precision |
| Preperation-1 |
100.0 |
100.4 |
| Preperation-2 |
100.4 |
100.0 |
| Preperation-3 |
100.5 |
100.7 |
| Preperation-4 |
100.2 |
100.7 |
| Preperation-5 |
101.1 |
99.7 |
| Preperation-6 |
100.0 |
100.6 |
| Mean |
100.4 |
100.4 |
| Std dev |
0.4 |
0.4 |
| %RSD |
0.4 |
0.4 |
Table 3: Precision and intermediate precision
Accuracy
The mean % recovery values for Maraviroc were found to be 99 – 101%. That proves methods accuracy.
| S. No |
50% |
100% |
150% |
| 1 |
100.1 |
99.7 |
99.4 |
| 2 |
99.7 |
100.1 |
99.2 |
| 3 |
99.5 |
100.1 |
99.3 |
| 4 |
99.9 |
99.9 |
99.4 |
| 5 |
100.0 |
100.7 |
99.1 |
| 6 |
99.8 |
99.6 |
99.3 |
| % RSD |
0.2 |
0.4 |
0.1 |
Table 4: Results of accuracy
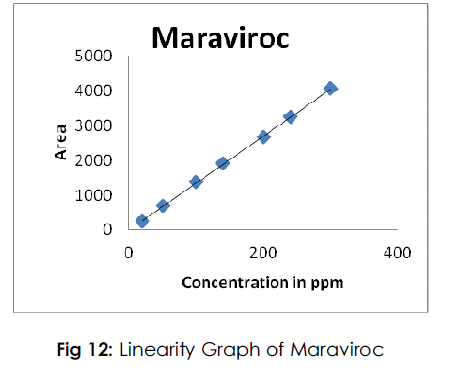
Fig 12: Linearity Graph of Maraviroc
Linearity
The linearity study reveals that the method is linear from 10 to 150%. The R2 values for all compounds found to be more than 0.9998.
| Conc. in ppm |
Area |
| 20 |
251.39598 |
| 50 |
694.20972 |
| 100 |
1369.08057 |
| 140 |
1904.83203 |
| 200 |
2673.5813 |
| 240 |
3267.24902 |
| 300 |
4054.84863 |
| Corr |
0.999890464 |
| Slope |
13.52003152 |
| intercept |
2.73773641 |
| Bias |
0.102399595 |
Table 5: Results of Linearity
Robustness
There were no changes in results when slight changes in chromatographic conditions were made, method is robust. Results were shown in table no 6.
| pH Variation |
6.8 |
7.0 |
7.2 |
| %RSD of RT |
0.37 |
0.34 |
0. 43 |
| %RSD of PA |
0.45 |
0.37 |
0.39 |
| T. Factor |
0.95 |
0.91 |
0.99 |
| T. Plates |
9345 |
9190 |
8956 |
| Mobile Phase variation |
55:45 |
60:40 |
65:35 |
| %RSD of RT |
0.65 |
0.37 |
0.37 |
| %RSD of PA |
0.55 |
0.42 |
0.34 |
| T. Factor |
1.28 |
0.91 |
0.89 |
| T. Plates |
8745 |
9428 |
9634 |
| Temperature Variation |
35°C |
30°C |
25°C |
| %RSD of RT |
0.38 |
0.42 |
0.45 |
| %RSD of PA |
0.49 |
0.51 |
0.42 |
| T. Factor |
0.99 |
0.91 |
0.97 |
| T. Plates |
9190 |
9712 |
8990 |
| Flow variation |
0.4 mL/min |
0.5 mL/min |
0.6 ml/min |
| %RSD of RT |
0.44 |
0.36 |
0.51 |
| %RSD of PA |
0.47 |
0.45 |
0.55 |
| T. Factor |
0.94 |
0.89 |
1.03 |
| T. Plates |
8890 |
9512 |
9365 |
Table 5: Results of Linearity
Conclusions
An UHPLC method was developed for the analysis of Maraviroc. The developed method is able to detect maraviroc within 4 min. The method is sensitive, specific, linear, precise, accurate, robust and easy to perform. This method can be used for the assay of Maraviroc in bulk drug and in formulations.
5754
References
- Dorr P, Westby M, Dobbs S, et al. Maraviroc (UK- 427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti- human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49(11):4721-4732.
- Rosario MC, Poland B, Sullivan J, Westby M, van der Ryst E. A pharmacokinetic- pharmacodynamic model to optimize the phase IIa development program of maraviroc. J Acquir Immune Defic Syndr. 2006;42(12):183-191.
- Westby M, Mori J, Smith-Burchnell C, et al. Maraviroc (UK-427,857)-resistant HIV-1 variants selected by serial passage, are sensitive to CCR5 antagonists and T-20. Antiviral Ther. 2005; 10(suppl): S72.
- V. Kalyana Chakravarthy and D.Gowri Sankar. Stability indicating HPLC method for determination of maraviroc and its degradants/impurities in bulk and pharmaceutical formulation. Rasayan J. Chem. 2012; 5 (1): 90-105.
- L. Satyanarayana, S.V. Naidu, M. Narasimha Rao, C. Ayyanna, and Alok Kumar. The Estimation of Maraviroc in Tablet dosage form by RP-HPLC. Research Journal of Pharmaceutical Dosage Forms and Technology 2011; 3(5): 230 -235.


















