Key words
|
| |
| Oxadiazole, microorganisms |
| |
Introduction
|
| |
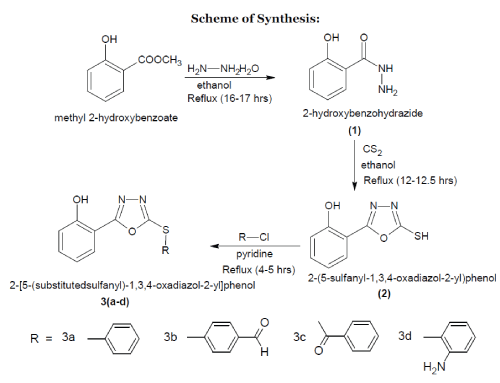
| The major drawback of current treatment of infectious diseases are challenging due to resistance to antimicrobial agents and their side effects. In order to overcome this situation, it is necessary to continue the search for new antibacterial agents. In recent scenario heterocycles plays a major role in drug synthesis. In that respect oxadiazole plays a significant role among other heterocycles. From the literature survey oxadiazole was found to be having diverse activity like anti-inflammatory, antimicrobial, antifungal, antiviral, analgesic, anti-mycobacterial, antidepressent and anticancer etc. So it was planned to synthesize a novel series of 1,3,4 oxadiazole derivatives and to check their activity as antimicrobial and antifungal agent.1-7 |
| |
Experimental
|
| |
| The entire chemicals were supplied by S. D. Fine Chem. (Mumbai), Finar Chem. Ltd (Ahmedabad) and Loba Chemie. Pvt. Ltd. (Mumbai). Melting points were determined by open tube capillary method and were uncorrected. Purity of compounds were checked by thin layer chromatography (TLC) on silicagel-G in solvent system hexane-ethyl acetate (1:1) and the spots were located under iodine vapours and UV light. IR spectra of all compounds were recorded on FT-IR 8400S Shimadzu spectrophotometer using KBr. Mass spectra were obtained using 2010EV LCMS Shimadzu instrument. |
| |
 |
| |
|
Synthetic procedure of 2-hydroxybenzohydrazine:-
|
| |
| A mixture of (12.94ml, 0.1mole) methyl salicylate and (10ml, 0.2mole) hydrazine hydrate were refluxed in 50ml ethanol for 17hours. The resultant mixture was concentrated, cooled and poured into crushed ice. The solid mass thus seperated out was dried and recrystallized from ethanol.7 |
| |
|
Synthetic procedure of 2-(5-sulfanyl-1,3,4-oxadiazol-2-yl)phenol:-
|
| |
| A mixture of (1.52g, 0.01mole) of 2- hydroxybenzohydrazine, (0.56g, 0.01mole) of KOH and 10ml of CS2 were refluxed in 50ml of 95% ethanol for 12-12.5hours. The resultant mixture was concentrated and cooled to room temperature, acidified with dil.HCl. and the crude product was filtered and recrystallized from ethanol.7 |
| |
|
General procedure of 2-(5-substituted sulfanyl-1,3,4-oxadiazol-2-yl)phenol derivatives:-
|
| |
| A mixture of (0.97g, 0.005mol) of 2-(5-sulfanyl- 1,3,4-oxadiazol-2-yl)-phenol and (0.005mol) of different aryl halides were refluxed in 25ml of pyridine solution for 3.5hours. The resultant mixture was cooled and poured into crushed ice. The solid mass is thus seperated out was dried and recrystallized from ethanol. |
| |
|
Spectral characterization data:-
|
| |
| 1. IR(KBr) [cm-1]:- -OH (3321), -NH2 (3257.55, 3245), >C=O (1710) |
| |
| 2. IR(KBr) [cm-1]:- -OH (3321), -C-S-H (2600), >C=N (1650), 2910 (Ar, C-H streching) |
| |
| 3a. IR (KBr) [cm-1]:- -OH (3328), -C-O-C- (1265), MS [m/z]=270 [M+] |
| |
| 3b. IR (KBr) [cm-1]:- -OH (3305), -CHO (1700, 2854), -C-O-C (1230), MS [m/z]=297 [M-1] |
| |
| 3c. IR (KBr) [cm-1]:- -OH (3285), >C=O (1681), C-O-C (1292), MS [m/z]=298 [M+], 1H NMR (DMSO-d6): δ: 6.9-8.2 (m, 9H, ArH), 10.6 (s, 1H, OH). |
| |
| 3d. IR (KBr) [cm-1]:- -OH (3217), -NH2 (3182, 3139), C-O-C (1260), MS [m/z]=285 [M+] |
| |
|
Antibacterial Activity
|
| |
| The microbiological assay was based upon a comparison of inhibition of growth of microorganisms by measured concentrations of test compounds with that produced by known concentration of a standard antibiotic. Two methods generally employed were turbidometric (tubedilution) method and filter paper disc method. In the turbidometric method inhibition of growth of microbial culture in a uniform dilution of antibiotic in a fluid medium is measured. It was compared with the synthesized compounds. Here the presence or absence of growth was measured. The cylinder plate method depends upon diffusion of antibiotic from a vertical cylinder through a solidified agar layer in a petridish or plate to an extent such that growth of added micro-organisms is prevented entirely in a zone around the cylinder containing solution of the antibiotics. The cup-plate method is simple and measurement of inhibition of microorganisms was also easy. Here we have used this method for antibacterial screening of the test compounds.8-12 |
| |
|
Name of Microorganism
|
| |
| Gram +ve microorganisms |
| |
| Staphylococcus aureus (MTCC No. 96) |
| |
| Pseudomonas aeruginosa (MTCC No. 1688) |
| |
| Bacillus subtillis (MTCC No. 121) |
| |
| Gram -ve microorganisms |
| |
| Escherichia coli (MTCC No. 521). |
| |
|
Preparation of medium:-
|
| |
| Nutrient agar 2% |
| |
| Peptone 1% |
| |
| Beef extract 1% |
| |
| Sodium chloride 0.5% |
| |
| Distilled water up to 100ml |
| |
| All the ingredients were weighed and added to water. This solution was heated on water bath for about one and half-hour till it became clear. This nutrient media was sterilized by autoclave at 121°C at 15psi. |
| |
|
Apparatus:-
|
| |
| All the apparatus like petridishes, pipettes, glass rods, test-tubes etc. were properly wrapped with papers and sterilized in hot air oven. |
| |
|
Antimicrobial screening method
|
| |
| • All the Petri dishes were sterilized in oven at 160°C for I hour. |
| |
| |
| • Agar media, borer and test solutions were sterilized in autoclave at 121°C at 15psi. |
| |
| • Molten sterile agar was poured in sterile petri dishes asceptically. |
| |
| • The agar was allowed to cool and the bacterial suspension was poured into the petridishes asceptically. |
| |
| • Placing the sterile filter paper discs in the agar plate and solution of the compounds was added by using pipette (0.1ml) in appropriate four quadrants of petridishes aseptically. |
| |
| Petridishes were incubated at 37°C for antimicrobial and 24ºC for antifungal for 24 hrs and observed the zone of inhibition.13-17 |
| |
|
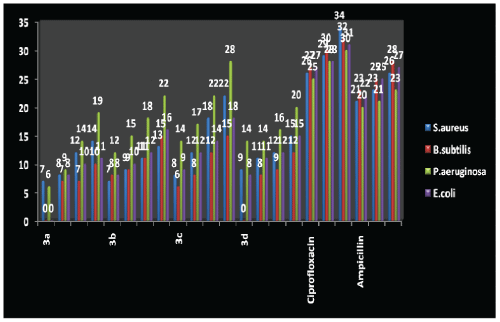
HISTOGRAM: ANTIMICROBIAL SCREENING BY ZONE OF INHIBITION IN MILIMETER (FILTER PAPER DISC METHOD)
|
| |
 |
| |
|
Antifungal activity:-13-17
|
| |
|
Preparation of standard solution
|
| |
| The standard drug fluconazole and ketoconazole were dissolved in appropriate quantity of DMF to obtain the concentration range of 100, 250 and 500μg/ml and the zone of inhibition was checked. |
| |
|
Preparation of test solution
|
| |
| Specified quantity (100mg) of the compound was accurately weighed and dissolved in 100ml of DMF to get the 1000μg/ml stock solution. Further dilution was made to obtain the concentration in the range 750μg/ml, 500μg/ml and 250μg/ml. |
| |
|
Fungi used
|
| |
| The synthesized compounds were screened for their antifungal activity against fungi Candida albicans (MTCC No. 22). |
| |
|
Preparation of Sabouraud Dextrose Broth Formula/Liter
|
| |
| Enzymatic digest of Casein 5g |
| |
| Enzymatic digest of Animal Tissue 5g |
| |
| Dextrose 20g |
| |
| Final pH 5.6 ±0.2 at 25 °C |
| |
| Purified water 1000ml |
| |
|
Procedure
|
| |
| 30g of the medium was suspended in 1000ml of purified water. The mixture was allowed to boil till it forms a homogeneous solution. The medium was autoclaved at 121°C for 15 minutes at 15psi. |
| |
|
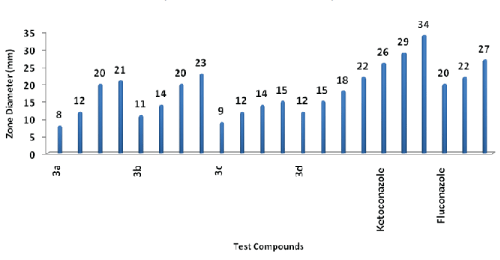
HISTOGRAM: ANTIFUNGAL SCREENING BY ZONE OF INHIBITION IN MILIMETER (FILTER PAPER DISC METHOD)
|
| |
 |
| |
|
HISTOGRAM: MIC of test compounds
|
| |
 |
| |
CONCLUSION
|
| |
|
Antibacterial activity
|
| |
| From the result it was found that 3c compound has maximum antibacterial activity against P. aeruginosa. Compounds 3b and 3c have found maximum activity against B. subtilis. Compound 3c has maximum activity against S. aureus. |
| |
|
Antifungal activity
|
| |
| Maximum antifungal activity against C. albicans was found in compound 3b. All compounds were less potent than standard drugs ampicillin, ciprofloxacin, fluconazole and ketoconazole. |
| |
ACKNOWLEDGEMENT
|
| |
| The author Palak K. Parikh is thankful to the project guide Prof. Dr. Dhrubo Jyoti Sen and the staff members of Shri Sarvajanik Pharmacy College, Mehsana, Gujarat to fulfil the project successfully. All the authors are thankful to the Quality Assurance Department of Shri Sarvajanik Pharmacy College, Mehsana for UV and IR spectral data, Oxygen Healthcare, Ahmedabad for Mass spectral data and Punjab University for NMR spectral datas. |
| |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
| |










