Keywords
Complex; Metal ions; [Cu(2(4-nitrophenyl)-4.5- diphenyl-1H-imidazole)2]; [Co(2(4-nitrophenyl)-4.5-diphenyl-1Himidazole) 3]; [Mn(2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole)3] complexes; Toxicity
Introduction
The imidazole compound is a compound that is frequently utilized in medicine. Imidazole is a five-membered ring compound (aromatic) composed of three carbon (C) atoms and two nitrogen (N) atoms. Imidazole is often used in pharmacy as a drug ingredient. For example, the compound Cimetidine which was developed by Smith, Kline, and French can be used to relieve stomach pain so that the person suffering the illness does not need an operation or even die because of it [1]. The other compound is Nitrofurantoin, which is a derivation of the imidazoline compound which has anti-urinary-tract-infection properties. In addition, there is also Sulconazole nitrate, which is a topical antifungal [2]. In the previous research, the ligands derived from the imidazole compound are often used as an antibacterial agent. N-alkyl imidazoles, such as 2-methyl imidazole and 2-methyl-4-nitro imidazole, are created as antibacterial agents. The compounds were tested against Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. The antibacterial effects of imidazole derivations increased in accordance with the number of carbon atoms on the alkyl chain. The substitution of 2-methyl and 2-methyl-4-nitro on the imidazole ring was also able to increase antibacterial effects [3]. Complex compounds have an important role in many biological systems. It has been often researched that metal complexes have a great influence on antimicrobial activities. Complex compounds of Zn(II), Cu(II), Co(II), and Ni(II) with an imidazole derivation (1,3-di(1H-imidazole-1-il)-2- propanol) have been reported." The metal complex compound has been tested against E. coli, P. aeruginosa, Klesbiella pneumonia and S. aureus using diffusion method. Research findings show that most complex compounds are more active than ligands on several species of bacteria [4]. The nature of three complex compounds from Cu(II), Co(II), and Pb(II) metal ions with a 4.5-diphenyl-2-(4-nitrophenyl)-1H-imidazole (DNPI) ligand has been reported. The structures of the three complex compounds are the same with 2(4-nitrophenyl)-4.5-diphenyl-1Himidazole. However, the activity of the complex compounds against cancer cells has not been further researched [5]. Based on the above explanation, research on the formation of complex compounds using the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole compound with Cu(II), Co(II), and Mn(II) metal ions needs to be conducted. Synthesis, characterization, and toxicity test are done to understand the activity of the three formed complex compounds that are derived from imidazole.
Experimental Section
Materials
Benzyl acetate, ammonium acetate, glacial acetic acid, nitrogen gas, 4-nitrobenzaldehyde, n-hexanes:ethylacetate=3:1 solvent, ethyl acetate, CoCl2.6H2O, ethanol, CuCl2.2H2O, MnCl2.4H2O, KBr pellet, HNO3, L-Cysteine Standard.
Instrumentation: FTIR Spectrometer, 1Procedure: Synthesis of the 2(4-nitrophenyl)-4.5-diphenyl-1Himidazole
ligand:H NMR Spectrometer, Atomic Absorption Spectrometer (AAS).
Procedure: Synthesis of the 2(4-nitrophenyl)-4.5-diphenyl-1Himidazole ligand: The 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand was synthesized using a previously reported method [6]. Benzyl (10 mmol) and ammonium acetate (0.1 mol) was added to a reflux flask containing glacial acetic acid (25 mL) and was stirred at a temperature of 80-100°C for 1 hour with nitrogen gas channeled in 4-nitrobenzaldehyde (10 mmol) in glacial acetic acid (5 mL) was added drop by drop for 15 minutes and was stirred for 4 hours. The reaction was monitored by a TLC with an n-hexanes:ethylacetate=3:1 solvent; after the reaction became perfect, the reaction was stopped and the reaction mixture was set aside at room temperature. The reaction mixture was poured into an ice bath (200 gr) and a yellow precipitate was obtained. The yellow precipitate was filtered with a vacuum pump and rinsed with cold distilled water. After being dry, the formed ligand was recrystallized using ethyl acetate. The formed ligand is dark yellow in color and was tested for melting points and characterized with FTIR, 1H NMR.
Synthesis of the cobalt(ii) metal complex with the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand (1)
The synthesis of the complex compound Co(II) with the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand was done using a previously reported method [7]. The synthesis of the Co(II) complex with the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand was done with a reflux tool. The CoCl2.6H2O metal of 0.0592 grams (0.25 mmol) was combined with 0.1985 grams (0.5 mmol) of the ligand and was put into a 100 mL reflux flask and given an ethanol solvent of 25 mL. The reaction mixture underwent reflux for 24 hours at a temperature of 60-80°C, and the solution was put into a desiccator and set aside for a few days to form crystals. The crystals were filtered and dried, and then weighed and analyzed.
Synthesis of the copper(ii) metal complex with the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand
The synthesis of Cu(II) complex compound with the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand was done using a previously reported method [5]. The synthesis of the Cu(II) complex with the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand was done with a set of reflux tool. CuCl2.2H2O of 0.0426 grams (0.025 mmol) was weighed and added into a round flask, and then 40 mL ethanol was added in and stirred at room temperature until dissolved. The 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand was weighed to get 0.1705 grams (0.05 mmol) of it and added to a beaker glass, dissolved in 15 mL ethanol, and stirred. The 2(4-nitrophenyl)-4.5-diphenyl-1Himidazole ligand was added drop by drop into the metal-filled flask and stirred at room temperature. The reaction mixture underwent reflux for 24 hours and then the solution was put into a desiccator and set aside for a few days to form crystals. The crystals were filtered and dried, and then weighed and analyzed.
Synthesis of the manganese(ii) metal complex with the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand
The synthesis of Mn(II) complex compound with the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand was done using a previously reported method [8]. The synthesis of the Mn(II) complex with the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand was done with a reflux tool. MnCl2.4H2O of 0.0507 grams (0.025 mmol) was weighed and added into a round flask, and then 15 mL ethanol was added in and stirred at room temperature until dissolved. The 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand was weighed to get 0.1986 grams (0.05 mmol) of it and added to a beaker glass, dissolved in 15 mL ethanol, and stirred. The 2(4-nitrophenyl)-4.5-diphenyl-1Himidazole ligand was added drop by drop into the metal-filled flask and stirred at room temperature. The reaction mixture underwent reflux for 24 hours, and then the solution was put into a desiccator and set aside for a few days to form crystals. The crystals were filtered and dried, and then weighed and analyzed.
Analysis using the FTIR spectrometer
The analysis using FTIR functions was done to determine the functional groups of a complex compound. The required ingredients are KBr as pellet add mixture and the Co(II), Cu(II), and Mn(II) complex compounds. A 1 mg sample was taken and mixed with KBr of 9 mg, and then placed in a press holder and pressed to create a very thin pellet. The resulted pellet is inserted into a compartment and analyzed in the infrared spectrum.
Analysis using the Atomic Absorption Spectrometer (AAS)
This analysis was done to find out the metal contents of Co(II), Cu(II), and Mn(II) in the formed complex compound. A standard metal solution was made at the concentration of 0, 2, 4, 6, 8 and 10 ppm, ready to be measured. A sample solution of the complex compounds of Co(II), Cu(II), and Mn(II) was created to 100 ppm. 0.005 grams of a complex was dissolved in 2 mL of concentrated HNO3, and then placed in a 50 mL flask which is topped up to the mark with distilled water. The sample mixture was then diluted to 80 ppm; 20 mL of the sample solution at 100 ppm concentrate is placed in a 25 mL flask, to which is added 1 mL HNO3 and distilled water to the mark. After both solutions are prepared, the spectrometry test is conducted.
Analysis of C, H, and N microelements
The equipment used for the analysis of microelements C, H, and N is first standardized using a L-Cysteine Standard (C5H12N2O4S2, C=29.99%, H=5.03%, N=11.66%, S=26.69%, and O=26.63%). 10 mg of the sample is placed in the aluminum foil, and then inserted into a punctured plate. Combustion was carried out using oxygen gas. Microelement analysis is performed, and the composition of C, H, and N contained in the sample is read out on a monitor.
Toxicity test (Brine Shrimp Lethality Test (BSLT))
Toxicity tests are performed to find out the value of LC50 (Lethal Concentration 50%, the concentration that causes 50% of deaths to test animals) from the complexes resulted from the synthesis. Test solutions created with concentration of 62.5 μg/mL, 125 μg/mL, 250 μg/mL, 500 μg/mL, 1000 μg/mL, and 2000 μg/mL are each taken to a volume of 0.15 μL and added to different tubes of 3 μL volumes. Seawater of 0.15 μL containing 10 juvenile shrimps are then added to each tube. The tubes are set aside for 24 hours and the number of juvenile shrimps that die are counted visually. Testing is done three times for each concentration.
Results and Discussion
Synthesis of the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand
The 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand was synthesized with precursors of benzyl, 4-nitrobenzaldehyde, and ammonium acetate. During the synthesis the mixture was exposed to channeled nitrogen gas so that the compound does not react with O2. The synthesis was performed at temperatures of 80-100°C so that the compound molecules can make maximal contact because the temperatures are optimal for the reaction [6]. The 4-nitrobenzaldehyde which was dissolved with glacial acetic acid is added to the reaction after the reaction has lasted for 1 hour. The reaction was continued for 4 hours and monitored by a TLC using an n- hexanes:ethylacetate=3:1 solution. The reaction was stopped when a single stain had been obtained on the TLC. After the reaction completed, the reaction mixture was put into an ice bath and left until all the ice had melted. The obtained yellow precipitate was the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand. The precipitate was filtered and rinsed with cold distilled water. The dry yellow precipitate was recrystallized using ethyl acetate. The ligand was monitored using three eluent TLC and continued with 2D TLC to ensure the ligand purity. The result of compound melting point test was 149°C. The yield of the 2(4-nitrophenyl)-4.5-diphenyl-1Himidazole ligand was found to be 77.22%.
The FTIR characterization result showed the distinct spectra of the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand; the FTIR spectra can be seen in Figure 1. The peak at around 3485.5 cm-1 on the FTIR spectrum is the stretching vibration of the N-H bond. Other FTIR spectrum parts that show the presence of nitrogen atoms in the ligand is also seen at wave number of 1685.67 cm-1 which shows the C=N bond; the C=N bond is the bond found in the imidazole ring. The peak at around 1108.99 cm-1 also shows the presence of a nitrogen atom on the ligand; the spectrum shows the presence of the C-N bond. The peak at around 1338.5 cm-1 shows the presence of the NO2 substituent in the phenyl group. Spectrum peaks at 3060.82 and 3031.89 show the presence of C-H sp2 bonds which are the bonds of the aromatic ring. The presence of aromatic ring bonds is also supported by the FTIR spectrum in the peaks at 1600.81cm-1 and 1487.01 cm-1 which shows the presence of the C=C bond. The peak at the wavenumber region of 765.69 cm-1 proves that there is a substituted benzene at the para position [6]. The peaks of the FTIR spectrum further confirm the suggested structure of the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand. Single-crystal XRD was used to ensure the ligand had been formed. The results of the single-crystal XRD can be seen in Figure 2.
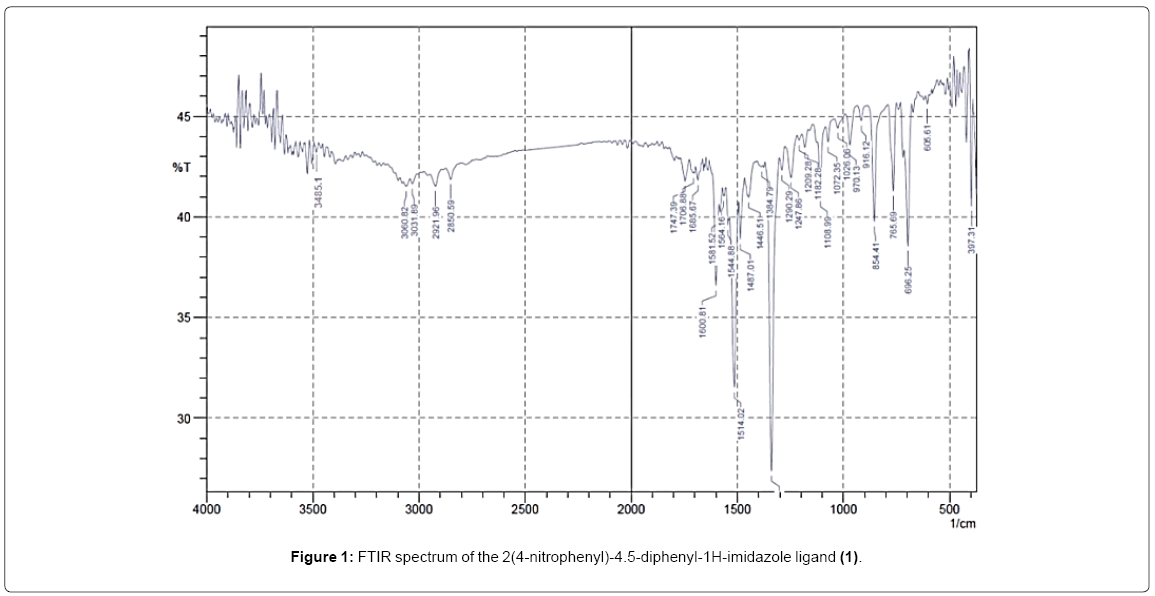
Figure 1: FTIR spectrum of the 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand (1).
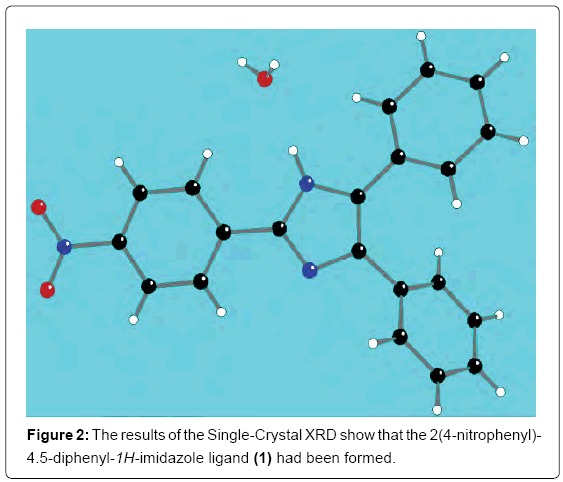
Figure 2: The results of the Single-Crystal XRD show that the 2(4-nitrophenyl)- 4.5-diphenyl-1H-imidazole ligand (1) had been formed.
Synthesis of the copper (II), cobalt (II), and manganese (II) metal complexes with the 2(4-nitrophenyl)-4.5-diphenyl-1Himidazole ligand
The synthesis of Cu(II), Co(II), and Mn(II) complex was done using a reflux system at the temperature of 70-80°C and was stirred for 24 hours. The use of temperature of 70-80°C and the stirring were aimed to quicken and optimize the reaction. The solutions were added to beaker glass and covered with punctured aluminum foil. The punctures on the aluminum foil was to enable ethanol vapor to evaporate to leave behind the crystal complexes of Cu(II), Co(II), and Mn(II). The solution was put into a desiccator and set aside for 7 days for the crystals to grow. The crystals of the formed Cu(II) and Mn(II) complexes were shiny orange in color. The formed Co(II) complex is colored yellow.
Analysis of the formation of the complexes with a UV-Vis spectrometer
Characterization with a UV-Vis spectrophotometer was to find out the maximum wavelength shift (λmax) on the metal compared to the formed complex compound. The complex compound is stated to have been formed if the maximum wavelength of the complex compounds is different from its metal ion. The measurements of the the maximum wavelength was done by dissolving the metal ion source and complex in acetone with the same concentrations. The MnCl2.4H2O solution was light pink in color while the solution of Mn(II)-2(4-nitrophenyl)- 4.5-diphenyl-1H-imidazole complex solution was orange. The CuCl2.2H2O solution was light green in color while the solution of Cu(II)-2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole complex solution was orange. The CoCl2.6H2O solution was dark blue in color while the solution of Co(II)-2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole complex was orange, than the maximaum wavelangeth was measured at the wavelength area of 200-800 nm. The maximum measured wavelength can be seen in Table 1.
| Compound |
Wavelength (nm) |
| MnCl2.4H2O |
286 |
| Mn(II)-2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole |
378 |
| CuCl2.2H2O |
346 |
| Cu(II)-2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole |
390 |
| CoCl2.6H2O |
673 |
| Co(II)-2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole |
389 |
Table 1: Results of the Maximum Wavelength Measurement of the Metal Ion Sources and Complexes.
Based on the analysis results using the UV-Vis spectrophotometer it was found that the maximum wavelength for MnCl2.4H2O was 286 nm, while the maximum wavelength for the Mn(II)-2(4-nitrophenyl)- 4.5-diphenyl--1H-imidazole complex was 378 nm." This proves that the Mn(II) complex had been formed because a wavelength shift had occurred from the ligand to the metal. The maximum wavelength of the complex was read at 378 nm because the color absorbed by the UV-Vis is violet, which is the complement of orange-yellow [9].
The maximum wavelength for the metal ion source CuCl2.2H2O was 346 nm and that of Cu(II)-2(4-nitrophenyl)-4.5-diphenyl-1Himidazole complex was 390 nm (Table 1). The Cu(DNPI)2 complex compound was measured using the UV-Vis with a DMF solution and appeared at wavelength 410 nm [5]. The complex color reflected was yellow and the color absorbed was purple which was at the wavelength of 340-450 nm.
The CoCl2âˆÂââ€Å¾Â¢6H2O compound had a maximum wavelength in the region of 673 nm while the Co(II)-2(4-nitrophenyl)-4.5-diphenyl-1Himidazole complex compound had a maximum wavelength of 389 nm. The color absorbed in the UV spectrum was the complementary color of the complex compound, i.e., violet, which had the wavelength range within 400-480 nm [10].
Analysis of CHN microelements
The analysis using the CHN analyzer was aimed at finding out the relative compositions of carbon, hydrogen, and nitrogen atoms from the complex compound crystals of Mn(II)-2(4-nitrophenyl)-4.5- diphenyl-1H-imidazole. The obtained relative composition percentages were then compared to theoretically calculated percentages to find out the molecular formula that is most appropriate for the formed complex compounds.
The molecular formula for [Mn(2(4-nitrophenyl)-4.5-diphenyl- 1H-imidazole)3] showed that the metal:ligand ratio resulted from the synthesis is 1:3; this did not correspond to the ratio during the synthesis of the complex compound which used a metal-ligand ratio of 1:2. The difference between metal:ligand ratio used in complex synthesis and the resulted complex was caused by the ionic radii of Mn(II) ion which is having a diameter of 97 pm and it could achieve d2sp3 hybridization, allowing it to bind 3 ligands yielding octahedral geometry [11].
The molecular formula for [Cu(2(4-nitrophenyl)-4.5-diphenyl- 1H-imidazole)3] showed that the metal:ligand ratio resulted from the synthesis was 1:2; this is similar with the ratio during the synthesis of the complex compound.
The molecular formula for [Co(2(4-nitrophenyl)-4.5-diphenyl- 1H-imidazole)2] showed that the metal:ligand ratio resulted from the synthesis was 1:3. This did not correspond to the ratio during the synthesis of the complex compound which used a metal:ligand ratio of 1:2. The difference of the metal:ligand ratio of the complex compound from the result of the synthesis was caused by the metal ion Co(II) also possess ionic radii 89 pm, which is similar to Mn(II). This radii makes this ion to achieve d2sp3 hybridization, allowing it to bind 3 ligands yielding octahedral geometry [11].
Analysis of Co (II), Cu (II) and Mn (II) metal ion contents with Atomic Absorption Spectrometry (AAS)
The Co(II), Cu(II), and Mn(II) metal ion contents obtained from the analysis results with atomic absorption spectrometry (AAS) was adjusted to the molecular formula obtained from the analysis results of the CHN analyzer. The result of the AAS analysis showed that the concentration of the Mn(II) metal ion found in the sample was 0.198 ppm with an absorbance of 0.0125. The obtained result showed that the closest and most appropriate molecular formula was [Mn(2(4- nitrophenyl)-4.5-diphenyl-1H-imidazole)3] with a Mn(II) metal ion content of 5.10%.
The result of the atomic absorption spectrometry (AAS) analysis showed that the concentration of the Cu(II) metal ion found in the sample is 0.7067 ppm with an absorbance of 0,0212 Å. The obtained result showed that the closest and most appropriate molecular formula was [Cu(2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole)2] with a Cu(II) metal ion content of 8.13%. The result of the AAS analysis for the compound Co(II)-2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole showed that the concentration of the Co(II) metal ion was 0.4816 ppm with an absorbance of 0.0070 Å. The obtained result showed that the closest and most appropriate molecular formula was [Co(2(4- nitrophenyl)-4.5-diphenyl-1H-imidazole)3] with a Co(II) metal ion content of 5.45%.
Analysis of functional groups and bonds with a FTIR spectrophotometer
Characterization using infrared (FTIR) spectrophotometry served to find out the functional groups and kinds of bonds contained in a complex compound in order to be able to predict the bond structure of the complex compound. Characterization using FTIR was done on wavenumber 4000-350 cm-1. This range of numbers would show the distinct spectra of complex compounds, in particular on fingerprint areas that become the distinct character of a complex so that it could be predicted whether a complex compound has been formed or not. FTIR spectra of complex compounds (Figure 3).
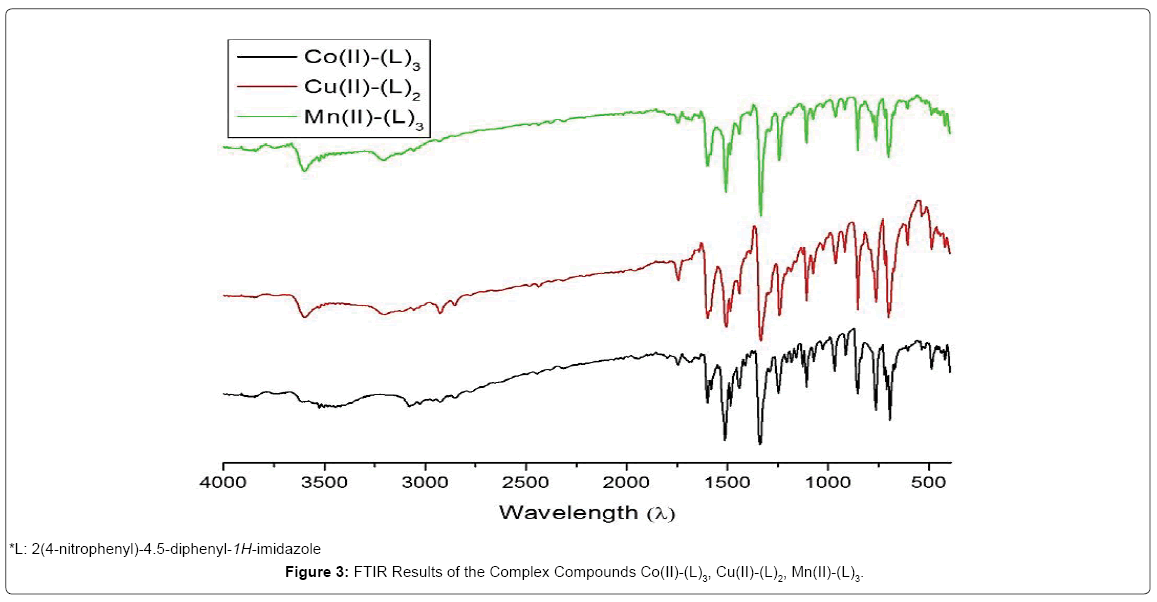
Figure 3: FTIR Results of the Complex Compounds Co(II)-(L)3, Cu(II)-(L)2, Mn(II)-(L)3.
On the infrared spectra, the peak at the 3602.78-3598.92 cm-1 region was the peak of the N-H bond. The peak at the 3078,18-3055,03 cm-1 region showed the C-H sp2 bond; the presence of an aromatic group was also confirmed by a peak at the regions of 1600.81 cm-1 and 1485.09 cm-1 which is the stretching vibration of the C=C bond. The existence of a nitrogen atom was shown by the peak in the region of 1677.95 cm-1 which is the C=N bond in the imidazole ring. The peak at the 1107.06 cm-1 region was the peak of the C-N bond. The peak at the 1334.65 cm-1 region is the peak of the NO2 substituent on the phenyl group. The peak at the 763.76 cm-1 region showed that there was substituted benzene at the para position [6]. The distinct peak of the complex compound was shown by the bond between metal and ligand. On the complex compound [Co(2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole)3] the bond between the Co metal and the N atom on imidazole was shown by wavenumber 408.88 cm-1 [12]. Meanwhile the bond between the Co metal and the O atom on nitro was shown by wavenumber 543 cm-1 [13].
The Cu-O bond in the [Cu(2(4-nitrophenyl)-4.5-diphenyl-1Himidazole) 2] complex was shown by wave number 964.34 cm-1 and the Cu-N bond on wavenumber 486.03 cm-1 [5]. In compound [Mn(2(4- nitrophenyl)-4.5-diphenyl-1H-imidazole)3] the region 486.03cm-1 showed the presence of a Mn-O bond. The bond between metal and ligand was also shown by the peak at the 326.06 cm-1 region which was the peak of the Mn-N bond [14].
Toxicity test with the Brine Shrimp Lethality Test (BSLT) method
Toxicity test was done to find out the LC50 value of [Cu(2(4- nitrophenyl)-4.5-diphenyl-1H-imidazole)2], [Mn(2(4-nitrophenyl)- 4.5-diphenyl-1H-imidazole)3] and [Co(2(4-nitrophenyl)-4.5-diphenyl- 1H-imidazole)3] complexes. The LC50 value was related to the biological activity and toxicity of the compounds. Toxicity test is a preliminary test for compounds to be used as anti-cancer agents [15]. The toxicity test in this research used the Brine Shrimp Lethality Test (BSLT) method, which is a toxicity test using the larvae of Artemia salina juvenile shrimp as the test animals.
The testing samples, which were [Cu(2(4-nitrophenyl)-4.5- diphenyl-1H-imidazole)2], [Mn(2(4-nitrophenyl)-4.5-diphenyl-1Himidazole) 3] and [Co(2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole)3] complexes were first dissolved with ethanol because the complexes dissolve perfectly in ethanol, and then distilled water was added to a concentration of 1000 ppm. The addition of distilled water was intended to make the shrimp larvae not poisoned by the ethanol solvent. From 1000 ppm, the solutions were thinned with distilled water to concentrations of 500, 250, 125, and 62.5 ppm. The test solutions were put into test plates that contained 10 Artemia salina shrimp larvae and were set aside for 24 hours. This test was done with a repetition of three times for each concentration. From the results of the toxicity test with the BSLT method, it was shown that the death percentage had a direct relationship with the test compound concentrations [16]. To obtain the LC50 value, a chart of the relationship was created with the test compound concentration on the x-axis and percentage of deaths as the y-axis. Based on the results of the toxicity test, the LC50 value for the compound [Mn(2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole)2] was found to be 182.79 ppm. This showed that at a concentration of 182.79 ppm the compound [Mn(2(4-nitrophenyl)-4.5-diphenyl- 1H-imidazole)3] was able to kill 50% of Artemia salina larvae. From the LC50 value, it was known that the complex compound [Mn(2(4- nitrophenyl)-4.5-diphenyl-1H-imidazole)3] was considered a toxic compound because the LC50 values <200 ppm.
In the compound [Cu(2(4-nitrophenyl)-4.5-diphenyl-1Himidazole) 2] the death of 50% of Artemia salina larvae occurred at a concentration of 125-250 ppm. This value was obtained from calculations using a linear regression equation based on the chart, so the obtained LC50 value was 229.66 ppm. The obtained LC50 values was higher than 200 ppm, and thus the complex of Cu(II) with 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole ligand was not toxic. Toxicity test results for the compound [Co(2(4-nitrophenyl)-4.5- diphenyl-1H-imidazole)3] showed an LC50 value of 265.79 ppm. This showed that at a concentration of 265.79 ppm the compound [Co(2(4- nitrophenyl)-4.5-diphenyl-1H-imidazole)3] was able to kill 50% of Artemia salina larvae. The compound [Co(2(4-nitrophenyl)-4.5- diphenyl-1H-imidazole)3] was a non-toxic compound because the LC50 value was >200 ppm [17].
Conclusion
The ligand 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole (1) has been successfully synthesized with a yield of 77.22%. This is confirmed by the results of FTIR and 1H NMR characterization that proves that the compound 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole (1) has been formed. Copper(II), cobalt(II) and manganese(II) complexes and 2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole (1) ligand have been successfully synthesized. The crystals of the resulting complexes were dark orange and yellow. The yields were 74.5% for the manganese(II) complex and 77.14% for the copper(II) complex. Based on the characterization of spectrometry, CHN analyzer, and FTIR, a prediction of the molecular formula for the formed complex compounds were obtained as [Cu(2(4-nitrophenyl)-4.5-diphenyl-1H imidazole)2], [Mn(2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole)3] and [Co(2(4-nitrophenyl)-4.5-diphenyl-1H-imidazole)3] complexes. Results of the biological activity test for [Mn(2(4-nitrophenyl)-4,5- diphenyl-1H imidazole)3], [Cu(2(4-nitrophenyl)-4,5-diphenyl-1Himidazole) 2], and [Co(2(4-nitrophenyl)-4,5-diphenyl-1H-imidazole)3] complexes showed the LC50 values of 182.79 ppm, 229,66 ppm, and 265.79 ppm. Based on the LC50 value, it was known that the [Mn(2(4- nitrophenyl)-4.5-diphenyl-1H-imidazole)3] complex compound was toxic. [Cu(2(4-nitrophenyl)-4,5-diphenyl-1H-imidazole)2] and [Co(2(4-nitrophenyl)-4,5-diphenyl-1H-imidazole)3] complexes were non-toxic.
Acknowledgments
Authors greatly acknowledge to Universiti Teknologi MARA, Malaysia for helping the characterization with the CHN analyzer, the Laboratory of Natural Product and Synthesis, Department of Chemistry Institute Teknologi Sepuluh Nopember for technical support.
References
- Clayden J, Greeves N, Warren S, Wothers P (2005)Organic Chemistry. 2ndedn. Oxford Press.
- Sudibyo RS (2012) Metabolit Sekunder: Manfaat dan Perkembangannya dalam Duia Farmasi. Universitas Gadjah Mada.
- Khabnadideh S, Rezaei Z, Khalafi-Nezhad A, Bahrinajafi R, Mohamadi R, et al. (2003) Synthesis of N-alkylated derivatives of imidazole as antibacterial agents. Bioorganic & Medicinal Chemistry Letters 13: 2863-2865.
- Ikram M, Rehman S, Faiz A (2010) Synthesis, characterization and antimicrobial studies of transition metal complexes of imidazole derivative. Bulletin of the Chemical Society of Ethiopia, p: 24.
- Kou S, Tang G, Tang T, Zhang Y, Song Y (2014) Synthesis, characterization and theoretical investigation of the structure, electronic properties and third-order optical nonlinearity of M (dnpi) 2 (M= Cu2+, Co2+ and Pb2+; dnpi= 4, 5-Diphenyl-2-(4-nitrophenyl)-1H-imidazole). Dyes and Pigments 104: 102-109.
- Jain AK, Ravichandran V, Sisodiya M, Agrawal RK (2010) Synthesis and antibacterial evaluation of 2–substituted–4, 5–diphenyl–N–alkyl imidazole derivatives. Asian Pacific Journal of Tropical Medicine 3: 471-474.
- Sandoval HL, Lemos ME, Velasco RG, Melendez IP, Marcias PG, et al. (2008)Synthesis and Antibacterial Evaluation of 2-substituted-4,5-diphenyl-N-alkyl Imidazole Derivatives. Journal of Inorganic Biochemistry 102: 1267-1276.
- Bouchoucha A, Terbouche A, Bourouina A, Djebbar S (2014) New complexes of manganese (II), nickel (II) and copper (II) with derived benzoxazole ligands: Synthesis, characterization, DFT, antimicrobial activity, acute and subacute toxicity. InorganicaChimica Acta 418: 187-197.
- Boiani L, Gerpe A, Arán VJ, de Ortiz ST, Serna E, et al. (2009) In vitro and in vivo antitrypanosomatid activity of 5-nitroindazoles. European Journal of Medicinal Chemistry 44: 1034-1040.
- Underwood RD (2002)Analisis Kimia KuantitatifEdisiKeenam. Erlangga, Jakarta.
- Kimia CR, Jilid D (2003)Erlangga. 3rd edn. Jakarta.
- Nakamoto K (1997)Infrared and Raman Spectra of Inorganic and Coordination Compounds. John Wiley & Sons Inc.
- Kalanithi M, Rajarajan M, Tharmaraj P, Sheela CD (2012) Spectral, biological screening of metal chelates of chalcone based Schiff bases of N-(3-aminopropyl) imidazole. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 87: 155-162.
- Martak F, Onggo D, Nugroho A (2014)Synthesis and Characterization of a Bimetallic Oxalate Based Magnet [(C4H9)4P][M(II)Cr(ox)3] M = Fe, Co, Ni, Cu. IndoJ Chem 14: 311-314.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DJ, et al. (1982) Brine shrimp: a convenient general bioassay for active plant constituents. Planta Medica 45: 31-34.
- Harmita MR,Buku A(2006)Analisis Hayati.Jakarta: BukuKedokteran EGC.
- Martak F, Utomo WP, Nugraheni ZV, Budi P (2016) Synthesis and Toxicity Test of M/Zn (II) Complexes [M= Mn (II), Co (II)] with Pyridine-2, 6-dicarboxylic Acid Ligand. Asian Journal of Chemistry 28: 1773.
22322
References
- Clayden J, Greeves N, Warren S, Wothers P (2005)Organic Chemistry. 2ndedn. Oxford Press.
- Sudibyo RS (2012) Metabolit Sekunder: Manfaat dan Perkembangannya dalam Duia Farmasi. Universitas Gadjah Mada.
- Khabnadideh S, Rezaei Z, Khalafi-Nezhad A, Bahrinajafi R, Mohamadi R, et al. (2003) Synthesis of N-alkylated derivatives of imidazole as antibacterial agents. Bioorganic & Medicinal Chemistry Letters 13: 2863-2865.
- Ikram M, Rehman S, Faiz A (2010) Synthesis, characterization and antimicrobial studies of transition metal complexes of imidazole derivative. Bulletin of the Chemical Society of Ethiopia, p: 24.
- Kou S, Tang G, Tang T, Zhang Y, Song Y (2014) Synthesis, characterization and theoretical investigation of the structure, electronic properties and third-order optical nonlinearity of M (dnpi) 2 (M= Cu2+, Co2+ and Pb2+; dnpi= 4, 5-Diphenyl-2-(4-nitrophenyl)-1H-imidazole). Dyes and Pigments 104: 102-109.
- Jain AK, Ravichandran V, Sisodiya M, Agrawal RK (2010) Synthesis and antibacterial evaluation of 2–substituted–4, 5–diphenyl–N–alkyl imidazole derivatives. Asian Pacific Journal of Tropical Medicine 3: 471-474.
- Sandoval HL, Lemos ME, Velasco RG, Melendez IP, Marcias PG, et al. (2008)Synthesis and Antibacterial Evaluation of 2-substituted-4,5-diphenyl-N-alkyl Imidazole Derivatives. Journal of Inorganic Biochemistry 102: 1267-1276.
- Bouchoucha A, Terbouche A, Bourouina A, Djebbar S (2014) New complexes of manganese (II), nickel (II) and copper (II) with derived benzoxazole ligands: Synthesis, characterization, DFT, antimicrobial activity, acute and subacute toxicity. InorganicaChimica Acta 418: 187-197.
- Boiani L, Gerpe A, Arán VJ, de Ortiz ST, Serna E, et al. (2009) In vitro and in vivo antitrypanosomatid activity of 5-nitroindazoles. European Journal of Medicinal Chemistry 44: 1034-1040.
- Underwood RD (2002)Analisis Kimia KuantitatifEdisiKeenam. Erlangga, Jakarta.
- Nakamoto K (1997)Infrared and Raman Spectra of Inorganic and Coordination Compounds. John Wiley & Sons Inc.
- Kalanithi M, Rajarajan M, Tharmaraj P, Sheela CD (2012) Spectral, biological screening of metal chelates of chalcone based Schiff bases of N-(3-aminopropyl) imidazole. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 87: 155-162.
- Martak F, Onggo D, Nugroho A (2014)Synthesis and Characterization of a Bimetallic Oxalate Based Magnet [(C4H9)4P][M(II)Cr(ox)3] M = Fe, Co, Ni, Cu. IndoJ Chem 14: 311-314.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DJ, et al. (1982) Brine shrimp: a convenient general bioassay for active plant constituents. Planta Medica 45: 31-34.
- Martak F, Utomo WP, Nugraheni ZV, Budi P (2016) Synthesis and Toxicity Test of M/Zn (II) Complexes [M= Mn (II), Co (II)] with Pyridine-2, 6-dicarboxylic Acid Ligand. Asian Journal of Chemistry 28: 1773.









