Short Communication
Anti-ageing reagents: Sulfo disaccharides co-work with klotho was prepared by organic synthesis. By comparing of these compounds by easiness of getting starting compounds, reaction steps, easiness of reactions, easiness of purification, activities, easiness of handling and solubility. p-methoyphenyl-3-sulfp-β-Dglucuronosyl-( 1-4)-2-acetoamino-3-deoxy-glucpyranoside 9294 is selected as best compound for research on anti-ageing.
Nabeshima found Klotho (anti-ageing gene) [1]. Since then many reports on klotho [2-29] are published.
Nabeshima found a sulfo disaccharide from mouse liver cell. This saccharide bind with Klotho I synthesized five sulfo disaccharide [30]. At the second paper [31], I have proposed the hypothesis that klotho make disaccharide from glucuronic acid and glucosamine on site and klotho (anti-ageing gene) co-work with his produced disaccharide (anti-ageing reagent) and provide Ca homeostasis and consequent anti-ageing and long life. This proposed mechanism must be proved by many others persons. I described synthetic procedure in detail for 9279, 9274, 9188 at first paper [30]. The synthesis of these sulfo disaccharide are not easy. I wish to describe synthetic method of 9294, most easily obtainable anti-ageing reagent at this paper.
Anti-ageing reagent: Sulfo disaccharide
To get proof of the hypothesis, we must get easily accessible disaccharide for the research to know how klotho and disaccharide are working. At this paper the easiness of synthesis and easiness of handling of 5 disaccharides were compared. It was found that p-Methoxyphenyl-3-sulfo-β-D-glucuronosyl-(1-4)-2-acetoamino- 2-deoxy-glucpyranoside 9294 was as a best compound for this purpose.
We can consider four possible structure A,B,C and D As an example of compounds C, 3-estrone3-sulfo -glucuronosyl (1-3)-2-acetoamino-2-deoxy-β-D-glucopyranoside 9279 were prepared. As an example of copound D Esteron-3-sulfoglucuronosyl) (1-4)-2-acetoamino-2-deoxy-glucopyranoside 9214, p-methoxyphenyl 3-sulfo-glucuronososyl (1-4)-2-acetoamino- 2-deoxy-glucopyranoside 9294 As an example of compound A, p-methoxtphenyl-3-sulfo-glucuronyl(1-4) 2-acetoamino-2- deoxy-galactopyranoside 9583 was prepared. The synthesis of p-methoyphenyl-3-sulfo-glucuronosyl (1-4) -2-acetoamino-2- deoxy-glucpyranoside 9294 was carried out as shown in Figure 1.
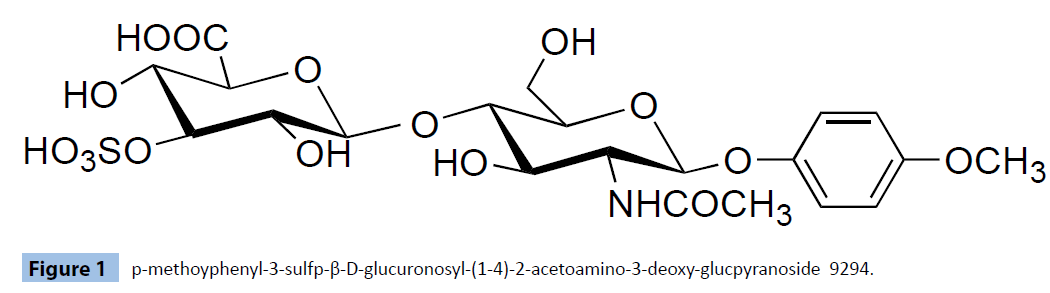
Figure 1: p-methoyphenyl-3-sulfp-β-D-glucuronosyl-(1-4)-2-acetoamino-3-deoxy-glucpyranoside 9294.
By the reaction of p-methoyphenyl-3,6-di-O-benzyl-2- acetoamino-2-deoxy-glucopyranoside with 1-bromo-2,3,4-tri- O-acetyl-D-glucuronate, (1-4) bonded glucoside was obtained. 3,6-di-Benzyl are cleaved by hydrogen reduction. Methyl group and acetoxy groups are cleaved by LiOH hydrolysis. Sulphonation by SO3-TEt3 gave desired p-methoyphenyl-3-sulfo – glucuronosyl- (1-4)-2-acetoamino-2-deoxy-glucpyranoside 9294.
Structure activity relationship
The binding activities of synthesized compounds, 9214, 9188, 9583, 9279, 9244 were measured. The activities of these 5 compounds were almost same irrespective of (1-4) or (1-3) bond, glucopyranoside or galactopyranoside, p-methoxyphenyl, or esteron, or serine.
Comparison of preparation steps
Estrone-3-sulfo-glucuronosyl (1-3)-(2-acetoamino-2-deoxyglucopyranoside) 9279 is prepared by 9 steps as shown at Figure 1 [30].
Serine-3-sulfo-glucuronosyl)-(1-3)-2-acetoamino-2-deoxy glucopyranoside 9244 is prepared by 9 steps as shown at Figure 2 [30].
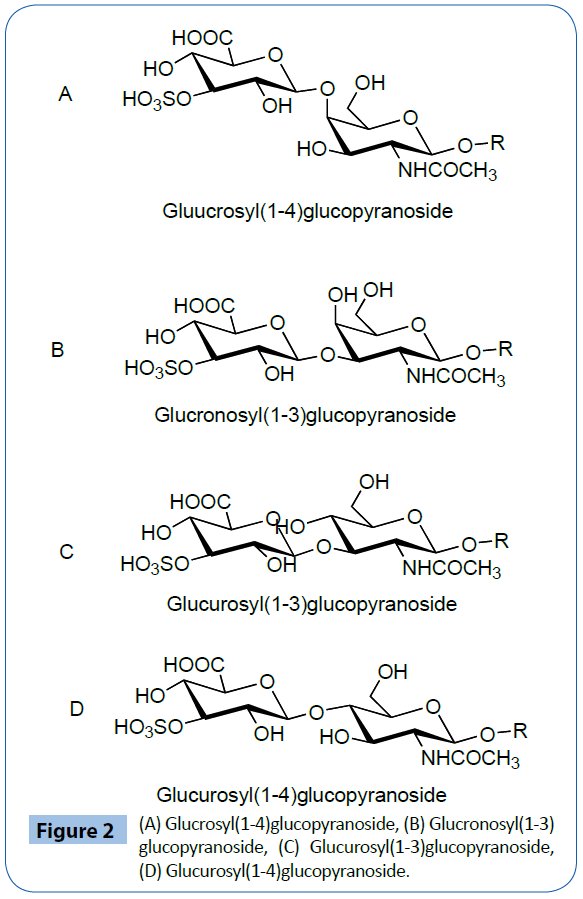
Figure 2: (A) Glucrosyl(1-4)glucopyranoside, (B) Glucronosyl(1-3) glucopyranoside, (C) Glucurosyl(1-3)glucopyranoside, (D) Glucurosyl(1-4)glucopyranoside.
3-Estrone 3-sulfo-glucronosyl)-(1-4)-2-acetoamino-2-deoxyglucopyranoside 9188 is prepared by 8 steps as shown at Figure 3 [30].
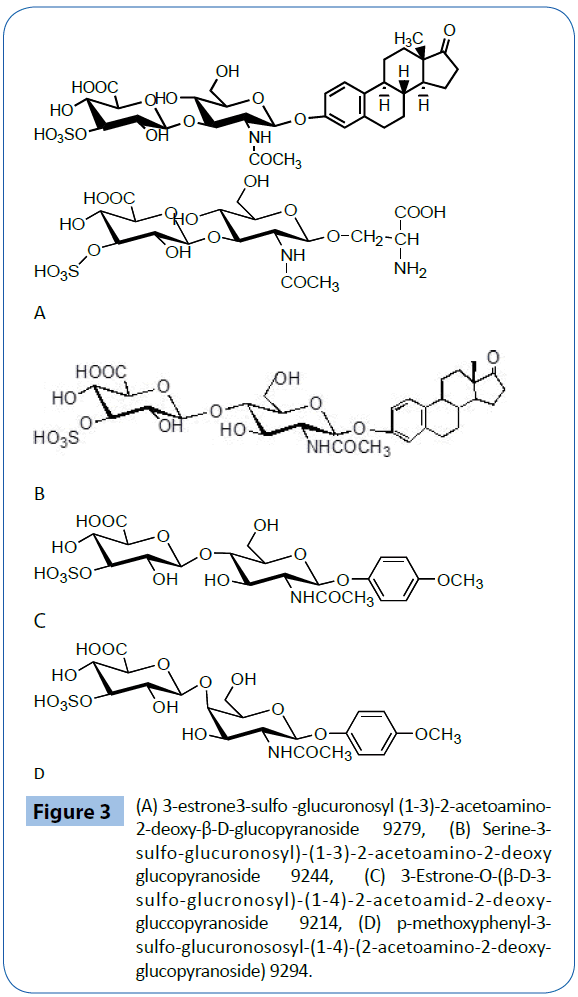
Figure 3: (A) 3-estrone3-sulfo -glucuronosyl (1-3)-2-acetoamino- 2-deoxy-β-D-glucopyranoside 9279, (B) Serine-3- sulfo-glucuronosyl)-(1-3)-2-acetoamino-2-deoxy glucopyranoside 9244, (C) 3-Estrone-O-(β-D-3- sulfo-glucronosyl)-(1-4)-2-acetoamid-2-deoxygluccopyranoside 9214, (D) p-methoxyphenyl-3- sulfo-glucuronososyl-(1-4)-(2-acetoamino-2-deoxyglucopyranoside) 9294.
Gln-His-Thr-3-sulfo-glucuronosyi-(1-3)-2-acetoamino-2-deoxyglucopyranoside 9205 is prepared by 9 steps as shown at Figure 4 [1]. p-Methoyphenyl-3-sulfo-glucuronosyl-(1-4) -2-acetoaminoglucpyranoside 9294 is prepared by only 4 steps as shown at Figure 1 of this paper.
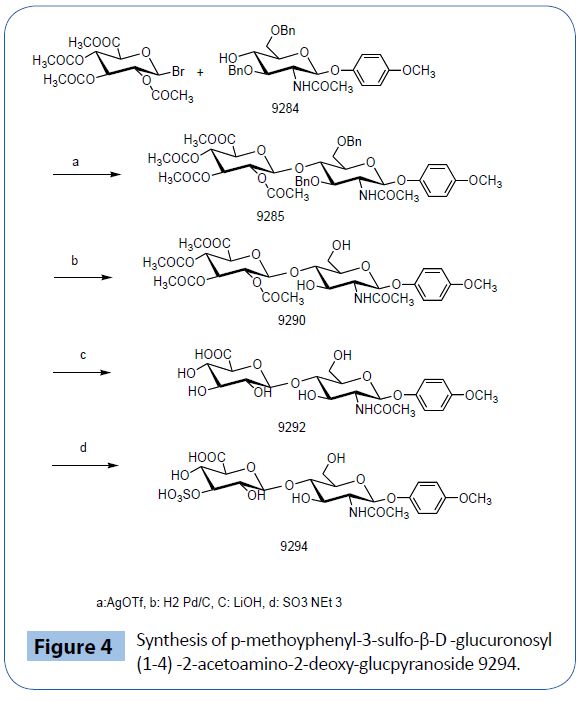
Figure 4: Synthesis of p-methoyphenyl-3-sulfo-β-D -glucuronosyl (1-4) -2-acetoamino-2-deoxy-glucpyranoside 9294.
Comparison of properties of disaccharides
p-Methoxyphenyl-3-sulfo-glucuronosyl (1-4)-2-acetoamino-2- deoxyy-glucpyranoside 9294 is soluble in organic solvent and litium or sodium salt is soluble in water and easy to purify by chromatography.
Estrone-3-sulfo-glucuronososyl-(1-3)-(2-acetoamino-2- deoxy-glucopyranoside) 9279 and 3-Estrone-O-(β-D-3-sulfoglucronosyl)-( 1-4)-2-acetoamid-2-deoxy-gluccopyranoside 9214 are insoluble in chloroform and water. And purification by chromatograph is very difficult.
Conclusion
Considering these facts, p-methoyphenyl-3-sulfo-β-Dglucuronosyl-( 1-4)-2-acetoamino-3-deoxy-glucpyranoside 9294 looks like a best compounds. But this is only best in 5 compounds. By expanding the kind of sugar, by changing or at glucosamine 1-position, discovery of much better anti-ageing reagents will be discovered.
Experimental part
Synthesis of p-methoxyphenyl-3-sulfo-glucuronososyl-(1-4)-(2- acetoamino-2-deoxy-glucopyranoside) 9294 [33-38]
(1) p-Methoxyphenyl-O-2,3,4-tri-O-acetyl methyl glucuronosyl)-(1-4)-2-acetoamino-2-deoxy-3,6-di- O-benzyl-glucopyranoside 9285 p-Methoxyphenyl- 2 - a c e t o a m i d o - 3 , 6 - d i - O - b e n z y l - 2 - d e o x y - β - D - glucopyranoside (obtained by the acetylation of p-methoxyphenyl-2-amino-3,6-di-O-benzyl-2-deoxy- β-D-glucopyranoside(Tokyo Kasei M1616)) 420 mg (0.828 mmole) was dissolved in CH2Cl2 6 ml and, silvertriflate 348 mg(1.65 mmole), Molecular sieve 3A 0.7g under dry nitrogen atmosphere cooled at -40°C? A solution of 1-bromo-2,3,4-tri-O-acetyl-D-glucuronate [38] 460 mg (1.28 mmole) and collidine 138 mg in 5 ml dichloromethane was added drop wise to the cooled solution. After 2 hours, warmed to room temperature over night with stirring. After addition of dichlomethane 10 ml, molecular sieve was filtered. Washing of the filtrate with 3% hydrochloric acid, saturated sodium bicarbonate, and water. The solution was dried and evaporated. The product was purified on silica gel with ethyl acetate and hexane 1:1.9285 647 mg (95%) was obtained.
(2) p-Methoyphenyl-(β-D-2,3,4-triacetyl-glucuronosyl)-(1-3)- 2-acetoamino-2-deoxy-glucpyranoside 9290, 9285 100.9 mg 0.122 mmol Pd/C 53 mg is dissolved in CH3OH. 5 ml stiirred under H2 at room temperature overnight. Pd/C is filtered and the reaction mixture is evaporate to get 9290 50.6 mg(64.5%).
(3) p-Methoyphenyl-β-D -glucuronosyl)-(1-4)-2-acetoamino- 2-deoxy-glucpyranoside 9292.
9290, 9.9 mg(0.0148 mmol) is dissolved in LiOH solution (LiOH 1.75 M aqueous solution 16.5 μl and THF 100 μl. After 5 minutes, the reaction mixture is evaporated under vacuum to get residue 10 mg of 9292.
Mass analysis showed 503.197. Theoretical exact mass is 503.1639.
(4) 3-Sulfo p-methoyphenyl-β-D-glucuronosyl)-(1-4)-2- acetoamino-2-deoxy-glucpyranoside 9294.
The solution of 9292, 5 mg (0.00856 mmol) and SO3-NEt3 4.65 mg [39] in dimethylformamide 50 μl are stirred for 2 hours and evaporated under vacuum. This process was repeated 3 times to get 6 mg 9294.
9294 activated the binding of klotho and FGF 23.
7721
References
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, et al. (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing.Nature 390: 45-51.
- Matsumura Y,Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, et al. (1998) Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein.BiochemBiophys Res Commun 242: 626-630.
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, et al. (2005) Suppression of aging in mice by the hormone Klotho.Science 309: 1829-1833.
- Arking DE,Krebsova A, Macek M Sr, Macek M Jr, Arking A, et al. (2002) Association of human aging with a functional variant of klotho.Proc Natl Acad Sci U S A 99: 856-861.
- Xiao NM, Zhang YM, Zheng Q, Gu J (2004) Klotho is a serum factor related to human aging.Chin Med J (Engl) 117: 742-747.
- Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M Dena B, et al. (2014) Life Extension Factor Klotho Enhances Cognition.Cell Reports 7: 1065-1076.
- Huang CL(2010) Regulation of ion channels by secreted Klotho: mechanisms and implications.Kidney Int 77: 855-860.
- MediciD, Razzaque MS, DelucaS, RectorTL, HouB, et al. (2008)FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. The Journal of Cell Biology 182: 459-465.
- Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y (2003)Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol 17: 2393-403.
- Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, et al.(2007)Alpha-Klotho as a regulator of calcium homeostasis. Science 316: 1615-1618.
- Fukumoto S(2009) [Chronic kidney disease (CKD) and bone. Regulation of calcium and phosphate metabolism by FGF23/Klotho].Clin Calcium 19: 523-528.
- Nabeshima Y(2000) Challenge of overcoming aging-related disorders.J DermatolSci 24 Suppl 1: S15-21.
- Kurosu H,Kuro-o M (2008) The Klotho gene family and the endocrine fibroblast growth factors.CurrOpinNephrolHypertens 17: 368-372.
- Tomiyama K, Maeda R, Urakawa I, Yamazaki Y, Tanaka T, et al. (2010) Relevant use of Klotho in FGF19 subfamily signaling system in vivo.Proc Natl Acad Sci U S A 107: 1666-1671.
- Tohyama O, Imura A, Iwano A,Jean-Noel Freund, Henrissat R, et al. (2004) Klotho is a novelß-glucuronidases capable of hydrolyzing steroid-ß-glucuronides. J.Biological Chemistry 279: 9777-9764.
- Shimoyama Y,Taki K, Mitsuda Y, Tsuruta Y, Hamajima N, et al. (2009) KLOTHO gene polymorphisms G-395A and C1818T are associated with low-density lipoprotein cholesterol and uric acid in Japanese hemodialysis patients.Am J Nephrol 30: 383-388.
- Choi BH, Kim CG, Lim Y, Lee YH, Shin SY (2010) Transcriptional activation of the human Klotho gene by epidermal growth factor in HEK293 cells; role of Egr-1.Gene 450: 121-127.
- Fukumoto S(2009) [Chronic kidney disease (CKD) and bone. Regulation of calcium and phosphate metabolism by FGF23/Klotho].Clin Calcium 19: 523-528.
- Razzaque MS(2009) FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player?Am J Physiol Renal Physiol 296: F470-476.
- Menon R, Pearce B, Velez DR, Merialdi M, Williams SM, et al. (2009) Racial disparity in pathophysiologic pathways of preterm birth based on genetic variants.Reprod Biol Endocrinol 7: 62.
- Prié D,Ureña Torres P, Friedlander G (2009) [Fibroblast Growth Factor 23-Klotho: a new axis of phosphate balance control].Med Sci (Paris) 25: 489-495.
- Torres PU,Prié D, Beck L, De Brauwere D, Leroy C, et al. (2009) Klotho gene, phosphocalcic metabolism, and survival in dialysis.J RenNutr 19: 50-56.
- Halaschek-Wiener J,Amirabbasi-Beik M, Monfared N, Pieczyk M, Sailer C, et al. (2009) Genetic variation in healthy oldest-old.PLoS One 4: e6641.
- Shimoyama Y,Nishio K, Hamajima N, Niwa T (2009) KLOTHO gene polymorphisms G-395A and C1818T are associated with lipid and glucose metabolism, bone mineral density and systolic blood pressure in Japanese healthy subjects.ClinChimActa 406: 134-138.
- Kurosu H,Kuro-o M (2008) The Klotho gene family and the endocrine fibroblast growth factors.CurrOpinNephrolHypertens 17: 368-372.
- Nabeshima Y(2008) [Discovery of alpha-Klotho and FGF23 unveiled new insight into calcium and phosphate homeostasis].Clin Calcium 18: 923-934.
- Chen SN,Cilingiroglu M, Todd J, Lombardi R, Willerson JT, et al. (2009) Candidate genetic analysis of plasma high-density lipoprotein-cholesterol and severity of coronary atherosclerosis.BMC Med Genet 10: 111.
- Nabeshima Y (2007) Molecular function of a-Klotho in calcium homeostasis. Igakunoayumi222: 225-230.
- Ozaki S (2015) Sulfo disaccharides co-working with Klotho. Studies on structure, structure activity relation and function. Wourld J of Pharmacy and Pharmaceutical Sciences 4:152-175.
- Ozaki (2015) Glucosamine Derivatives. Sulfo disaccharides co-working with Klotho. Nutrition and Food Science5: 416.
- Yamazaki F,Kitajima T, Nukada T, Ito Y, Ogawa T (1990) Synthesis of an appropriately protected core glycotetraoside, a key intermediate for the synthesis of "bisected" complex-type glycans of a glycoprotein.Carbohydr Res 201: 15-30.
- Slagshek Ted M, Nakahara Y, Ogawa T, Kamerling JP, VliegenhartJoannnes FG (1994) Carbohydrate Research.
- Guo ZW, Ito Y, Nakahara Y, Ogawa T (1998) Synthetic study on a novel Asn-linked core structure: synthesis of a pentasaccharide alpha-D-Man-(1-->3)-[alpha-D-Man-(1-->6)]-beta-D-Man-(1-->4)- [beta-D-GlcNAc-(1-->60]-beta-D-GlcNAc-->OMp.Carbohydr Res 306: 539-544.
- Wang P, Wang J, Guo T, Li Y (2010) Synthesis and cytotoxic activity of the N-acetylglucosamine-bearing triterpenoidsaponins.Carbohydr Res 345: 607-620.
- Wang ZG, Zhang XF, Ito Y, Nakahara T, Ogawa T(1996) Carbohydrate Research.
- Hasegawa A, Ito K, Ishida R, Kiso M (1995) The use of single-molecule force control operation carried restriction endonuclease EcoRI the quantitative measurement of the rate of the chemical reaction. J Carbohydrate Chem14: 352-368










