Upma Narain1* and Arvind Gupta2
1Tejas Microdiagnostics, Allahabad, Uttar Pradesh, India
2Division of Nephrology, Moti Lal Nehru Medical College, Allahabad, Uttar Pradesh, India
Corresponding Author:
Dr. Upma Narain
Microbiologist and Immunologist
Tejas Microdiagnostics, 62, Jawahar Lal Nehru Road
Tagore Town, Allahabad, Uttar Pradesh–211002, India
Tel: +91 - 9415253337
E-mail: upmanarain@gmail.com
Received Date: September 11, 2016; Accepted Date: October 31, 2016; Published Date: November 07, 2016
Citation: Narain U and Gupta A. Superficial Fungal Infections: A 16 Years Study in ESRD Patients. Arch Clin Microbiol. 2016, 7:6. doi:10.4172/1989-8436.100060
Copyright: © 2016 Narain U et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: Mounting prevalence of end-stage renal disease is a major public health problem worldwide, resulting in millions of individuals affected. It is important to note that between 50% to 100% of such group have been found to have at least one or the other dermatological disorder. In the present study we have tried to analyze patients with end stage renal disease who had developed superficial fungal infections.
Methods and Findings: In between January 2000 to June 2016, analysis have been made on 2112 patients with end stage renal disease, who were undergoing haemodialysis at least thrice a week for a minimum of three months at our centre. Out of the above 2112 patients, 1500 suspected cases of superficial fungal infections we identified, 1040 cultures tested positive. The macroscopic examination of the scalp, skin and the nails revealed 0.2% Tinea capitis, 28.8% Pityriasis versicolor, 16.7% Tinea corporis, 5.3% Tinea mannum, 32.4% Onychomycosis, 13.5% Tinea cruris and 3.1% Tinea pedis. Culture examination highlighted 29.1% Trichyopyton rubrum, 22.9% Trychophyton mentagrophyte, 5.1% Trychophyton violaceum, 0.9% Trichophyton verrucosum, 0.1% Microsporum canis, 3.1% Epidermophyton flucossum, 3.1% Trychophyton tonsurans, 6.9% Scopulariopsis brevicaulis and 28.8% Malassezia. The predominant clinical abnormality observed was Onychomycosis and the prevalent fungal isolate was Trichophyton rubrum.
Conclusion: The current study illustrates that a notable number of patients with the end stage renal disease had a prevalence of superficial fungal infection; hence, a prompt recognition of skin lesion and identification of these superficial fungal infections alarm us to undertake early and judicious management in order to reduce the associated morbidity and thereby improve the quality of life in the said patients.
Keywords
End stage renal disease; Superficial fungal infections; Dermatophytes; Nondermatophytes; Haemodialysis; Yeast.
Introduction
Secondary immune failure in uremia is multi-faceted and is influenced by uremic intoxication per se, by altered renal metabolism of immunologically active proteins and specific effects of renal replacement therapy. Large interindividual variability shows the importance of individual factors [1]. Patients with end stage renal disease (ESRD) suffer from a variety of symptoms with very low quality of life accompanied by a particularly high prevalence of dermatological disorder [2]. Superficial mycoses are prevalent worldwide. They arise from a pathogen that is restricted to the stratum corneum, with little or no tissue reaction [3]. Dermatophytes are not part of the normal flora of the skin. Due to their keratinophilic and keratinolytic nature, they are able to use cutaneous keratin as a nutrient producing the infection in this way. Although the infection is confined to the superficial keratinized tissues, it induces a humoral and a cellular immune response [4-6]. The lipophilic yeast Malassezia is part of the normal cutaneous micro flora of most warm-blooded vertebrates. The normally commensal yeast may become a pathogen whenever alternation of the skin surface microclimate or host defense occurs [7,8]. Much has been reported about the cutaneous changes and invasive fungal infections among these patients but dedicated description of superficial fungal infections lack in literature. Hence the aim of this study is to evaluate the prevalence of superficial fungal infections in ESRD patients at our Centre.
Materials and Methods
It is a retrospective study involving patients undergoing haemodialysis at least thrice a week for a minimum of three months at our Centre who developed superficial fungal infections over a period of 16 years, from Jan 2000 to March 2016. Out of 2112 ESRD patients, 1500 ESRD patients developed superficial fungal infections. Dermatological evaluation and confirmation of the presenting lesions were done by the dermatologist. In all cases, data related to the age, sex, duration of the lesions, occupation, personal habits etc. were noted. After a detailed clinical examination, the physical features of the scalp, skin and nails were recorded. A lot of care has been particularly taken to record the past history of superficial mycotic infections.
Before obtaining a specimen, the infected areas were cleansed by swabbing them liberally with alcohol to eliminate as many bacteria as possible, because they can overgrow and inhibit the growth of dermatophytes. Scrapings and clippings were collected from the diseased portions of the scalp, skin and nails. When both the skin and the nails were affected, specimens were collected from both the sites.
Each specimen was divided into two parts; one was taken for direct microscopic examination after 10% KOH solution treatment and the second was inoculated on sabouraud dextrose agar (M286) and sabouraud cycloheximide chloramphenicol agar (M664). Two successive cultures were performed to establish the colonization of the pathogen because successive sampling rarely demonstrates the same contaminant. Cultures were routinely incubated at 25–30°C and examined daily for up to 4 weeks. The identification of the individual fungus was based on standard methods such as microscopy, morphology, colonial characterization and pigment production, rate of growth and biochemical test [9].
Results
Out of 2112 ESRD patients, a total of 1500 clinically suspected cases of superficial fungal infections were undertaken for mycological studies during the period spanning from Jan 2000 to June 2016 at our centre. The base line and the demographic data of all 1500 patients are summarized (Table 1).
| Demographic features |
| Age |
59.19 ± 9.099 |
| Gender |
Male1150 (76.7%)
Female 350 (23.3%) |
| Haemodialysis vintage (months) |
21.01 ± 8.045 |
| Primary diagnosis |
| Diabetes |
728 (48.5%) |
| Glomerulonephritis |
532 (35.5%) |
| Hypertension |
200 (13.3%) |
| Unknown |
40 (2.7%) |
| Laboratory test |
| Kt/V* |
1.2 ± 0.1 |
| S. creatine (mg/dl) |
5.4 ± 0.3 |
| S. albumin (g/dl) |
3.2 ± 0.4 |
| Haemoglobin (g/dl) |
10.0 ± 1.0 |
| BUN (mg/dl) |
40.0 ± 2.0 |
* Values expressed in mean ± SD. Kt/V parameter used for measurement of the adequacy of haemodialysis treatment
Table 1: Demographic features of ESRD patients.
Out of this total of 1500 suspected cases of superficial mycosis, 1040 cases were culture positive; the rest 460 cases did not show any fungal growth on culture media after an incubation period of 4 weeks hence they were considered as negative. A complete illustration of Tinea infections, the spectrum of lesions and spectrum of fungal isolates of the 1040 culture positive cases has been depicted in Figures 1-3 respectively. Culture isolates in relation to the site of involvement are shown (Table 2). For the 1040 culture positive patients the predominant clinical abnormality observed was Onychomycosis and the prevalent fungal isolate was Trichophyton rubrum. The duration of the lesions varied from one month to 4 months but a majority of these cases were of less than two month duration.
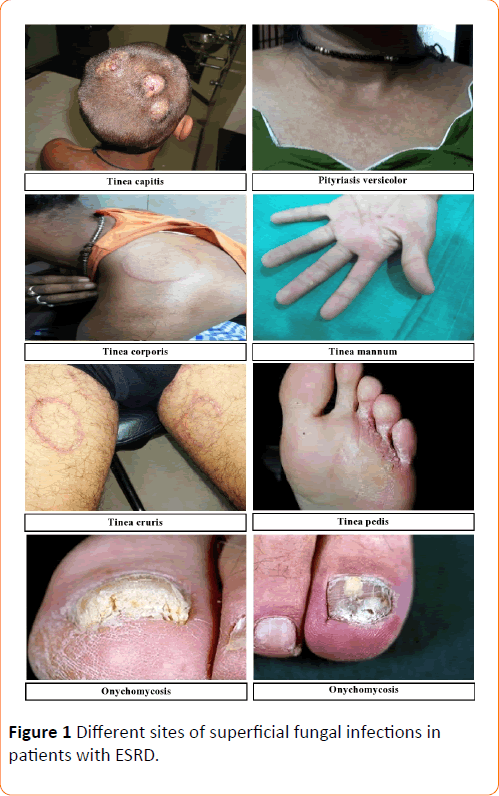
Figure 1: Different sites of superficial fungal infections in patients with ESRD.
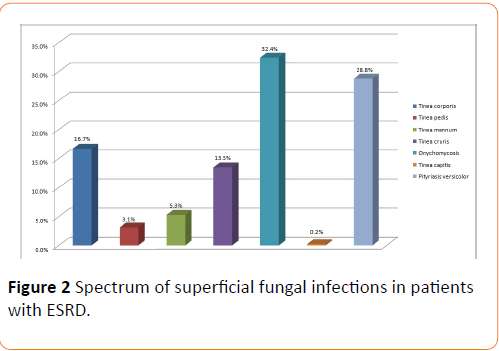
Figure 2: Spectrum of superficial fungal infections in patients with ESRD.
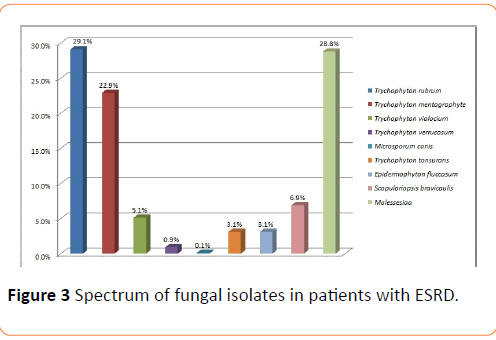
Figure 3: Spectrum of fungal isolates in patients with ESRD.
| Fungus |
Cutaneous Infection |
Total |
| Tinea corporis |
Tineapedis |
Tineamannun |
Tinea cruris |
Onycho- mycosis |
Tinea capitis |
Pityriasis versicolor |
| Trychophyton rubrum |
121 |
10 |
30 |
61 |
80 |
0 |
0 |
302 |
| 11.70% |
0.9% |
2.9% |
5.9% |
7.7% |
0.0% |
0.0% |
29.1% |
| Trychophyton mentagrophyte |
52 |
23 |
25 |
79 |
59 |
0 |
0 |
238 |
| 5.0% |
2.2% |
2.4% |
7.6% |
5.7% |
0.0% |
0.0% |
22.9% |
| Trychophytonviolocium |
0 |
0 |
0 |
0 |
54 |
0 |
0 |
54 |
| 0.0% |
0.0% |
0.0% |
0.0% |
5.1% |
0.0% |
0.0% |
5.1% |
| Trychophyton verrucosum |
0 |
0 |
0 |
0 |
8 |
2 |
0 |
10 |
| 0.0% |
0.0% |
0.0% |
0.0% |
0.8% |
0.1% |
0.0% |
0.9% |
| Microsporumcanis |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
2 |
| 0.0% |
0.0% |
0.0% |
0.0% |
0.1% |
0.0% |
0.0% |
0.1% |
| Trychophytontonsurans |
0 |
0 |
0 |
0 |
31 |
1 |
0 |
32 |
| 0.0% |
0.0% |
0.0% |
0.0% |
3.0% |
0.1% |
0.0% |
3.1% |
| Epidermophytonfluccosum |
0 |
0 |
0 |
0 |
32 |
0 |
0 |
32 |
| 0.0% |
0.0% |
0.0% |
0.0% |
3.1% |
0.0% |
0.0% |
3.1% |
| Scopulariopsisbravicaulis |
0 |
0 |
0 |
0 |
71 |
0 |
0 |
71 |
| 0.0% |
0.0% |
0.0% |
0.0% |
6.9% |
0.0% |
0.0% |
6.9% |
| Malessesiaa |
0 |
0 |
0 |
0 |
0 |
0 |
299 |
299 |
| 0.0% |
0.0% |
0.0% |
0.0% |
0.0% |
0.0% |
28.8% |
28.8% |
| Total |
173 |
33 |
55 |
140 |
337 |
3 |
299 |
1040 |
| 16.7% |
3.1% |
5.3% |
13.5% |
32.4% |
0.2% |
28.8% |
100.0% |
Table 2: Culture isolates in relation to the site of involvement.
The macroscopic examination of the scalp, skin and the nails of these 1040 patients further revealed 0.2% Tinea capitis, 28.8% Pityriasis versicolor, 16.7% Tinea corporis, 5.3% Tinea mannum, 32.4% Onychomycosis, 13.5% Tinea cruris and 3.1% Tinea pedis. Culture examinations revealed 29.1% Trichyopyton rubrum, 22.9% Trychophyton mentagrophyte, 5.1% Trychophyton violaceum, 0.9% Trichophyton verrucosum, 0.1% Microsporum canis, 3.1% Epidermophyton flucossum, 3.1% Trychophyton tonsurans, 6.9% Scopulariopsis brevicaulis and 28.8% Malassezia.
Discussion
Patients with end stage renal disease commonly show a spectrum of dermatological disorders and each disorder has its own unique presentation and treatment approach. Superficial fungal infections arise from a pathogen that is restricted to the stratum corneum, with little or no tissue reaction [3]. The host immune response against infections caused by the Dermatophytes depends on factors such as the host defenses against metabolites of the fungus, the virulence of the infecting strain or species, the anatomical site of infection and local environmental factors [10].
Out of the total of 1500 suspected cases of superficial mycosis, 1040 cases were of positive culture and the rest 460 cases did not show any fungal growth on culture media after an incubation period of 4 weeks. The possible reasons for a negative fungus culture might be the following: the presence of non-viable hyphae elements in the distal region of the diseased nail and in the margins of the skin lesions, an uneven colonization of lesion with the fungus and if an antifungal treatment had been given prior to the collection of the specimen. Low culture positivity rate was also reported in previous studies, in 2004 and 2005, the MRL received 5312 and 5137 dermatology specimens. Out of the above noted figures 2321 (45%) and 2277 (43%) were positive by direct microscopy, 1538 (30%) and 1553 (29%) were positive by culture and 1430 (28%) and 1415 (27%) were positive by both microscopy and culture [11].
Hypercytokinemia is a typical feature of uremia, likely due to accumulation of pro-inflammatory cytokines as a consequence of decreased renal elimination and/or increased generation following induction by uremic toxins, oxidative stress, volume overload, comorbidities etc. [12,13]. On the background of uremia- induced inflammatory activation of monocytes, dialysis therapy causes additional abnormalities. It activates complement and leads to further induction of cytokines such as IL-6 and TNFα [1].
Susceptibility to dermatophytes is variable and may be related to the variations in the composition of sebum, fatty acids, skin surface, carbon dioxide tension, presence of moisture or presence of inhibitors for the growth of dermatophytes in sweat or serum such as transferring [14,15].
Factors related to the fungus also contribute to the development of infection. Dermatophytes are able to penetrate keratinized cells by producing enzymes such as keratinases. It is suggested that Trichophyton rubrum has the ability to suppress the expression of TLR receptors in keratinocytes and LC necessary for stimulation of Th1-type cell response. Consequently, there would be marked expression of DC-SIGN in macrophages of the epidermis and dermis, which occurs in Th2- type responses, which are inadequate to fight fungal infection. This would allow a chronic and extensive infection caused by this dermatophyte to set in [3]. Trichophyton mentagrophyte has two enzyme isotypes. Malassezia species produce lipases which may aid in the digestion of fats in sebum. The Fungus that cause nail diseases do not all produce keratinases and some appear to be able to invade the nail plate only if there is a preexisting abnormality, such as peripheral vascular disease, trauma etc. Therefore, small changes in the host defense are important for allowing organisms to invade the skin [16]. Virulence factors of dermatophytes contribute to the modulation of the host immune response and can be expressed throughout the whole infectious process [17,18]. Several studies suggested that the immunosuppressive properties of the mannans is responsible for the chronicity of dermatophytosis especially caused by Trichophyton rubrum and is less inflammatory in individuals with impaired functions of T lymphocytes [19].
Different dermatophyte species vary in their ability to stimulate an immune response: organisms such as Trichophyton rubrum cause chronic or relapsing infections, whereas other fungi induce resistance to re-infection [20,21]. In our study two patients exhibited Tinea corporis lesions caused by Trichophyton rubrum during the period of one year. Thus, the fungus/host interaction exerts influence on the degree of inflammatory reaction which will define the clinical presentation and duration of the lesion in these patients.
The present study lightens the findings that a significant number of patients with the end stage renal disease has a prevalence of superficial fungal infections, hence, prompt recognition of these skin lesions and the identification of these superficial fungal infections need our prompt awareness so that early and judicious management may be taken to reduce the associated morbidity and thereby improve the quality of life in such patients.
17376
References
- Girndt M, Sester U, Sester M, Kaul H, Kohler H (1999) Impaired cellular immune function in patients with end-stage renal failure. Nephrol Dial Transplant 14: 2807-2810.
- Shafiee MA, Akbarian F, Memon KK, Aarabi M, Boroumand B (2015) Dermatologic Manifestations in End-stage Renal Disease. IJKD 9: 339-353.
- Schwartz RA (2004) Superficial fungal infections. The Lancet 364: 1173-1182.
- DeBoer DJ, Moriello KA, Cooley AI (1991) Immunological reactivity to intradermal dermatophyte antigens in cats with dermatophytosis. Vet Dematol 2: 59-67.
- DeBoer DJ, Moriello KA (1993) Humoral and cellular responses to Microsporumcanis in naturally occurring feline dermatophytosis. J Vet Med Mycol 31: 121-132.
- DeBoer DJ, Moriello KA (1995) Investigations of a killed dermatophyte cell-wall vaccine against infection with Microsporumcanis in cats. Res Vet Sci 59: 110-113.
- Ashbee HR (2006) Recent developments in the immunology and biology of Malassezia species. FEMS Immunol Med Microbiol 47: 14-23.
- Ashbee HR (2007) Update on the genus Malassezia. Med Mycol 45: 287-303.
- Narain U, Gupta A (2016) Peritoneal Dialysis Related Candida Peritonitis: A 16-year Single-Centre Experience. Arch ClinMicrobiol 7: 2.
- Criado PR, Dantas KC, Benini LV, Oliveira CB, Takiguti FA, et al. (2011) Superficial mycosis and the immune response elements. An Bras Dermatol 86: 726-731.
- Borman AM, Campbell CK, Fraser M, Johnson EM (2007) Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Medical Mycology 45: 131-141.
- Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, et al. (1998) Immunologic function and survival in hemodialysis patients. Kidney Int 54: 236-244.
- Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, et al. (2005) IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia–the good, the bad, and the ugly. Kidney Int 67: 1216-1233.
- Calderon RA (1989) Immunoregulation in dermatophytosis. Crit Rev Microbiol 16: 339-368.
- King RD, Khan HA, Foye JC, Greenberg JH, Jones HE (1975) Transferrin, iron, and dermatophytes. I. Serum dermatophyte inhibitory component definitively identified as unsaturated transferrin. J Lab Clin Med 86: 204-212.
- Hay RJ (1993) Fungi and Skin Disease. London: Gower Medical Publishing.
- Hay RJ, Calderon RA, Mackenzie CD (1988) Experimental dermatophytosis in mice: correlation between light and electron microscopic changes in primary, secondary and chronic infections. Br J ExpPathol 69: 703-716.
- Acorci-Valério MJ, Bordon-Graciani AP, Dias-Melicio LA, Golim MA, Nakaira-Takahagi E, et al. (2010) Role of TLR2 and TLR4 in human neutrophil functions against Paracoccidioidesbrasiliensis. Scandinavian Journal of Immunology 71: 99-108.
- Mignon B, Tabart J, Baldo A, Mathy A, Losson B, Vermout S (2008) Immunization and dermatophytes. CurrOpin Infect Dis 21: 134-140.
- MacGregor JM, Hamilton A, Hay RJ (1992) Possible mechanisms of immune modulation in chronic dermatophytosis - an in vitro study. Br J Dermatol 127: 233-238.
- Vermout S, Tabart J, Baldo A, Mathy A, Losson B, et al. (2008) Pathogenesis of dermatophytosis. Mycopathologia 166: 267-75.








