Key words
|
| |
| Quinazolin-3(2H)-one; Quinazolin-3-phenyl hydrazone; Anti-bacterial; Anti-fungal. |
| |
Introduction
|
| |
| The clinical potential of microbial product as therapeutic agents was first investigated by Pasteur and Joubert, who recorded their observation and speculations in 1877. The golden age of antibiotics began with the production of penicillin in 1941. The treatment of infectious diseases still remains an important and challenging problem because of a combination of factors including emerging infectious diseases and the increasing number of multi-drug resistant microbial pathogens with particular relevance for Gram positive bacteria [1-5]. We have found that quinazolines and condensed quinazolines exhibit potent pharmacological activities [6-13]. On the other hand, the considerable biological and medicinal activities of thiazole and their derivatives have attracted continuing interest over the years because of their varied biological activities [14], recently found application in drug development for the treatment of allergies [15], hypertension [16], schizophrenia [17], bacterial [18], HIV infections [19], and more recently for the treatment of pain [20], Our analogue-based design encompasses the synthesis of 5-(2'-hydroxy phenyl)- 6,7,8,9 tetra hydro-5H-(1, 3) thiazolo-2-(4’-substituted benzylidine) (2, 3-b) quinazolin-3-phenyl hydrazone derivatives to be tested for their in vitro antimicrobial properties against Gram positive and Gram negative bacteria and fungus. |
| |
Materials and Methods
|
| |
|
Materials
|
| |
| Synthetic starting material, reagents and solvents were of analytical reagent grade or of the highest quality commercially available and were purchased from Aldrich Chemical Co., Merck Chemical Co. and were dried when necessary. |
| |
| The melting points were taken in open capillary tube and are uncorrected. IR spectra were recorded with KBr pellets (ABB Bomem FT-IR spectrometer MB 104 ABB Limited Bangaluru, India). Proton (1H) NMR spectra (Bruker 400 NMR spectrometer Mumbai, India) were recorded with TMS as internal references. Mass spectral data were recorded with a quadrupol mass spectrometer (Shimadzu GC MS QP 5000, Chennai, India), and microanalyses were performed using a vario EL V300 elemental analyzer (Elemental Analysensysteme GmbH Chennai, India). The purity of the compounds was checked by TLC on pre-coated SiO2 gel (HF254, 200 mesh) aluminium plates (E.Merck) using ethyl acetate: benzene (1:3) and visualized in UV chamber. IR, 1H-NMR, mass spectral datas and elemental analyses were consistent with the assigned structures. |
| |
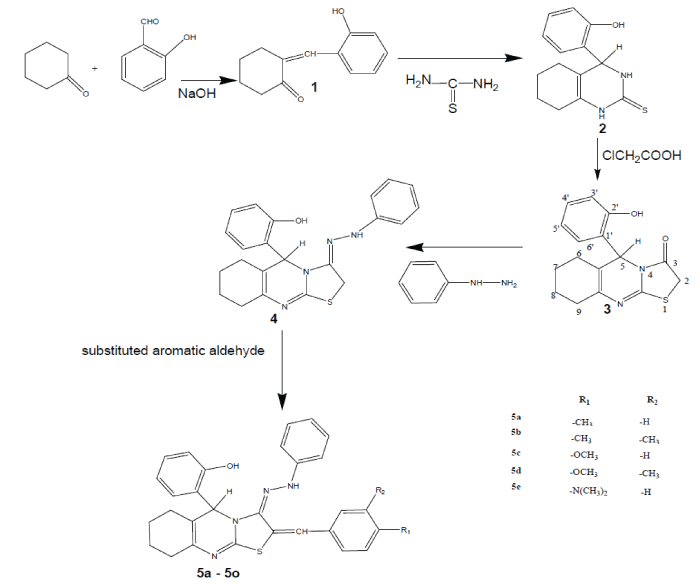
| General Procedures. The Synthesis of compounds starting compound 5-(2'-hydroxy phenyl)-6,7,8,9 tetra hydro-5H- (1,3)thiazolo (2, 3-b) quinazolin-3(2H)-one 3 prepared by the equimolar quantities of each (3.8g 0.039 mol) of cyclohexanone and salicylaldehyde (0.039 mol) were taken in a beaker, to this sodium hydroxide solution was added to make the solution alkaline, this was shaken and kept aside. The solid thus obtained, was filtered, washed with water and recrystallized from absolute ethanol; it yields 84% of 2- hydroxy benzylidine cyclohexanone ring 1. A mixture of 2-hydroxy benzylidine cyclohexanone ring 1 (0.039 mol) thiourea (0.03 mol) and potassium hydroxide (2.5g) in ethanol (100 ml) was heated under reflux for 3 h. The reaction mixture was concentrated to half of its volume, dilute with water, then acidified with dilute acetic acid and kept overnight. The solid thus obtained, was filtered, washed with water and recrystallized from ethanol to give 79% of 4-hydroxy phenyl 3, 4, 5, 6, 7, 8-hexahydro quinazolin-2-thione 2. The chloroacetic acid (0.096 mol) was melted on a water bath and thione (0.009 mol) added to it portion wise to maintain its homogeneity. The homogeneous mixture was further heated on a water bath for 30 min and kept overnight. The solid thus obtained was washed with water until neutralized and crystallized from ethanol to give 5-(2'- hydroxy phenyl)- 6,7,8,9 tetra hydro-5H- (1, 3) thiazolo (2, 3-b) quinazolin-3(2H)-one 3 [21]. A mixture of 3 (0.002 mol), treated with phenyl hydrazine (0.002 mol) anhydrous sodium acetate (0.002 mol) and glacial acetic acid (10 ml) were dissolved in 10ml of warm ethanol and refluxed for 30 min. After standing for approximately 24h at room temperature, the product were separated by filtration, vaccum dried and recrystallized from warm ethanol to yields 5-(2'- hydroxy phenyl)- 6,7,8,9 tetra hydro-5H- (1, 3) thiazolo (2, 3-b) quinazolin-3-phenyl hydrazone 4. |
| |
| Equimolar quantities (0.002 mol) of compound 4 clubbed with substituted benzaldehyde (0.002 mol) and anhydrous sodium acetate (0.002 mol) in glacial acetic acid (10 ml) was heated under reflux for 4h. The reaction mixture was kept overnight and the solid, thus separated, was filtered, washed with water and recrystallized from ethanol to furnish of 5-(2'-hydroxy phenyl)- 6,7,8,9 tetra hydro-5H-(1,3) thiazolo-2-(4’- substituted benzylidine) (2, 3-b) quinazolin-3-phenyl hydrazone (5a-e). The spectral data IR, 1H NMR, mass spectroscopy and elemental analyses were used to ascertain the structures of all the compounds. |
| |
| The analytical data for the prepared compounds are given below. 1H NMR spectra were recorded for all the target compounds. The 1H NMR spectra were recorded for the representative key intermediate. The 5-(2'- hydroxy phenyl)-6,7,8,9 tetra hydro-5H- (1,3)thiazolo (2, 3-b) quinazolin-3(2H)-one (3). Yield: 71%; m.p.153-155°C; IR cm-1: 3402 (phenolic OH), 3046 (Ar-CH), 1719 (C=O), 1462 (C=C); 1H-NMR (CDCl3) δ: 9.91 (s, 1H; Ar-OH), 6.61-6.89 (m,4H Ar- H), 5.71 (s, 1H; -CH) 3.76 (s, 2H; -CH2) 1.6-2.42 (m, 8H; CH2, CH2, CH2, CH2 ). EI-MS (m/z): 300 (M+); (Calcd for C16H16N2O2S; 300.38). Anal. Calcd for C16H16N2O2S; C, 63.98; H, 5.37; N, 9.32. Found: C, 63.81; H, 5.28; N, 9.43. |
| |
| 5-(2'-hydroxy phenyl)- 6,7,8,9 tetra hydro-5H- (1, 3) thiazolo (2, 3-b) quinazolin-3-phenyl hydrazone (4). Yield: 79%; m.p.162-164°C; IR cm-1: 3467 (O-H), 3065 (Ar-CH), 1541 (C=C), 1610 (C=N), 1333 (N-H bending), 3378 (N-H stretching); 1H-NMR (CDCl3): δ 9.74 (s, 1H, H-2', Ar-OH), 7.12 (s, 1H, N-H), 6.98-7.36 (m, 9H, Ar-H), 5.82 (s, 1H, H-5), 2.97 (s, 2H, thiazole), 1.59-2.47 (m, 8H, 4 × CH2); EI-MS (m/z): 390 (M+); (Calcd for C22H22N4OS; 390.5). Anal. Calcd for C22H22N4OS; C, 67.67; H, 5.68; N, 14.32. Found: C, 67.56; H, 5.59; N, 14.43. |
| |
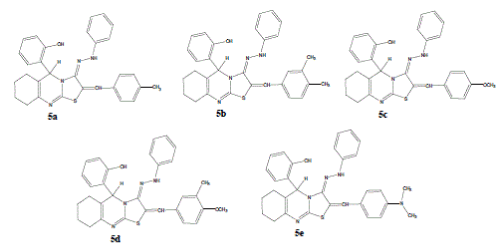
| 5-(2'-hydroxy phenyl)- 6,7,8,9 Tetra hydro-5H- (1, 3) thiazolo -2-(4'-methyl benzylidine) (2, 3-b) quinazolin-3-phenyl hydrazone (5a). Yield: 71%; m.p.144-146°C; IR cm-1: 3421 (O-H), 3027 (Ar-CH), 1419 (C=C), 1629 (C=N),1316 (N-H bending), 3304 (N-H stretching); 1H-NMR (CDCl3): δ 9.74 (s, 1H, H- 2', Ar-OH), 7.57 (s, 1H, N-H), 6.69-7.24 (m, 13H, Ar- H), 6.23 (s, 1H, =CH), 5.61 (s, 1H, H-5), 2.31 (s, 3H, – CH3), 1.36-2.41 (m, 8H, 4 × CH2); EI-MS (m/z): 492 (M+); (Calcd for C30H28N4OS; 492.63). Anal. Calcd for C30H28N4OS; C, 73.14; H, 5.73; N, 11.37. Found: C, 73.16; H, 5.64; N, 11.27. |
| |
| 5-(2'-hydroxy phenyl)- 6,7,8,9 Tetra hydro-5H- (1, 3) thiazolo -2-(3',4'-dimethyl benzylidine) (2, 3-b) quinazolin-3-phenyl hydrazone (5b). Yield: 72%; m.p.173-175°C; IR cm-1: 3429 (O-H), 3019 (Ar-CH), 1413 (C=C), 1648 (C=N), 1334 (N-H bending), 3313 (N-H stretching); 1H-NMR (CDCl3): δ 9.93 (s, 1H, H- 2', Ar-OH), 7.62 (s, 1H, N-H), 6.79-7.24 (m, 12H, Ar- H), 6.26 (s, 1H, =CH), 5.74 (s, 1H, H-5), 2.34 (s, 6H, – CH3), 1.36-2.41 (m, 8H, 4 × CH2); EI-MS (m/z): 506 (M+); (Calcd for C31H30N4OS; 506.66). Anal. Calcd for C31H30N4OS; C, 73.49; H, 5.97; N, 11.06. Found: C, 73.28; H, 5.82; N, 11.18. |
| |
| 5-(2'-hydroxy phenyl)- 6,7,8,9 Tetra hydro-5H- (1, 3) thiazolo -2-(4'-methoxy benzylidine) (2, 3-b) quinazolin-3-phenyl hydrazone (5c). Yield: 74%; m.p.157-159°C; IR cm-1: 3464 (O-H), 3027 (Ar-CH), 1494 (C=C), 1626 (C=N),1306 (N-H bending), 3396 (N-H stretching); 1H-NMR (CDCl3): δ 9.87 (s, 1H, H- 2', Ar-OH), 7.22 (s, 1H, N-H), 6.72-7.23 (m, 13H, Ar- H), 6.36 (s, 1H, =CH), 5.62 (s, 1H, H-5), 3.78 (s, 3H – OCH3), 1.46-2.42 (m, 8H, 4 × CH2); EI-MS (m/z): 508 (M+); (Calcd for C30H28N4O2S; 508.63). Anal. Calcd for C30H28N4O2S; C, 70.84; H, 5.55; N, 11.02. Found: C, 70.75; H, 5.46; N, 11.21. |
| |
| 5-(2'-hydroxy phenyl)- 6,7,8,9 Tetra hydro-5H- (1, 3) thiazolo -2-(4'-methoxy-3'-methyl benzylidine) (2, 3-b) quinazolin-3-phenyl hydrazone (5d). Yield: 79%; m.p.185-187°C; IR cm-1: 3444 (O-H), 3029 (Ar- CH), 1486 (C=C), 1627 (C=N),1311 (N-H bending), 3389 (N-H stretching); 1H-NMR (CDCl3): δ 9.87 (s, 1H, H-2', Ar-OH), 7.38 (s, 1H, N-H), 6.72-7.23 (m, 12H, Ar-H), 6.36 (s, 1H, =CH),5.62 (s, 1H, H-5), 3.78 (s, 3H –OCH3), 3.72 (s, 3H –CH3), 1.46-2.42 (m, 8H, 4 × CH2); EI-MS (m/z): 522 (M+); (Calcd for C31H30N4O2S; 522.66). Anal. Calcd for C31H30N4O2S; C, 71.24; H, 5.79; N, 10.72. Found: C, 71.36; H, 5.84; N, 10.78. |
| |
| 5-(2'-hydroxy phenyl)- 6,7,8,9 Tetra hydro-5H- (1, 3) thiazolo -2-(4' dimethyl amino benzylidine) (2, 3- b) quinazolin-3-phenyl hydrazone (5e). Yield: 73%; m.p.162-164°C; IR cm-1 : 3441 (O-H), 3035 (Ar-CH), 1417 (C=C), 1653 (C=N),1336 (N-H bending), 3376 (N-H stretching); 1H-NMR (CDCl3): δ 9.86 (s, 1H, H- 2', Ar-OH), 7.89 (s, 1H, N-H), 6.72-7.23 (m, 13H, Ar- H), 6.46 (s, 1H, =CH), 5.74 (s, 1H, H-5), 2.28 (s, 6H, – CH3), 1.39-2.43 (m, 8H, 4 × CH2); EI-MS (m/z): 521 (M+); (Calcd for C31H31N5OS; 521.68). Anal. Calcd for C31H31N5OS; C, 71.37; H, 5.99; N, 13.42. Found: C, 71.49; H, 5.86; N, 13.31. |
| |
|
Antimicrobial Screening
|
| |
| The biological evaluation of synthesized compound was performed using the disk diffusion method [22]. In the present study four gram-positive, four-gram negative, and one fungus were selected. The gram positive strains were Bacillus lentus (ATCC 155), Bacillus cereus (ATCC 11778), Micrococcus luteus (ATCC 4698), Staphylococcus albus (ATCC 9144); gram negative strains were Escherichia coli (ATCC 25922), Klebsiella aerogenes (ATCC 2853), Salmonella paratyphi (ATCC 11298), Proteus vulgaris (ATCC 9029) and fungus Candida albicans (ATCC 2091). The strain was confirmed for its purity and identity by the gram-staining method and it was further characterized by chemical reaction. The selected strains were preserved by periodical subculturing on agar slant and storing them under frozen condition; for the study fresh 24 hours broth cultures were used. Each bacterial and fungal pure culture was transferred into 100 ml of Muller Hinton nutrient broth and Sabouraud’s dextrose broth, respectively. The inoculated broths were incubated at 37°C for 24 hours and 27°C for 72 hours for bacteria and fungus respectively. After incubation, inocula were standardized to 108 colony-forming units (CFU)/ml for bacteria and 106 CFU/ml for fungus by colony forming unit method. Muller Hinton agar media was prepared by using Beef infusion 300 g, Casein acid hydrolysation 17.5 g, starch 1.5 g, and agar 17 g. Accurately weighed quantities of these ingredients were suspended in 1,000 ml of distilled water. They were boiled to dissolve completely. The pH was adjusted to 7.3 ± 0.2 at 25°C. It was then sterilized by autoclaving at 15 lbs. pressure (121°C for 15 minutes). The prepared Muller Hinton agar medium was transferred into sterile Petri plates; 200 µl of the standardized bacterial inoculums and fungus inoculum were spread on agar medium using sterile cotton swab. The synthesized product of thiazolo quinazoline derivatives were dissolved in suitable chloroform solvent to a final concentration of 50 µl of drug solution, each disk absorbed approximately 10 µl of the drug. The drug was impregnated on disk and placed on the inoculated agar medium. Ciprofloxacin and clotrimidazole were used as a standard for the antibacterial and antifungal activity, respectively. All the bacterial Petri plates were kept in an incubator and the fungal Petri plate was kept at room temperature for approximately 18 hours. Then the zones of inhibition were measured. |
| |
Results and discussion
|
| |
|
Chemistry
|
| |
| The synthesized series of heterocycles, 5a-e by the reaction of 3 with appropriate phenyl hydrazine and aromatic aldehydes in the presence of anhydrous sodium acetate and glacial acetic acid as presented in Scheme 1. The IR, 1H-NMR, mass spectroscopy and elemental analyses for the new compound is in accordance with the assigned structures. From the structural investigation, IR spectra showed the absence of C=O peak at 1715-1740 cm-1and appearance of a strong intensity band in the IR spectra of compound 4 in the range of 1610–1655 cm-1 attributable to C=N peak provides a strong evidence for the condensation and also confirms the formation of the azomethine 4. The proton magnetic resonance spectra of thiazolo quinazoline and their corresponding derivatives have been recorded in CDCl3. In this 5a-e NH signal of thiazolo (2, 3-b) quinazolin-3-phenyl hydrazone moiety appear range at 7.22 – 7.89 (s) ppm respectively. The position and presence of NH signal in the 1H-NMR spectra of final compounds conforms the secondary NH proton in thiazolo quinazoline moiety. This clearly envisages that thiazole-3-one moiety involve in thiazolo (2, 3-b) quinazolin-3-phenyl hydrazone formation. All these observed facts clearly demonstrate that the 3rd position of keto group in thiazole ring is converted into secondary amino group as indicated in |
| |
|
Antimicrobial
|
| |
| The antimicrobial screening of all the compounds showed an excellent zone of inhibition against both gram-positive and gram-negative bacteria than standard ciprofloxacin. Similarly, the zone of inhibition on the fungal strain showed a stronger activity than the standard clotrimidazole. New derivatives of 5-(2'- hydroxy phenyl)- 6,7,8,9 tetra hydro-5H-(1, 3) thiazolo-2-(4’-substituted benzylidine) (2, 3-b) quinazolin-3-phenyl hydrazone 5a-e series exhibits stronger inhibition on gram-negative Escherichia coli compared with other bacterial strains. On the other hand, Candida albicans zone was highly inhibited by title compounds 5a-e, which proves the efficiency of antifungal activity than antibacterial activity. The range of inhibition on Staphylococcus albus comparatively smaller than other bacterial species. Discussing the antimicrobial activity against individual organisms, it was clear that all the compounds have significant inhibitions. It was found that the Escherichia coli and Candida albicans were highly susceptible to killing by the synthesized 5-(2'-hydroxy phenyl)- 6,7,8,9 tetra hydro-5H-(1, 3) thiazolo-2-(4’-substituted benzylidine) (2, 3-b) quinazolin-3-phenyl hydrazone 5a-e derivatives. The observed data on the antimicrobial activity of the synthesized compounds and standard drugs are given in Table 1. |
| |
Tables at a glance
|
 |
| Table 1 |
|
| |
Figures at a glance
|
 |
| Figure 1 |
 |
| Scheme 1 |
|
| |








