Keywords
Traditional Chinese medicine decoction for restoration; Partial hepatectomy; VEGF-A; PROX1; Edema; Regeneration
Introduction
TCM decoction for restoration is composed by the winetreated rhubarb, Bupleurum, Angelica, semen persicae, safflower, pangolins, Radix trichosanthis, and licorice, mainly used for the treatment of traumatic injuries in chest or abdomen, by alleviating hepatic blood stasis, easing swelling and pain, anti-inflammation, and improving microcirculation. The mouse experiment on the pain caused by the hot plate indicated that the TCM decoction could elevate threshold of pain, prolong the writhing latency and reduce the times of writhing, suggesting the analgesic effect of TCM decoction; the analgesic efficacy was demonstrated 30 min after TCM decoction administration, and lasted till 120 min. The dimethyl benzeneinduced auricle swelling test on mice indicated that TCM decoction significantly suppressed auricle swelling and the efficacy at high dose was equivalent to Aspirin. In addition, the abdominal capillary permeability showed that TCM decoction decreased the optical density value of peritoneal lavage fluid, indicating the anti-inflammatory effect of TCM decoction. The modern TCM studies showed that Bupleurum had antiinflammation and anti-exudation effects; rhubarb reduced permeability, fragility and exudation of capillary; and licorice had the cortisone-like effects of anti-inflammation and antiexudation. All of these are the major components of TCM decoction for restoration, which might be the mechanism basis for anti-inflammation of TCM decoction [1]. The residual liver tissue after partial hepatectomy (PH) was manifested as inflammatory edema and regenerative repair in sequential pathological stages, which allowed the PH as an ideal model to test blood circulation promoting, stasis eliminating, swelling and pain relieving effect of TCM decoction [2,3]. The overt congestion and swelling of residual liver tissue was observed at early stage after PH; meanwhile, the expression of vascular endothelial growth factor (VEGF-A) was also up regulated which could significantly increase the permeability of vascular endothelial cells [4]. It was also reported that Prox1, one of the key regulator for liver tissue development and hepatocyte regeneration [5]. could up regulate VEGF-A expression via HIF [6]. It was indicated by animal study that TCM decoction could significantly decrease the auricle swelling induced by xylene, and inhibit the increased permeability of abdominal capillary vessels [1]. However, the key molecules or mechanism of TCM decoction on the swelling and degeneration of post-traumatic liver tissue were unclear yet; and it was speculated by us that the alleviated swelling of liver tissue at early stage after PH by TCM decoction might be associated with the altered expression level of VEGF-A and Prox1. Therefore, in the current study, the pathological feature of residual liver tissue after PH was recorded after TCM decoction administration; meanwhile, the expression levels of VEGF-A which participates the capillary permeability and tissue edema, and hepatocyte regenerative factor Prox1 were detected to investigate the molecular mechanism of TCM decoction on inflammation and edema.
Material and Methods
Traditional Chinese medicine
TCM decoction is composed of Bupleurum (9 g), Radix Trichosanthis (9 g), Angelica (9 g), safflower (6 g), licorice (6 g), pangolins (6 g), wine-treated rhubarb (12 g), and wine-treated Semen Persicae (9 g), which were all purchased from the TCM pharmacy of Affiliated Hospital of Shandong College of Traditional Chinese Medicine. The decoction was prepared by the pharmacy according to the traditional method after herb soaking, decoction, filtering, and concentration. The crude drug had a final concentration of 0.26 g/mL in the decoction, and was vacuum packed and stored in the refrigerator.
Experimental animals
Kunming male and female mice (18 to 26 g) of SPF grade were provided by the animal facility of Shangdong University (Permission No. SCXK(Lu)20090001). The animal utilization license number was SYXK(Lu)20100011. Animals were housed at 21-23 °C with constant humidity and a 12 h light/12 h dark lighting cycle. Mice had free access to food and water. Before the experiments, the mice were randomly apportioned to either a control or liver TCM group. Ten mice in each group. Donkey anti-rabbit IgG fluorescent antibodies were obtained from Antgene (Wuhan, China); rabbit anti-mouse VEGF-A IgG, rabbit anti-mouse RPOX1 IgG, 0.01 M PBS (pH 6.0) and the DAB Immunoassay kit were purchased from Boster Biological Technology (Wuhan, China). The DP-72 microscope was from Olympus (Japan). The animal experiments were performed on the basis of institutional, local and national guidelines on animal research and ethics.
Establishment of a mouse model of PH
The mouse abdomen was opened along the midline after anesthesia with ketamine and xylazine at 50 mg/kg and 40 mg/kg, respectively, with a cut of 3.0 cm, to expose the left hepatic lobe; partial liver tissue resection was performed with an electronic knife of approximately 0.2 g before sequential abdominal muscle and skin suturing. The mice were placed in constant temperature incubators for body temperature recovery until awakening. Treatment group mice were orally administrated TCM decoction at 10 g/kg, twice a day.
Sampling and specimen preparation
Ten mice in each group were sacrificed every other day from postoperative day 1 to 8; the remaining left lobe was removed and fixed with 4% formaldehyde, paraffin embedded, and sliced at 4 μm. Histological morphology was observed after H&E staining, and VEGF-A and PROX1 protein levels were detected by immunohistological staining and double staining.
Immunohistochemistry
Antigen retrieval was carried out in 0.01 mol/L citrate buffer at 121°C for 15 min after dewaxing. The slices were incubated with first antibodies (rabbit anti-mouse VEGF-A or PROX1) at a dilution of 1:100 at 4°C for 12 h, after treatment with 3% hydrogen peroxide to inactive endogenous peroxidase. This was followed by incubation with HRP labeled goat anti-rabbit IgG at 37°C for one hour. Finally, the slices were developed by DAB and photographed under a DP72 microscope. 50 high power fields (averaging 3.6 × 1012 nm2) were imaged by microscopy at 400× from each slice. The micrographs were semi-quantitatively analyzed by Image-Pro Plus 6.0, and averaged signal intensities were statistically analyzed. In the double staining of fluorescent immunohistochemistry, the slices were incubated with rabbit anti-mouse PROX1 (1:100) at 4°C for 24 h after dewaxing and antigen retrieval, and incubated with Cy3 labeled donkey antirabbit IgG; subsequently, the slices were incubated with goat anti-mouse VEGF-A (1:100) 4°C for 24 h, and incubated with FITC labeled donkey anti-rabbit IgG. The slides were analyzed under a DP-72 fluorescent microscope.
Statistical analysis
Microsoft Excel was used for statistical analyses in the current study; t test was used for group comparisons, and P<0.05 was considered statistically significant.
Results
Histological morphology alteration of residual liver tissue after PH, with or without TCM decoction treatment
Compared with its normal structure (Figure 1a), the hepatic lobule was structurally disordered after PH, with large amounts of hepatocytes swelling with vacuoles, accompanied with lightened color; the sinus was filled with red blood cells, with the liver cells completely losing their normal morphology (Figure 1b). However, the liver tissue specimens from the TCM decoction treatment group presented normal hepatic lobule and liver cells with mild swelling and lightened color; the nuclei, cytoplasm, and sinus were all recognizable, and only reduced amounts of red blood cells were found in the sinus. Though liver morphology was not as neat and clear as in the normal tissue, it was far better compared with that of the control group (Figure 1e). Subsequently, along with postoperative recovery, the lobule was gradually recovered with only some hepatocytes remaining swollen and light in color; some hepatocytes had double nuclei indicating they were in a phase of division and proliferation, while hepatic cords and blood sinus were structurally disordered with moderate amounts of red blood cell deposition (Figure 1c). However, in the TCM decoction treatment group, no obviously proliferating hepatocytes were found; hepatic cells and blood sinus were also recognizable, with no obvious blood red cell deposition. Taken together, these findings indicated that liver tissue regeneration was significantly inhibited by TCM decoction (Figure 1f). At the late stage of PH, the hepatic lobule structure gradually recovered despite large amounts of proliferating hepatocytes remaining with lightened color and red blood cell deposition (Figure 1d). Meanwhile, in the TCM decoction group, the hepatic lobule structure returned to normal, with hepatic cells and blood sinus clearly recognized; hepatocytes were stained normally, with no proliferating hepatocytes or cellular volume increased (Figure 1g).
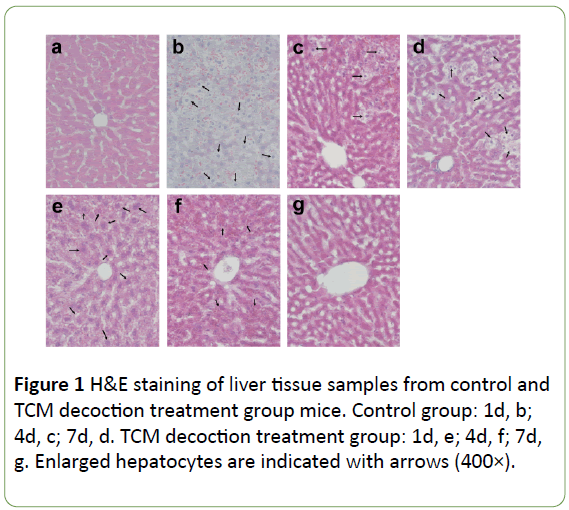
Figure 1: H&E staining of liver tissue samples from control and TCM decoction treatment group mice. Control group: 1d, b; 4d, c; 7d, d. TCM decoction treatment group: 1d, e; 4d, f; 7d, g. Enlarged hepatocytes are indicated with arrows (400×).
VEGF-A protein expression in residual liver tissue after PH with or without TCM decoction treatment
In the control group, VEGF-A expression was widely distributed (and stained dark), mainly around enlarged hepatocytes and the central vessels surrounding the cells (Figures 2a and 2b) at the early stage post-PH; subsequently, it accumulated around the enlarged hepatocytes, with particularly dark color (Figures 2c and 2d). In the TCM decoction group, VEGF-A expression was limited, with staining intensity relatively low (Figures 2e-2h). Averaged light densities of VEGF-A indicated that VEGF-A expression was significantly inhibited after postoperative administration of TCM decoction (Figure 3).
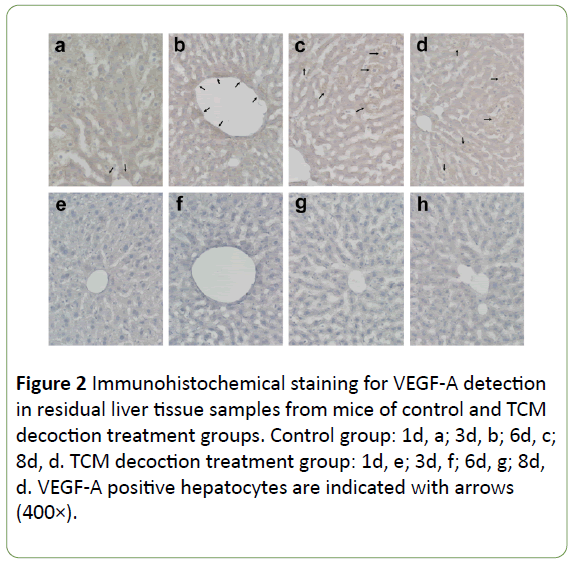
Figure 2: Immunohistochemical staining for VEGF-A detection in residual liver tissue samples from mice of control and TCM decoction treatment groups. Control group: 1d, a; 3d, b; 6d, c; 8d, d. TCM decoction treatment group: 1d, e; 3d, f; 6d, g; 8d, d. VEGF-A positive hepatocytes are indicated with arrows (400×).
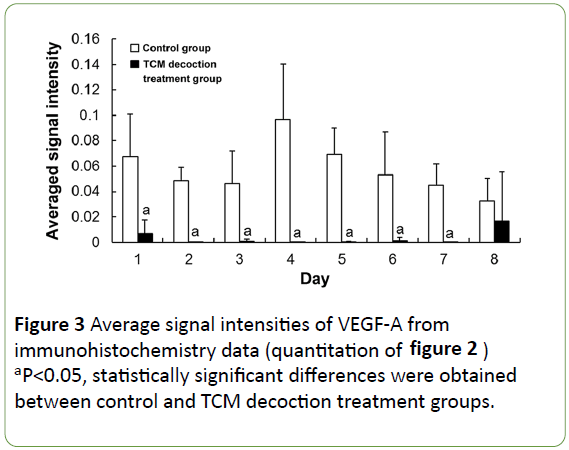
Figure 3: Average signal intensities of VEGF-A from immunohistochemistry data (quantitation of figure 2) aP<0.05, statistically significant differences were obtained between control and TCM decoction treatment groups.
PROX1 protein expression in residual liver tissue after PH with or without TCM decoction treatment
The expression of PROX1 was evenly distributed in the cytoplasm of some hepatocytes in the normal condition, stained with light color and lower signals found in the nucleus (Figure 4a). Large amounts of PROX1 positive cells were accumulated on the liver surface after PH, showing dark staining; moreover, most PROX1 was translocated into the nucleus instead of remaining in the cytoplasm (Figure 4b). However, no obvious PROX1 positive cells were found on the liver surface after PH in mice treated with TCM decoction (Figure 4e). In the residual liver tissue, the few PROX1 positive hepatocytes were normal in size in the control group; however, little PROX1 signals were found in enlarged hepatocytes. Meanwhile, large amounts of PROX1 positive cells were found in mice treated with TCM decoction; PROX1 was evenly distributed in the cytoplasm, similar to normal hepatocytes. Subsequently after PH, PROX1 positive hepatocytes showed small size, with nuclei stained dark (Figure 4c) in the control group; Meanwhile, large amounts of PROX1 positive cells were still found in the TCM decoction group, with PROX1 evenly distributed in the cytoplasm, similar to normal hepatocytes; the nuclei were not intensively stained (Figure 4f). Later on after PH, PROX1 was distributed among hepatocytes with various sizes in control group animals; dark staining was found in smaller hepatocytes, with relatively light and lightest staining in the middle size and enlarged ones (Figure 4d). However, most PROX1 positive cells were found in the hepatic blood sinus (Figure 4g), indicating PROX1 induced hepatocyte proliferation in control group mice, while participating in the formation of hepatic blood sinus in the TCM decoction treatment group. Average signal intensities of PROX1 signals indicated that PROX1 expression was significantly inhibited by TCM decoction treatment except at postoperative days 1 and 8 (Figure 5).
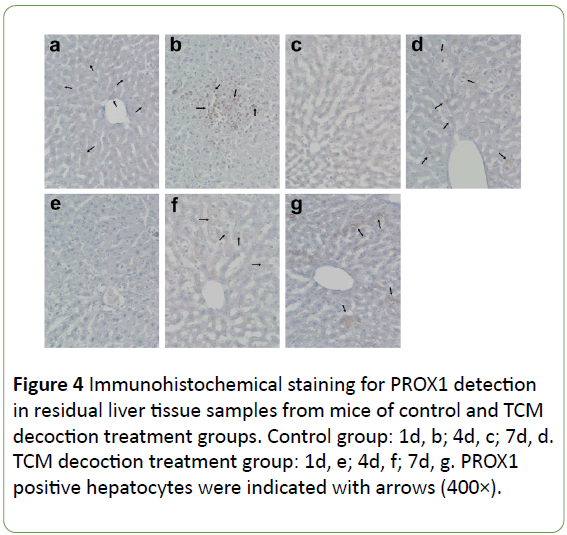
Figure 4: Immunohistochemical staining for PROX1 detection in residual liver tissue samples from mice of control and TCM decoction treatment groups. Control group: 1d, b; 4d, c; 7d, d. TCM decoction treatment group: 1d, e; 4d, f; 7d, g. PROX1 positive hepatocytes were indicated with arrows (400×).
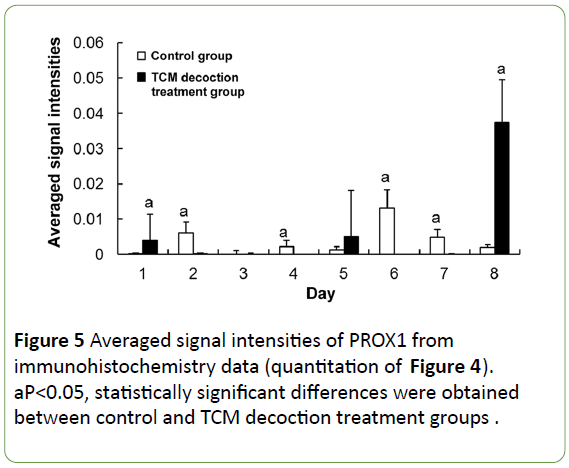
Figure 5: Averaged signal intensities of PROX1 from immunohistochemistry data (quantitation of Figure 4). aP<0.05, statistically significant differences were obtained between control and TCM decoction treatment groups.
Co-expression of PROX1 and VEGF-A in residual liver tissue after PH
Immunofluorescence confocal microscopy indicated that PROX1 and VEGF-A co-expressed in the same hepatocytes (Figure 6), implying a close correlation between these proteins; therefore, it is highly possible that both of PROX1 and VEGF-A participated in post-traumatic liver edema, degeneration, and regeneration process, whereas TCM decoction could significantly downregulate both molecul e s (Figures 3 and 5). Our findings indicated that PROX1 and VEGF-A signaling pathways might be therapeutic targets of TCM decoction for swelling reduction and pain relief.
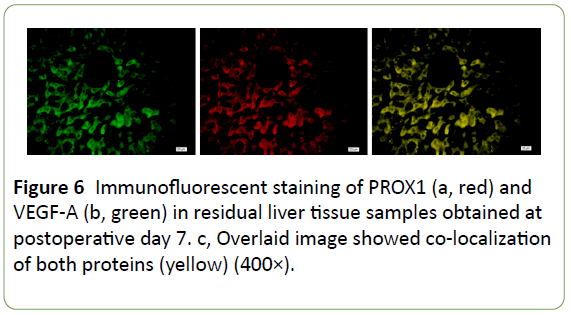
Figure 6: Immunofluorescent staining of PROX1 (a, red) and VEGF-A (b, green) in residual liver tissue samples obtained at postoperative day 7. c, Overlaid image showed co-localization of both proteins (yellow) (400×).
Discussion
‘Decoction for restoration’ is known as ‘Number one patent formulation’ for traumatology, and was highly valued by ancient physicians. The decoction effectively promotes blood circulation and removes blood stasis, reducing swelling and releasing pain, and is suitable for the treatment of traumatic injury with congestion beneath the rib and unbearable pain [1]. The clinical applications of this decoction have been constantly widened: it is also used as anti-inflammatory and analgesic [7]. However, the molecular mechanisms underlying its pharmacological effects remain unclear. Therefore, in the current study, the pharmacological effects of this decoction were assessed from the perspective of molecular morphology.
Degeneration and regeneration after injury are essential in tissue repair, e.g. edema, necrosis and regeneration [8]. Some regeneration related key activation or inhibitory factors have been discovered in recent years. For instance, VEGF family members specifically regulate endothelial cell division and proliferation, inhibiting their apoptosis [9]. Meanwhile, VEGF significantly increases capillary permeability with an efficacy of 50,000 fold that of the same dose of Histamin [10]. Most studies focused on the vascular regeneration effects of VEGF at the late stage of tissue injury, and it was overlooked that tissue swelling could be caused by VEGF expression at the early stage of tissue injury by increasing capillary permeability [11-13]. This study showed for the first time that overt swelling of residual liver tissue post PH was associated with increased expression of VEGF-A at the early stage of injury, and congestion within the hepatic blood sinus was also found. On the other hand, VEGF-A expression was significantly reduced by TCM decoction, with no obvious congestion within the residual hepatic lobule although a moderate swelling was noted. Reduced swelling and released membrane tension in residual liver tissue after injury were achieved by TCM decoction treatment via down-regulation of VEGF-A, which could be a key molecular mechanism underlying the effects of TCM decoction.
Hypoxia inducible factor (HIF) is one of the most sensitive factors upregulating VEGF-A; meanwhile, the expression of VEGF-A is indirectly enhanced by PROX1 via increased HIF expression [6]. In the current study, not only post-traumatic liver swelling was reduced by TCM decoction, but hepatocyte regeneration was also significantly inhibited. PROX1, an indispensable regulator participating in the development of multiple organs [14,15], plays a vital role in hepatocyte proliferation and migration [5]. Under normal conditions, PROX1 was evenly distributed in the cytoplasm; meanwhile, it was rapidly transferred to the nucleus in control group mice after PH, with the expression of VEGF-A also significantly increased. Interestingly, TCM decoction treatment resulted in inhibited translocation of PROX1 from cytoplasm to nucleus, significantly decreasing PROX1 levels. These findings indicated that PROX1 might be a key target gene of TCM decoction in inhibiting hepatic swelling and proliferation, reducing tissue and cell hypoxia after injury.
The reduced VEGF-A expression in residual liver tissue after PH by TCM decoction treatment might be associated with PROX1, as shown above. Importantly, immunofluorescence confocal microscopy indicated that PROX1 and VEGF-A were coexpressed and co-localized in the same hepatocyte. Meanwhile, PROX1 expression was also significantly reduced most of the time in residual tissue after TCM decoction treatment. Taken together, these findings suggest that PROX1 and VEGF-A signaling pathways might be the key pathways targeted by TCM decoction to reduce swelling and release pain.
In the current study, TCM decoction effects on liver tissue swelling, degeneration, and regeneration were observed in a mouse model of PH. VEGF-A and PROX1 expression levels were assessed by immunohistological methods, and pathways targeted by TCM decoction were identified for the first time, providing a basis for further in-depth studies and extended clinical application. TCM decoction is an empirical formula for circulation promotion and blood stasis removal, with complex composition. Identification of effective components was not carried out in the current study, and future studies are warranted to elucidate the key compounds of this preparation, which might interact with PROX1 and VEGF-A directly or indirectly. This would be of great value for the clinical use of TCM decoction for the treatment of swelling caused by various conditions or even malignancies (including liver cancer) with high incidence and unfavorable prognosis.
Acknowledgement
None.
Competing Interests
All authors declare that they have no any conflict of interests.
Authors’ Contributions
Study concept and design: Fanwei Meng, Sai-sai Tang, Chunting Yan, Jie Liu and Wenhui Liu; analysis and interpretation of data: Fanwei Meng; drafting of the manuscript: Fanwei Meng and Sai-sai Tang; and sample collection: Chunting Yan, Jie Liu and Wenhui Liu.
Funding Sources
This study was supported by grants from the project of Shandong province administration of Traditional Chinese Medicine (No. 2013-149).
22623
References
- Shi MY, Duan L, Yi JP (2004) Preliminary pharmacological research on TCM decoction for restoration. J Wuhan Uni (Medical Sciences) 25: 58-61.
- Kim RD, Stein GS, Chari RS (2001) Impact of cell swelling on proliferative signal transduction in the liver. J Cell Biochem 83: 56-69.
- Kuttippurathu L, Patra B, Hoek JB, Vadigepalli R (2016) A novel comparative pattern count analysis reveals a chronic ethanol-induced dynamic shift in immediate early NF-kappaB genome-wide promoter binding during liver regeneration. Mol Biosyst 12: 1037-1056.
- Meng F (2014) Vascular endothelial growth factor C induces LYVE-1(+) endothelial cells to reconstruct hepatic sinusoid during liver regeneration. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 30: 1039-1042.
- Sosa-Pineda B, Wigle JT, Oliver G (2000) Hepatocyte migration during liver development requires Prox1. Nat Genet 25: 254-255.
- Liu Y1, Zhang JB, Qin Y, Wang W, Wei L, et al. (2013) PROX1 promotes hepatocellular carcinoma metastasis by way of up-regulating hypoxia-inducible factor 1alpha expression and protein stability. Hepatology 58: 692-705.
- Liu AL, Bi AL (2002) Novel application of TCM decoction for restoration. Shaanxi trad Chin med 23: 558.
- Lancerotto L, Orgill DP (2014) Mechanoregulation of Angiogenesis in Wound Healing. Adv Wound Care (New Rochelle) 3: 626-634.
- Shibuya M (2014) VEGF-VEGFR Signals in Health and Disease. Biomol Ther (Seoul) 22: 1-9.
- Koczy-Baron E, Kasperska-Zajac A (2014) The role of vascular endothelial growth factor in inflammatory processes. Postepy Hig Med Dosw (Online) 68: 57-65.
- Rocha SF, Schiller M, Jing D, Li H, Butz S, et al. (2014) Esm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circ Res 115: 581-590.
- Al Masri AA, El Eter E (2012) Agmatine induces gastric protection against ischemic injury by reducing vascular permeability in rats. World J Gastroenterol 18: 2188-2196.
- Scaldaferri F, Vetrano S, Sans M, Arena V, Straface G, et al. (2009) VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology 136: 585-595.e585.
- Risebro CA, Searles RG, Melville AA, Ehler E, Jina N, et al.(2009) Prox1 maintains muscle structure and growth in the developing heart. Development 136: 495-505.
- Lavado A, Oliver G (2007) Prox1 expression patterns in the developing and adult murine brain. Dev Dyn 236: 518-524.











