Keywords
Testosterone; Renal disease; Hemodialysis; Testicular volume; Children
Introduction
In ESRD, gonadal hormones undergo several pathological changes which result in retardation of pubertal development [1]. These hormonal disturbances result in failure to thrive and delay the pubertal development [2]. About one third of male adolescents with CRF has a delayed puberty. Many pathophysiological mechanisms can explain pubertal delay in these patients [1] including hormonal disturbance in the hypothalamohypophyseal gonadal axis, toxic effects of uremia on peripheral tissues, testicular injury and disordered secretion and function of follicular stimulating hormone (FSH) and luteizing hormone (LH) which is known also as interstitial cell stimulating hormone (ICSH) secretion [3].
The impact of intensified dialysis on pubertal growth is controversal. Some authors reported that pubertal growth is substantially more impaired in dialysis patients compared with patients with chronic renal failure (CRF) stages 3-5. On other side, other authors concluded that over the last two decades, there are improvement in sexual maturation and in pubertal height gain in CKD patients incuding ESRD under renal replacement therapy. An explanation for this controversy is Growth hormone treatment which increases prepubertal height gain but pubertal timing and total pubertal height gain are not affected in these patients. Other suitable measures for improving pubertal growth and final height and avoiding height deficit before and even after kidney transplantation, are the use of efficacious immunosuppressive strategies for optimized graft function and early withdrawal, or even complete avoidance, of steroid therapy [4]. The aim of the work was to assess the plasma level of total testosterone hormones and testicular volume in pediatric patients with ESRD under regular haemodialysis and their correlation with duration of hemodialysis.
Subjects and Methods
This work was conducted on forty pediatric patients with ESRD under regular hemodialysis The range of their ages was 10-18 years. There mean +SD was 14.6+2.67 years. They were selected from the Pediatric Department of Tanta University Hospital and Zagazig University Hospital in Egypt at the period between July 2016 to July 2017. 40 healthy age and sex matched children and adolescents were selected as controls. This research was done after the approval of research ethical committee of the Tanta University Hospital and Zagazig University Hospital and after getting informed consents from parents of included subjects.
All subjects were undergoing regular HD, indication of HD was when estimated creatinine clearance is equal or less than 15 ml/ min/ 1.73 m3 3 times weekly. Duration of each session of HD ranged from for 3 to 4 hours. Children were put on Fresenius 4008-B Hemodialysis machine (Germany). Its blood flow rate was calculated according to the equation (2.5 × weight (kg) + 100 ml/min). We used polysulphane hollow fiber dialyzers which should be suitable for the body surface area (BSA) of the children (Meaning Fresenius F3=0.4 m2, F4=0.7 m2, F5=1.0 m2 and F6=1.2 m2). To improve the adequacy of dialysis, we used bicarbonate dialysis solutions. All children on regular HD were under regular supportive treatment including subcutenous erythropoietin (EPO) in a dose of 50 IU/Kg/session, parentral (intravenous) iron in a dose of 100 mg/Kg weekly, oral folic acid tablet in a dose of 1 mg/day, oral calcium syrup or tablets in a dose of 1gm/ day, oral vitamin D drops or capsules (one alpha) in a dose of 1-5 nanogram/Kg daily, oral antihypertensive medications for hypertensive patients and oral magnesium capsiles which act as as phosphate binder for patients with hyperphoshatemia.
Inclusion criteria
All male patients diagnosed as ESRD and treated by maintenance HD regularly.
Exclusion criteria
History of hormonal disorders (eg. Type 1 Diabetes mellitus), who received hormonal treatment, past history of drugs which can affect serum testosterone hormone levels or male children who already get signs of pubertal development before onset of CRF.
All patients and controls were subjected to:
Full history
It includes age, past history of diseases and medications, dialysis duration, developmental history, the exact age and order of sequences of the body and psychological changes of pubertal development, age at first appearance of axillary and pubic hair.
Thorough clinical examination
1. Body measures for evaluation of dietetic and developmental status
• Weight: It was measured provided minimal dress by electronic weight scale in Kilograms.
• Height: by measurement of the distance between the vertex of the skull to the base of the heel in centimeters using a stadiometer in standing position.
• Body mass index (BMI): It was calculated by following equation [5]:
BMI=weight (kg)/[height (m)]2
• Mid arm circumference (MAC): It was measured by circumference of the left upper arm at the mid-point between the tip of the shoulder (olecranon process) and the tip of the elbow (the acromium process) in centimeters.
1. Vital measurements focusing on systolic and diastolic arterial blood pressure which was aevaluated by auscultatory method by using a mercury sphygmomanometer, the semi setting position was preferable after ten minutes of relaxation. It was better to measure it in the non fistula arm using an appropriate cuff size. The mean value of three successive readings in three following days was considered.
2. Pubertal assessment: Genital system was examined in details for evaluating of sexual development. Tanner's classification [6,7] was applied which assessed:
• Pubic and axillary hairs.
• The length and width of the right and the left testicles was measured by metered tape.
• The stretched penile length in the flaccid state was measured with a rigid tape from the pubopenile skin junction to the top of the penis, excluding the prepuce under maximal but not painful extension.
• The penile circumference was measured at the base of the penis "close to the pubis" with a measuring tape.
• For obese males, the abdominal adipose tissue was shifted manually to one side to measure penile length and circumference) [7].
The early appearance of axillary and pubic hair was known as adrenarche occurred between ages of 6 and 10 years. It might be transient and disappeared before the onset of true puberty. Boys begin puberty at ages 11-12 years on average and usually complete puberty by ages 16-17 years [8]. Average onset of the first ejaculation for males at age 13 years [8]. Pubertal delayed was identified if there was absence of manifestations of onset of puberty by the expected age of their appearance or failure of appropriate progression of manifestations of puberty after puberty has commenced. Boys are considered to have delayed puberty if they reach the age of 13 years without evidence of pubertal changes [8].
1. Assessment of testicular volume
• Orchidometer: It consisted of a string of twelve numbered wooden or plastic beads of increasing size from about 1 to 25 milliliters. The beads are compared with the testicles of the patients, and the volume is read off the bead which matches most closely in size. Pre-pubertal sizes are 1-3 ml, pubertal sizes are considered 4 ml and up and adult sizes are 12-25 ml. Small testes can indicate either primary or secondary hypogonadism [9].
• Testicular ultrasound: The patient is placed in a supine position with the legs slightly spread apart. The scrotum is placed in a sling designed from a towel to improve exposure and should be supported and immobilized on a rolled towel placed between the patient’s thighs. The penis is covered with a towel and the towel is taped to the abdominal wall. High frequency broadband linear transducer (7.5-10.0 MHz) that can perform both power and spectral Doppler ultrasonography is used. The scrotum and its contents are scanned in three planes, along the longitudinal, axial and transverse axis. Measurements are taken in these planes.
Testicular volume formula was used: L × W × H × 0.71 [10].
Laboratory investigation
1. Routine laboratory investigations: Complete Blood Count (CBC) by an automated analyzer, Blood urea, Blood Urea Nitrogen (BUN) and serum creatinine, serum albumin and serum electrolytes (Ionized calcium, Potassium and Phosphorus ).
2. Measurement of serum total Testosterone levels by Kit supplied by (TOSOH BIOSCIENCE) [11] to assess androgen status of the subjects to distinguish eugonadal from hypogonadal. It included the circulating Testosterone in form of combination of free (unbound) circulating (2%), albumin bound (54%) and sex hormone binding globulin (SHBG) (44%) bound Testosterone. Bioavailable Testosterone is the free and albumin-bound Testosterone which is available to tissues for biological action. Serum Total Testosterone measurements in this study were performed by liquid chromatography tandem mass spectrometry in the morning as Testosterone levels exhibit biological variability due to episodic secretion from testes and circadian variation with peak concentrations in the morning [11].
Specimen collection and handling
Just before session of HD, samples of venous blood morning were withdrawn. 5 ml of them were collected using sterile needles through gentle venipuncture of puncture site under complete aseptic technique.
The collected samples were divided into the following fractions:
1. 2 ml which was put on 20 uL EDTA solution as anticoagulant for CBC including differential white blood cells count which was done on Leishman stained peripheral blood smear with evaluation using ERMA PCE-210 N cell-counter from Erma, Inc. Japan [12].
2. 3 ml was put in a plain tube without adding anticoagulant to allow coagulation of the sample. It was put in a water bath at 37oC. After coagulation, centrifugation was done at rate of 1500 xg for ten minutes. The separated serum was collected in a tube for the assessment of serum testosterone levels [13].
Statistical methods
We use statistical package for social science (SPSS) version 18.0 for evaluation and interpretation of data. Data was tabulated in forms of range, mean ± standard deviaton, number and percent, t student test and Chi square test were used. Pearson’s correlation coefficient (r) was also done where the r value was interpreted as follow:
Weak if < 0.25
Mild if ≥ 0.25 -<0.5
Moderate if ≥ 0.5 -< 0.75
Strong if ≥ 0.75
P value was considered significant if ≤ 0.05 [14].
Results
Table 1 reported the demographic characteristicsof the patients and controls regarding age, dialysis duration and anthropometric measures and systemic blood pressures.
| Parameters |
Patients |
Control |
Statistical test |
P. value |
| Age (years) |
Range |
10-18 |
10-18 |
t=0.200 |
0.657 |
| Mean ± SD |
14.63 ± 2.66 |
14.25 ± 2.64 |
| Duration of dialysis (years) |
Range |
3-10.8 |
- |
- |
- |
| Mean ± SD |
7.1 ± 2.47 |
| Weight (kg) |
Range |
20-55 |
35-78 |
25.146 |
0.001* |
| Mean ± SD |
36.43+10.51 |
54.85+12.63 |
| Height (cm) |
Range |
102-162 |
141-180 |
14.561 |
0.001* |
| Mean ± SD |
137.75+17.86 |
156.75+11.03 |
| Body Mass Index |
Range |
13.6-32.6 |
17-31 |
4.664 |
0.037* |
| Mean ± SD |
19.14+4.68 |
21.91+3.31 |
| Mid Arm Circumference (cm) |
Range |
13-26 |
21-30 |
33.403 |
0.001* |
| Mean ± SD |
19.75+3.71 |
25.55+2.52 |
Systolic blood pressure
(mm Hg) |
Range |
120-160 |
100-130 |
17.366 |
0.001* |
| Mean ± SD |
139.25+10.04 |
114.50+7.59 |
Diastolic blood pressure
(mm Hg) |
Range |
80-110 |
60-80 |
20.829 |
0.001* |
| Mean ± SD |
95.5+7.42 |
74+5.98 |
X2: Chi square test
P. Value < 0.05 significant
Table 1: Demographic data of the studied patients and control groups.
The range of age of our studied patients was 10-18 years with mean 14.6+2.7 in patients group and 14.3+2.6 in control group. There were no significant differences between Group I and Group II regarding age. Duration of dialysis in patients group ranged from 3.0-10. 8 years with mean 7.1+2.47.
As regard anthropometric measures (weight, height, BMI and MAC) of patients group was significantly lower than in control group (P<0.05).
Both systolic and diastolic blood pressures of the patients group were significantly higher in than in control group (P<0.05).
There were significant differences regarding Tanner staging of the studied groups with delayed puberty in Group I compared with Group II (P<0.05) (Table 2).
| Tanner's stage |
Patients |
Controls |
Total |
| I |
N |
20 |
8 |
28 |
| % |
50% |
20% |
35% |
| II |
N |
10 |
10 |
20 |
| % |
25.00% |
25% |
25% |
| III |
N |
5 |
4 |
9 |
| % |
12.50% |
10% |
11.25% |
| IV |
N |
4 |
14 |
18 |
| % |
10% |
35.00% |
22.50% |
| V |
N |
1 |
4 |
5 |
| % |
2.50% |
10.00% |
6.25% |
| Total |
N |
40 |
40 |
80 |
| % |
100.00% |
100.00% |
100.00% |
| Chi-square test |
X2 |
26.34 |
| P-value |
0.001* |
P. Value < 0.05 significant
Table 2: Tanner's stage distribution between the studied patients and control groups.
Table 3 reported routine laboratory findings of the studied subjects, there was statistically significant increase in levels of serum creatinine, blood urea, BUN, serum potassium and serum phosphorus levels in the studied patients if compared to controls (P<0.05). On the other side, there was statistically significant decrease in levels of hemoglobin percent, hematocrit values, platelet count, total leucocytic count count, serum albumin and serum ionized calcium in the studied patients when compared to healthy controls (P<0.05).
| Parameters |
Patients |
Control |
T. test |
P. value |
| Blood Urea (mg/dl) |
Range |
100.8-257 |
21.7-36.7 |
53.71 |
0.001* |
| Mean ± SD |
168.33 ±50.03 |
28.99 ± 4.84 |
| Serum Creatinine (mg/dl) |
Range |
4.9-10.3 |
0.5-1.4 |
87.349 |
0.001* |
| Mean ± SD |
7.76 ± 1.53 |
0.91 ± 0.27 |
| BUN (mg/dl) |
Range |
45-115 |
9.6-16.3 |
59.093 |
0.001* |
| Mean ± SD |
76.01 ± 22.30 |
12.81 ± 2.19 |
| HB (g/dl) |
Range |
7.2-12.4 |
11.2-13.9 |
46.311 |
0.001* |
| Mean ± SD |
10.45 ± 1.44 |
12.84 ± 0.74 |
| HCT % |
Range |
21.5-37.9 |
33.6-42 |
37.711 |
0.001* |
| Mean ± SD |
31.61 ± 4.48 |
38.37 ± 2.31 |
| PLT × 1000/cmm |
Range |
133-414 |
250-435 |
39.727 |
0.001* |
| Mean ± SD |
223.05 ± 81.65 |
357.90 ± 49.89 |
| WBC × 1000/cmm |
Range |
3.4-9.1 |
4.1-12.5 |
4.575 |
0.039* |
| Mean ± SD |
5.74 ± 1.77 |
7.05 ± 2.08 |
| Serum Albumin (g/dl) |
Range |
2.8-4.6 |
3.6-5.5 |
19.727 |
0.001* |
| Mean ± SD |
3.69 ± 0.54 |
4.52 ± 0.64 |
| Serum Ionized Ca (mg/dl) |
Range |
2.44-5.2 |
4.2-5.5 |
21.671 |
0.007* |
| Mean ± SD |
4.08 ± 0.69 |
4.89 ± 0.36 |
| Serum K (mmol/l) |
Range |
3.5-6.1 |
3.5-5.2 |
20.841 |
0.001* |
| Mean ± SD |
5.14 ± 0.74 |
4.22 ± 0.52 |
| Serum P (mg/dl) |
Range |
4.2-8.8 |
3.5-6.1 |
18.39 |
0.001* |
| Mean ± SD |
5.95 ± 1.03 |
4.71 ± 0.77 |
P.Value<0.05 significant; HB haemoglobin %; HCT Hematocrite value; PLT Platelet count; BUN: Blood Urea Nitrogen; Ca: Calcium; P: phosphorus; K: Potassium
Table 3: Routine investigations of the studied patients and control groups.
Table 4 clarified serum levels of total serum testosterone and testicular volume in the studied patients and control groups, the total serum testosterone levels and testicular volume in the patients group was statistically significantly lower in patients than in the control group (P<0.05).
| Parameters |
Patients |
Control |
T. test |
P. value |
| Serum testosterone (ng/ml) |
Range |
0.20-7.16 |
0.40-8 |
8.131 |
0.006* |
| Mean ± SD |
1.01 ± 1.61 |
2.73 ± 2.66 |
| Testicular volume (ml) |
Range |
0.58-18.40 |
1.80-20 |
6.177 |
0.016* |
| Mean ± SD |
4.45 ± 4.92 |
8.66 ± 7.08 |
P.Value<0.05 significant
Table 4: Serum levels of testosterone and testicular volume in the studied patients and control groups.
Table 5 and Figures 1 and 2 showed that there were significant negative correlations between the duration of hemodialysis and serum levels of total testosterone and the testicular volume in the patients ( P value=0.011 and 0.001 respectively ).
| Parameter |
r |
p |
| Serum testosterone (ng/ml) |
-0.457 |
0.011* |
| Testicular volume (ml) |
-0.908 |
0.001* |
P. Value<0.05 significant
Table 5: Correlation between the dialysis duration and serum testosterone levels and testicular sizes in patents group.
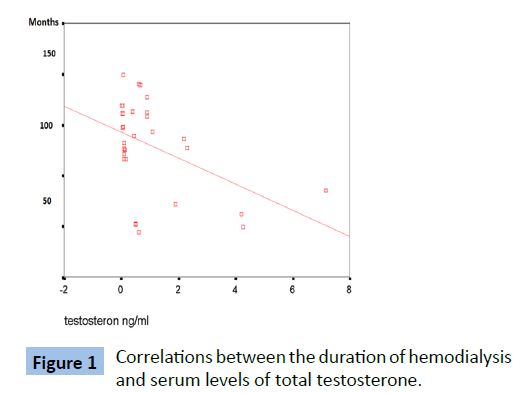
Figure 1: Correlations between the duration of hemodialysis and serum levels of total testosterone.
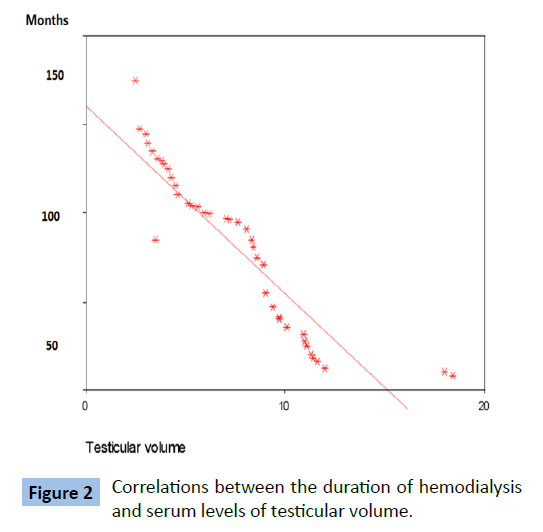
Figure 2: Correlations between the duration of hemodialysis and serum levels of testicular volume.
Discussion
Pubertal development is frequently delayed in children with CRF. Both neuroendocrine and peripheral alterations due to uremia have been hypothesized to explain the impairment in the pituitary gonadal axis [2].
ESRD was reported to be complicated by testicular injury and diminished testosterone levels together with impaired regulation of FSH and ICSH secretion [15]. In this work, plasma level of total male Testosterone hormone and testicular volume were done to evaluate testicular functions in boys with ESRD under regular HD. Very few researches have previously been performed to evaluate the hypothalamo-hypophyseal testicular axis in children and adolescents with ESRD [2].
Growth markers were assessed in this work, reporting that weight, height, BMI and MAC were statistically significantly lower in diseased subjects when compared to healthy controls. This may be multifactorial, related to poor nutritional status, comorbidities such as anemia, chronic kidney disease mineral bone disorders (CKD-MBD), alterations in hormonal responses, as well as to aspects of treatment such as steroid exposure [16]. Our results were supportive for previous data from multiple centers [17].
Belgorosky et al. [18] longitudinally analyzed growth curves of 29 adolescents with various degrees of CKD and concluded that the growth spurt of their patients started with an average delay of 2.5 years.
The patients in this work, the dialysis children showed statistically significant lower hemoglobin percent and hematocrite values when compared to healthy children. This was in accordance with Harmon and Jabs, who concluded that ESRD was associated with renal anemia which may be attributed to EPO deficiency, diminished RBCs life span or ecessive hemorrage [19].
Chatterjee et al. [20] explained the association of anemia with disturbed hypothalamo-pituitary-gonadal axis by multiple factors including chronic ill health, chronic hypoxia, underweight and low BMI. In addition, iron overload (due to repeated blood transfusion) may cause siderosis of both the pituitary gland causing secondary gonadal failure and testes causing primary gonadal failure.
In our work, There was significant differences regarding Tanner staging of the studied patients and controls, with pubertal delay in patients when compared with group (2) (P<0.05). This was in agreement with Harold et al. [21] and Castellano et al. [16] who stated that progress of puberty occurs in children with ESRD, although it was delayed for their actual chronological age.
As sequence of disturbances of pubertal development [22]. Sexual dysfunction is common in adolescents with ESRD [2].
Ehrich et al reported that about half of their patients under HD achieved the pubertal milestones later than the range of their controls [23].
The Cooperative Study for Pubertal Development reported that in CKD, delayed puberty was observed both in children on dialysis and after renal transplantation where the onset of puberty was delayed by 2-2.5 years on average [24].
Other authors reported that the start of genital maturation was delayed by 1.8 years in uremic and 2.5 years in transplanted boys. Full genital maturation was achieved in these patients with a delay of 2.2 and 3.2 years respectively. Rizzoni et al reported that once started, puberty appeared to proceed at a normal rate. However, in individual patients, particularly on long term dialysis, pubertal maturation may arrest for years [25].
Schaefer et al. [26] reported that, unlike the development of secondary sexual characteristics which is delayed but not permanently halted in CKD, these changes do not appear reversible after renal transplantation.
But our results regarding delayed puberty in patients were not in agreement with Haffner D & Zivicnjak M who conducted that most children requiring RRT before puberty in the last 20 years presented with normal or only slightly delayed pubertal onset and attributed these results to adequate nutrition, RRT and growth hormone therapy [4].
In our study, serum total testosterone was found significantly lower in patients than in controls with significant negative correlations between serum total testosterone and dialysis duration. Palmer explained hypogonadism in ESRD by primary gonadal damage, presence of circulating LH (ICSH) receptor inhibitor that might results in gonadal-cell resistance and impaired feedback mechanism at the hypothalamic pituitary level, in addition to presence of excessive prolacin secretion [15].
Our results were in agreement with Oertel et al. [17], who stated that testosterone levels were lower in their patents with CRF with or without dialysis. In accordance with our results, Schaefer et al. [25] and Schmidt et al. [27] who reported similar results. Many previous publications explained delayed puberty and hypogonadism in pediatric population by presence of chronic hemolytic anemia which may be encountered in children and adolescents with ESRD. Kyriakou et al. [28] and Skordis et al. [29] stated that, delayed puberty and hypogonadism were the commonest endocrine complications in patients with chronic hemolytic anemia and are often unavoidable as hypothalamus, and pituitary damage is progressive, even when intensive therapy was given. Kurtoglu, et al. [30] and Perera, et al. [31] found that inadequate therapy of chronic hemolytic anemia lead to iron deposition in secretory gonadotrophin cells of pituitary gland or secretory cells of gonads resulting in primary gonadal failure or hypogonadotropic hypogonadism [30,31]. Other possible causes of hypogonadotrophic hypogonadism in pediatric age may include underlying type 1 diabetes mellitus, hypothyroidism or associated zinc deficiency [30,31]. In this study, there was significantly lower testicular volume in Group I when compared to controls with significant negative correlations between testicular volume and dialysis duration. This is in agreement with Soliman, et al. [32], who evaluated growth parameters and sexual maturation in a large cohort of children and adolescents with chronic illness (110 Sickle cell disease and 72 thalassemia) compared with 200 normal age matched children and they found that one quarter of their boys above the age of 14 years had absence of testicular development also they found that their patients who had spontaneous testicular development had significantly smaller testicular volume than normal controls.
Unfortunetly, Palmer et al. [15] stated that disturbances in pituitary-gonadal axis rarely normalized with initiation of hemodialysis or peritoneal dialysis, moreover, they may progress and explained these data by possibility that plasticizers in dialysis tubing, such as phytate may play a role in propagating the abnormalities in pituitary-gonadal axis.
Also Schmidt et al. [27] stated that impairment of hypothalamopitutary- gonadal axis is not reversed by initiation of otherwise effective hemodialysis or peritoneal dialysis therapy.
20830
References
- Gupta V, Lee M (2012) Growth hormone in chronic renal disease. Indian J Endocrinol Metab 16: 195-203.
- Dötsch J, Nüsken K, Benz K, Dittrich K, Plank Ch, et al. (2004) Endocrine dysregulation in adolescents with chronic renal failure. Transplantationsmedizin 16: 19.
- Chan JCM, Williams DM, Roth KS (2002) Kidney failure in infants and children. Pediatr Rev 23: 47-60.
- Haffner D, Zivicnjak M (2017) Pubertal development in children with chronic kidney disease. Pediatr Nephrol 9: 763-766.
- Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, et al. (2008) Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes 32: 959-966.
- Tanner JM, Whitehouse RH (1976) A note on the bone age at which patients with true isolated GH deficiency enter puberty. J Clin Endocrinol Meta 41: 788.
- Tomova A, Deepinder F, Robeva R, Lalabonova H, Kumanov P, et al. (2010) Growth and development of male external genitalia. Arch Pediatr Adolesc Med 164: 1152-1157.
- Garibaldi L (2004) Physiology of puberty. In: Behrman RE, Kliegman RM, Nelson. Pediatrics, 17th edn.,pp: 1862-1864.
- Prader A (1966) Testicular size: Assessment and clinical importance. Triangle 7: 240-243.
- Sharath Kumar C, Najafi M, Vineeth VS, Malini SS (2013) Assessment of testicular volume in correlation with sperminogram of infertile males in south India. Adv Stud Biol 5: 327-335.
- Gilbert Barness E, Barness LA (2003) Clinical use of pediatric diagnostic tests, 1st edn., Lippincott Williams & Wilkins. p: 689.
- George-Gay B, Parker K (2003) Understanding the complete blood count with differential. J Perianesth Nurs 18: 96-114.
- Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS (2004) Measurement of total serum testosterone in adult men: Comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab 89: 534-543.
- Khothari CR (2012) Research methodology, methods and Techniques, New Age International, In: Khothari CR(ed), 2nd edn., New Delhi, pp: 95-97.
- Palmer BF (1999) Sexual dysfunction in uremia. J Am Soc Nephrol 10: 1381-1388.
- Castellano M, Turconi A, Chaler E, Rivarola MA, Belgorosky A (1993) Hypothalamic-pituitary-gonadal function in prepubertal boys and girls with chronic renal failure. J Pediatr 122: 46-51.
- Oertel PJ, Lichtwald K, Häffner S, Rauh W, Schönberg D, et al. (1983) Hypothalamo-pituitary-gonadal axis in children with chronic renal failure. Kidney Int 24: 34-39.
- Belgorosky A, Ferraris JR, Ramirez JA, Jasper H, Rivarola MA (1991) Sex hormone-binding globulin and serum non sex hormone binding globulin-bound testosterone fractions in prepubertal boys with chronic renal failure. J Clin Endocrinol Metab 73: 107-110.
- Harmon WE, Jabs KL (1998) Chronic renal failure. In: Paediatric Nephrology.4th edn., Williams and Wilkins, pp: 1151-1154.
- Chatterjee R, Katz M (2000) Reversible hypogonadotrophic hypogonadism in sexually infantile male thalassaemic patients. Clinical Endocrinol 53: 33-42.
- Harold KM, Srivastava LS, Burstein S (1983) Hypergonadoropism in peripubertal boys with chronic renal failure. Pediatrics 72: 3.
- Haffner D, Wuhl E, Schaefer F, Nissel R, Tonshoff B, et al. (1998) The German study group for growth hormone treatment in CRF. J Am Soc Nephrol 9: 1899-1907.
- Ehrich JHH, Rizzoni G, Brunner FP (1991) Combined report on regular dialysis and transplantation in Europe, 1989. Nephrol Dial Transplant 6: 37-47.
- Schaefer F, Hamill G, Stanhope R, Preece MA, Scha¨rer K (1991) Cooperative study on pubertal development in chronic renal failure. Pulsatile growth hormone secretion in peripubertal patients with chronic renal failure. J Pediatr 119: 568-577.
- Schaefer F, van Kaick, Veldhuis DJ (1994) Change in the kinetics and biopotency of luteinizing hormone in hemodialyzed men during treatment with recombinant human erythropoietin. J Am Soc Nephrol 5: 1208-1215.
- Schaefer F, Walther U, Ruder H (1991) Reduced spermaturia in adolescent and young adult patients after renal transplantation. Nephrol Dial Transplant 6: 840.
- Schmidt A, Luger A (2002) Sexual hormone abnormalities in male patients with renal failure. Nephrol Dial Transplant 17: 368-371.
- Kyriakou A, Skordis N (2009) Thalassemia and aberrations of growth and puberty. Medit J Hemat Infect Dis 1: 2035-3006.
- Skordis N (2011) Endocrine investigations and follow up in thalassemia. Thalassemia Reports 1: 79-82.
- Kurtoglu UA, Kurtoglu E, Temizkan KA (2012) Effect of iron overload on endocrinopathies in patients with beta-thalassemia major and intermedia. Endokrynol Pol 63: 260-263.
- Perera NJ, Lau NS, Mathews S, Waite C, Ho PJ, et al. (2010) Overview of endocrinopathies associated with beta thalassaemia major. Intern Med J 40: 689-696.
- Soliman AT, ElZalabany M, Amer M, Ansari BM (1999) Growth and pubertal development in transfusion-dependent children and adolescents with thalassaemia major and sickle cell disease: Acomparative study. J Trop Pediatr 45: 23-30.








