Keywords
C. pneumonia; Adhesion; Macrophage; Collagen type-IV
Introduction
Chlamydia pneumoniae is a respiratory human pathogen. Most of people at least once infected in live and frequently reinfected [1]. In population, 40-50% adults were detected antibody against C. pneumoniae which positively correlate with coronary artery diseases [2]. In vitro experiment showed that C. pneumoniae infected human endothelial cell and stimulated expression of selectin-E, ICAM-1, VCAM-1 [3]. Additionally, C. pneumoniae growth in cells line-macrophage and some endothelial cells line and in artery aortic smooth muscle cells (SMC) [4,5].
Adhesin is a protein which is required in adherence and infection of the host cell process. Adherence is an early step of pathogenesis mechanism [6]. Detection of molecule adhesin is a new thing from this research. Adherence a pathogen to endothelial cell can explain its involvement in atherogenesis theory (“responses to injury”) [7,8].
C. pneumoniae was associated with atherogenesis and its clinical manifestation. Seroepidemiologic study showed relationship between high titer antibody to C. pneumoniae with coronary artery disease, myocardial infarction, carotid artery disease and cerebrovascular disease. C. pneumoniae was found in atherosclerotic lesions, such as in endothelial cell, macrophage, and SMC [3]. Result of seroepidemiologic study, 25 (92.59%) of 27 acute myocardial infarction (AMI) patients were detected high titers IgG to C. pneumonaie [9,10].
Lipopolysacharide (LPS) of C. pneumoniae can induce monocyte differentiation into macrophage. Moreover, LPS causes the formation of in vitro foam cell when it is exposed by low density lipoprotein (LDL). Chlamydia lives and replicates in monocyte/ macrophage, and stimulate activation of chronic immunity, releasing TNF-α, IL-1β, IL-6 and IFN-γ [11]. LPS and the release of TNF-α may interfere with the release of nitric-oxide (NO) and prostacyclin, and hereinafter cause the change in endothelial cell [12].
Heat shock protein 60 C. pneumoniae (cHSP60) is homolog to human vascular protein, causing autoimmunity and endothelial cell injury [13]. The cHSP60 was found in the human carotid atheroma [2]. Human HSP60 and cHSP60 induce TNF, macrophages matrix metalloproteinase 9 (MMP-9) and cellular toxicity through complement or antibody-dependent cellular cytotoxicity. Vehmann-Kreula et al. have proved chlamydial protein (outer membrane protein 2/omp2, major outer membrane protein/ MOMP, cHSP60) induced macrophage MMP-9 [14].
Mostly ACS is as Results ruptures of labile atherosclerotic plaques and rarely from superficial thrombus which is caused by endothelial cell damage [15]. Rupture atherosclerotic plaques frequent associated with proteolytic enzymes that able to degrade extracellular matrix (ECM). The most proteolytic enzymes related to rupture of plaques is MMP [16].
Macrophage infiltration is one of plaques instability factors, predisposition for fibrous cap rupture. Other factors are thin fibrous cap, low collagen synthesis, exccentric position of fibrous cap, large lipid core [17]. Macrophage is able to degrade ECM through phagocytosis or secretion of proteolytic enzymes such as plasminogen activator and MMP. Macrophage is a major source of the MMPs [15,17]. Cytokine TNF-α and IL-1β promotes MMP macrophage and SMC productions. Worthly et al. explained although some enzymes involve in ECM destruction, but only MMP-9 have strong relation with plaques rupture. MMP-9 is able to degrade matrix components that cannot be degraded by others proteolytic enzymes [18,19].
Increasing MMP-9 is indication of a disease going worse [17]. Increasing MMP-9 serum occurs in AMI patients, unstable angina and its level appropriate to increasing of C-reactive protein (CRP) serum [20]. Increasing MMP-9 in atherosclerotic lesion proved a fact that active synthesis of MMP-9 by macrophage and SMC has closed relation with the increasing ECM degradation and plaques rupturing. Loftus et al. said that MMP-9 may be used as candidate for pharmacotherapy purpose and plaques stabilization in coronary artery disease patients and prevents acute ischemic syndrome [21].
Others than MMP production, activated macrophages induce expresses of effectors Fas-L resemble to T cell cytotoxic and NK cell. Inducible nitric oxide synthase (iNOS) pathway induces Fas expression on SMC surfaces, and SMC apoptosis [22]. SMC is a major cell produces collagen as a major component of ECM and fibrous cap have responsibility in fibrous cap stability [15]. The major component ECM is collagen. Major collagen in the body is type I-V. Collagen type-IV is have reticulum dimensional structure and as major component of base membrane all organs [23].
Commonly, collagen is present as non-fibrous form with triplehelix shape, so that its rigid structure is resistant to proteolysis. However, there is non-helical (globular) area for collagen type-IV which is very flexible and sensitive to proteolysis. Collagen type- IV contain 6 different polypeptides (α-1 until α-6). Each α consists of 3 domains, such as 7S, central helix and NC1. Proteolysis is cleavage NCI domain that can be detected in serum including its fragments [23].
Vehmaan-Kreula et al. proved that some C. pneumoniae proteins induced expression of MMP-9 [14]. In this work, the role of C. pneumoniae in plaques atherosclerotic rupturing was evaluated by detecting its adhesin availability and followed by test of adhesin ability in inducing degradation of collagen type-IV through macrophage activation and increasing MMP-9. Furthermore this research may useful in early diagnosis for human infected by C. pneumoniae as a risk factor of atherosclerosis and AMI.
Methods
Sample
This experiment used four samples of monocyte-derived macrophage obtained from healthy individual and C. pneumoniae that was obtained from ATCC-VR 1310 and was propagated in HEp-2 cell line (ATCC- CCL 23). The macrophage was then exposed by the optimum dose of adhesin 61 kDa of OMP C. pneumoniae for different periods as treatment, such as 0.5 h (group 1); 2 h (group 2); 24 h (group 3); 48 h (group 4); and 72 h (group 5). The optimum dose was determined by the best dose inducing production of macrophage MMP-9. A group without the adhesin exposure was used as a negative control.
Observations of the macrophage activation, which were indicated by the decrease in macrophage apoptosis, the increase in ROI, TNF-α, IL-1ß, MMP, MMP-9 productions, and phagocytosis activity, were performed after the adhesin exposure.
Inclusion factors of the samples are: hsCRP <1 mg/L [24]; non-smokers, non-hypertension, non-hypercholesterolemia (cholesterol total < 200 mg/dL, LDL<100 mg/dL, trigliseride <150 mg/Dl, HDL>40 mg/dL), non-diabetes mellitus, non-menopause women, male<55 years in age, BMI<25. If the culture of macrophage cells is unviable or contaminated by microorganism, the samples were not used in this experiment since they are categorized as the exclusion factor.
Procedures
Propragation of C. pneumoniae
C. pneumoniae was propagated in HEp-2 cell according to ATTCC procedure [25]. The growth of C. pneumoniae was detected using immunocytochemistry.
Isolation of EB from HEp-2 cell
HEp-2 cells were harvested at 3rd day and the cells were disrupted using glass bead. This isolation procedure was conducted according to Yamaguci method [10].
Isolation of OMP C. pneumoniae
The isolation procedure was done according to Barenkamp method and the OMP was characterized based on its molecular weight by mean of SDS-PAGE [26]. The protein 61 kDa was isolated from the OMP according to our previous research [27]. Briefly, protein 61 kDa is an immunogenic protein, hemaglutinin, and acts as an adhesin molecule. As immunogenic protein, it could be detected by immunoblotting method in acute myocardial infarction antibody patients. This protein is able to agglutinate human erythrocytes, so called as hemagglutinin. Commonly a hemagglutinin is an adhesin molecule, and to prove it, adhesion test directly and indirectly to human vein endothelial cells (HUVECs) were conducted. Indirect method was done by reacting the bacteria with specific antibody to protein 61 kDa before adhesion test, and the result showed that the protein inhibited adhesion and infection bacteria to HUVECs. Such Results proved that the protein 61 kDa is an adhesin molecule.
Isolation of protein from the OMP of C. pneumoniae
The immunogenic protein was cut from gel SDS-PAGE. The protein then was isolated by electro elution method. The result was dialyzed using sterilized deionized water for 2 x 24 hours then was kept at -400C.
Determination of the adhesin 61 kDa OMP C. pneumoniae exposure dose
For this purpose, culture macrophage cell was exposed with various dose of the protein. As parameter determination, the production of MMP-9 was measured using ELISA method to monoclonal antibody MMP-9. Supernatant of macrophage growth was colected after 2 and 48 jam cultered [14].
PMBCs purification and culture of monocyte
Peripheral blood mononuclear cells (PMBCs) were isolated from buffy coat heparinised blood (Institutional Review Board for Human Subjects, National Institute of Allergy and Infectious Diseases) as the following procedures: buffy coat was obtained by employing gradient centrifugation in the presence of ficol-phage. Then, buffy coat was collected, washed twice with PBS, and added with serum-free medium (RPMI 1640 contain 25 mM HEPES 25). Cells were cultured in 24 wells micro plate, 4x105 - 5x105 cells/well and incubated at 370C, 5% CO2. After 2 hours cultured, cells were washed using serum-free medium RPMI 1640. Isolated cells, as PMBCs, were then cultured in RPMI 1640 (contain 25 mM HEPES; 10% FBS; 10 μg per ml gentamycine). In this case, the chamber was coated with gelatine, as recommended by Hassan et al. [7]. With this technique, more than 90% the monocytes can be isolated (high monocyte purity), good cell viability, less contamination by platelets.
Induction of monocytes differentiation
For this purpose, the macrophage culture medium (RPMI 1640 + 25 mM HEPES + 10%; gentamicin 10 μg per ml) was added with 10-7 M phorbol myristate acetate (PMA). For MMP detection, serum-free medium was used along with incubation at 37°C, 5% CO2. The differentiation of monocytes into macrophage required 7 days [14].
Macrophage exposure with adhesin of the OMP C. pneumoniae
The macrophage culture was washed, then the medium was changed with serum-free and protein-free medium without PMA. The culture was incubated at 37°C, 5% CO2. The culture was divided into 2 groups such as Trial Group and Control Group. Trial Group was exposed with the adhesin in different time exposures of 0.5, 1.5, 24, 48 and 72 hours.
Detection of TNF- α and IL-1β productions
Detection of TNF- α and IL-1β productions containing in supernatant of macrophage culture was confirmed by ELISA method.
Detection phagocytosis activity
Latex bead (3.2 μm in diameter) was used to detect the macrophage activity [28].
Detection of ROI production
ROI production was detected base on Formosan formation [29].
Detection of MMPs productions
To detect MMP production, gelatine-zymography-non reducing SDS-PAGE method introduced by Vehmaan-Kreula et al. was used [14]. The gel was stained using coomasic brilliant blue R-250 for detection of gelatinolytic activity.
Identification and isolation of MMP-9
MMP-9 identification was done by immunoblotting (western blot) method to MMP-9 monoclonal antibody [30]. Isolation the MMP-9 was done by cut the gel SDS-PAGE in appropriate place.
Detection of macrophage apoptosis
To induce apoptosis, the macrophage culture was exposed with H2O2 in various concentrations (5 mM, 10 mM, 15 mM, 20 mM, and 25 mM). According to Laochumroonvorapong et al., 10 mM H2O2 is optimal dose to induce macrophages apoptosis [31]. Cover slip of macrophage growth was removed, and fixed with methanol. To detect the apoptosis, fixed macrophages were stained with immunocytochemistry technique to caspase-3 monoclonal antibody.
Observation collagen type-IV fragmentation by MMPs and MMP-9
The ability of MMPs and MMP-9 to degrade the collagen was done using collagenase assay according to Romanelli et al. [32].
Results
Detection the ability of the adhesin 61 kDa OMP C. pneumoniae to enhance macrophage activation
Outer membrane protein C. pneumoniae contain 16 protein fragments, including minor and major proteins. Protein 61 kDa OMP C. pneumoniae is a major protein (Figure 1), corresponding to an immunogenic, hemaglutinin and an adhesin. According to the theory “responses to injury”, adhesion to endothelial cell may cause injury because its function facilitates a pathogen to adhere on surface of the host cell. It is supposed that the adhesin contribute to atherogenesis. In this work, we investigated the other roles of the adhesin in atherogenesis and in atherosclerotic plaques rupturing, by examine the ability of the adhesin in macrophage activation.
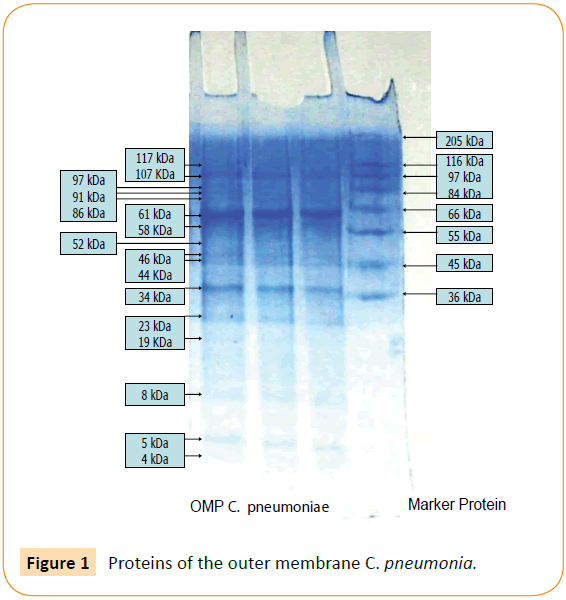
Figure 1: Proteins of the outer membrane C. pneumonia.
The optimum dose of adhesin was determined by the highest titre of MMP-9 production. The optimum dose was found to be 0.025 μg/ml. Then, macrophage culture was exposed with optimum dose of adhesin 61 kDa OMP C. pneumoniae in difference times (0.5, 2, 24, 48, and 72 h). The Results showed that adhesin 61 kDa OMP C. pneumoniae able to enhance macrophage activation parallel to the long-time exposure. It could inhibit macrophage apoptosis, induce increasing production of MMPs, MMP-9 (Figure 2). Production of MMPs and MMP-9 was given in Figures 3 and 5. While detection of MMPs in negative control was presented in Figure 4. The adhesin induces the increase of IL-1β, significantly. However, the increase in TNF-α production is not significantly observed (by one way ANOVA, p < 0.05) as shown in Table 1.
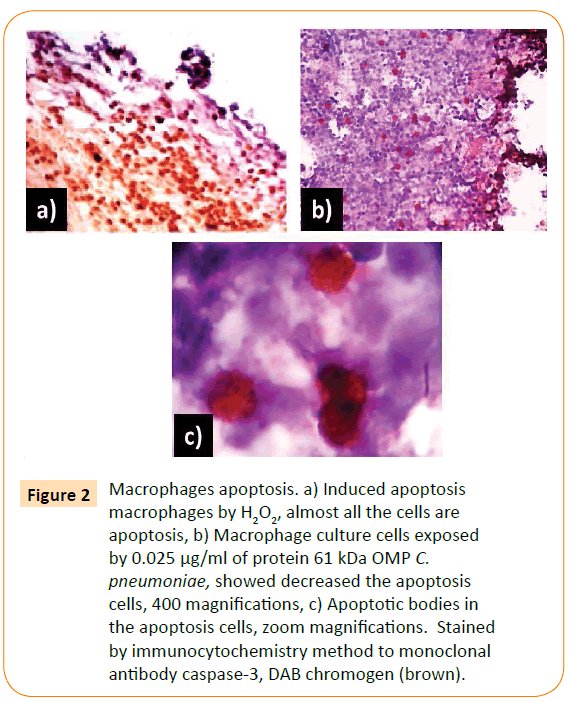
Figure 2: Macrophages apoptosis. a) Induced apoptosis macrophages by H2O2, almost all the cells are apoptosis, b) Macrophage culture cells exposed by 0.025 μg/ml of protein 61 kDa OMP C. pneumoniae, showed decreased the apoptosis cells, 400 magnifications, c) Apoptotic bodies in the apoptosis cells, zoom magnifications. Stained by immunocytochemistry method to monoclonal antibody caspase-3, DAB chromogen (brown).
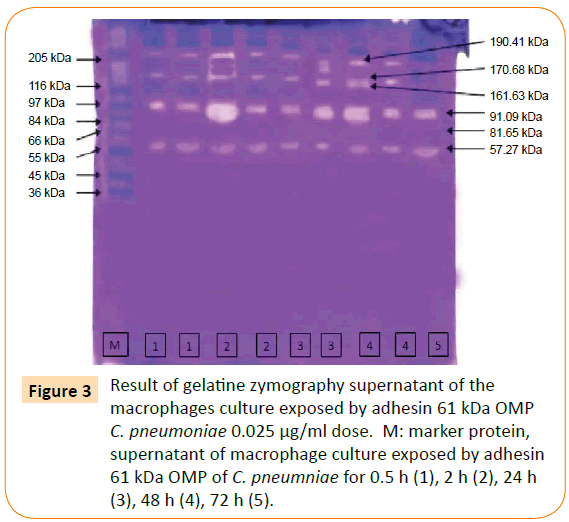
Figure 3: Result of gelatine zymography supernatant of the macrophages culture exposed by adhesin 61 kDa OMP C. pneumoniae 0.025 μg/ml dose. M: marker protein, supernatant of macrophage culture exposed by adhesin 61 kDa OMP of C. pneumniae for 0.5 h (1), 2 h (2), 24 h (3), 48 h (4), 72 h (5).
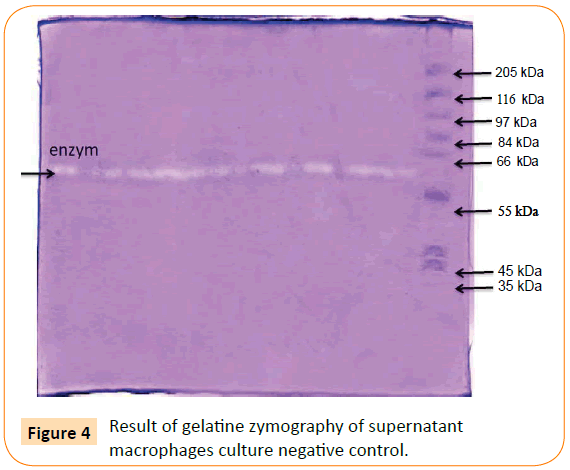
Figure 4: Result of gelatine zymography of supernatant macrophages culture negative control.
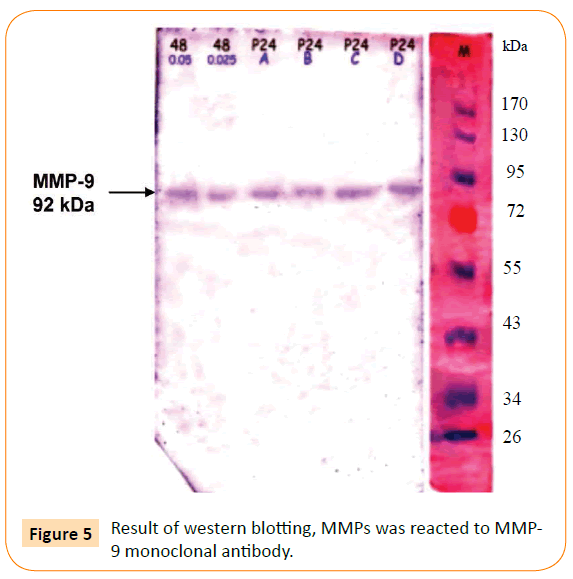
Figure 5: Result of western blotting, MMPs was reacted to MMP-9 monoclonal antibody.
| Group |
Optical Density in average |
| |
IL-1β |
| Time of exposure |
0.5 hr. |
2 hr. |
24 hr. |
48 hr. |
72 hr. |
| OD |
1.032 |
1.138 |
1.261 |
1.113 |
1.082 |
| Negative control |
0.906 |
0.896 |
0.946 |
0.924 |
0.904 |
| |
TNF-α |
| OD |
0.673 |
0.740 |
0.859 |
0.754 |
0.801 |
| Negative control |
0.859 |
0.858 |
0.764 |
0.734 |
0.721 |
Table 1: Optical Dencity ELISA IL-1β and TNF-α macrophages exposed by 0.025 μg/ml adhesin 61 kDa OMP C. pneumonia.
By one way ANOVA (α= 0.05), the production of IL-1ß macrophages exposed by 0.025 μg/ml adhesin 61 kDa OMP C. pneumoniae increased significantly, but insignificant increase was resulted for production of TNF-α. The highest titer of both cytokines was found after 2 h exposure.
Figure 2 showed that almost all macrophages exposed by H2O2 for 6 h are apoptosis. H2O2 is one of free radicals caused damaged components of cells, including proteins, membranes cell, lipid, DNA, RNA, enzymes, saccharides. The macrophages apoptosis decreased with the increase in time exposure of adhesin.
From Figures 3 and 4 it can be found that exposure of adhesin induced increasing MMPs production, but not in negative control since only one enzyme is produced (indicated by one line enzyme).
Figure 5 presents the immunoblotting result of the MMPs reacted with MMP-9 monoclonal antibody, which is aimed to detect production of MMP-9 after exposure with adhesin. As can be seen, the result showed one line specific reaction of MMP-9 and its antibody.
Detection ability the MMP-9 (produced by macrophage exposed by adhesion 61 kDa OMP C. pneumoniae) to degrade collagen type-IV
MMP-9 was reacted to biotin labelled collagen type-IV. The point of this study was to prove the involvement of the adhesin 61 kDa OMP C. pneumoniae in rupturing of plaque atherosclerotic. The result showed that MMPs and MMP-9 able to degraded collagen type-IV. MMPs degraded collagen type-IV into 19 fragments (Figure 6), while MMP-9 consists of 15 fragments (Figure 7).
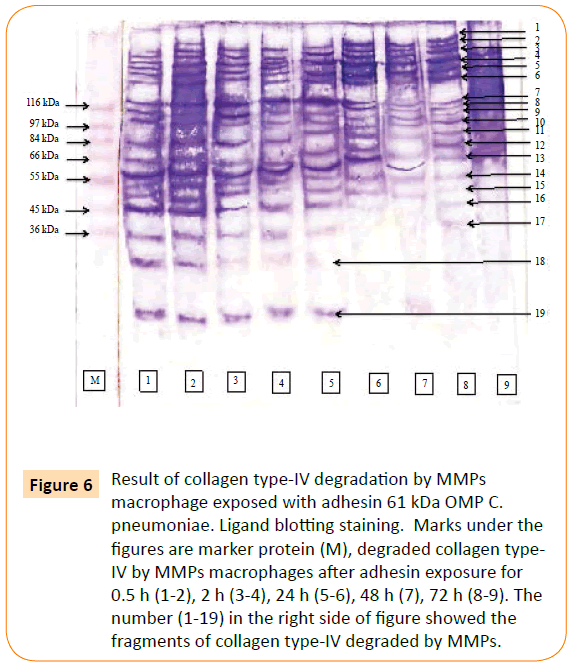
Figure 6: Result of collagen type-IV degradation by MMPs macrophage exposed with adhesin 61 kDa OMP C. pneumoniae. Ligand blotting staining. Marks under the figures are marker protein (M), degraded collagen type-IV by MMPs macrophages after adhesin exposure for 0.5 h (1-2), 2 h (3-4), 24 h (5-6), 48 h (7), 72 h (8-9). The number (1-19) in the right side of figure showed the fragments of collagen type-IV degraded by MMPs.
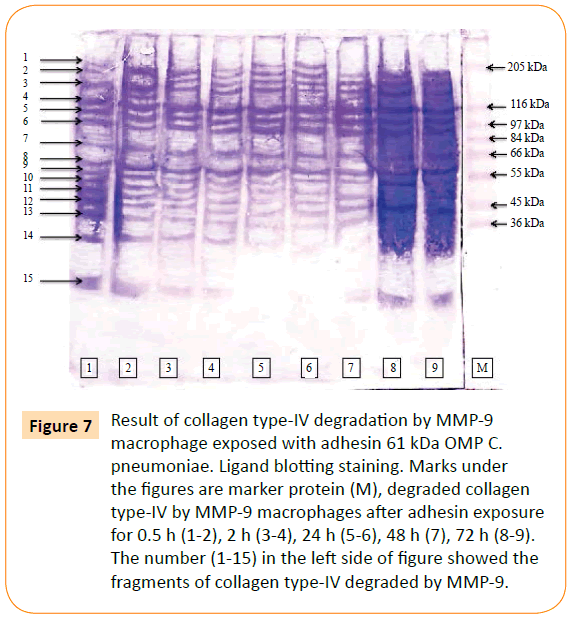
Figure 7: Result of collagen type-IV degradation by MMP-9 macrophage exposed with adhesin 61 kDa OMP C. pneumoniae. Ligand blotting staining. Marks under the figures are marker protein (M), degraded collagen type-IV by MMP-9 macrophages after adhesin exposure for 0.5 h (1-2), 2 h (3-4), 24 h (5-6), 48 h (7), 72 h (8-9). The number (1-15) in the left side of figure showed the fragments of collagen type-IV degraded by MMP-9.
The MMPs was reacted with collagen type-IV to detect the potency MMP in collagen degradation. Fragments of collagen type-IV as the result of degradation by MMPs were characterized base on their molecular weight. These fragments were 179.15 kDa, 171.94 kDa, 157.80 kDa, 145.02 kDa, 133.27 kDa, 122.48 kDa, 115.39 kDa, 114.56 kDa, 107.88 kDa, 99.15 kDa, 91.12 kDa, 80.26 kDa, 70.70 kDa, 64.97 kDa, 50.43 kDa, 46.33 kDa, 42.57 kDa, 39.12 kDa and 19.07 kDa.
The ability of MMP-9 after the chlamydial adhesion exposure in degradation of collagen type-IV proved that this MMP could also be causing base membrane. Fragments of collagen type-IV as the result of degradation by MMP-9 were characterized base on their molecular weight. These fragments were 157.80 kDa, 145.02 kDa, 133.27 kDa, 122.48 kDa, 107.88 kDa, 99.15 kDa, 91.12 kDa, 80.29 kDa, 64.97 kDa, 58.71 kDa, 57.23 kDa, 50.43 kDa, 46.33 kDa, 39.12 kDa and 19.07 kDa.
Discussion
This research has recognized 61 kDa OMP C. pneumoniae as adhesin that fasilitate C. pneumoniae adherence to human endothelial cell, proving the role of C. pneumoniae in atherogenesis appropriate to atherogenesis theory “responses to injury” [7,8]. This result is showing a new finding of our research.
Injury caused endothelial disfunction, induces expression of adhesin molecules and promotes foam cell formation, early step of atherogenesis [33]. The adhesin 61 kDa OMP C. pneumoniae was able to activate macrophage. It is indicated by its ability for inhibiting macrophage apoptosis, and increasing ROI, TNF-α, MMPs, MMP-9.
Geng et al. reported, for intracellular living C. pneumoniae inhibit macrophage apoptosis through induction of IL-10 [34]. Depletion of endogenous IL-10 abolished the apoptosis resistance of C. pneumoniae-infected mononuclear cells. In comparison to TNF-α and IL-1ß production, IL-10 was produced in longer time. After human monocyte exposed by LPS, IL-10 was initially produced within 24-48 hours after exposure, while TNF-α and IL-1ß required 4-8 hours [35]. These Results revealed that adhesin 61 kDa OMP C. pneumoniae may able to induce IL-10 production and inhibit macrophage apoptosis. To the best of our knowledge, this is the new finding that structural antigen of C. pneumoniae (adhesin OMP 61 kDa) could inhibit macrophage apoptosis. ROI production increased in activated immune cells as well as macrophages, as a protective host response. Immune cells generate free radicals in the process of microbial killing mechanisms, especially through oxygen dependent phagocytosis.
Production of MMPs increased after exposed by the adhesin, and in all time exposure investigated, it showed similar pattern. However, in the negative control only one MMP was found. This may not be inducible enzyme, but a constitutive enzyme which is produced in low level.
Antigens of C. pneumoniae have proven involving in atherogenesis; (a) LPS acts as cytotoxic to endothelial cell, (b) cHSP60, which was homolog to vessel protein, caused the atherosclerosis through the autoimmune reaction. Additionally, C. pneumoniae infected directly to endothelial cell. Such Results may contribute to atherogenesis. MOMP, omp2, cHSP60 have proven induces MMP-9 production as well as the adhesin 61 kDa [14]. This MMP-9 is able to degrade collagen type-IV. Collagen type-IV is major component of base membrane under of endothelial cell. The degradation collagen type-IV causes instable of atherosclerotic plaques integrity, causing rupture of plaques, and leading to myocardial infarction. These antigens may be synergism causing atherosclerosis.
Conclusion
Adhesin 61 kDa OMP of C. pneumoniae induce degradation collagen type-IV through enhancing macrophage activation and MMP-9 production significantly. It could be concluded that the adhesion 61 kDa OMP C. pneumoniae may play role in rupture of plaques atherosclerotic through degradation of collagen type-IV.
Acknowledgements
The author would like to thank Prof. Dr. Djanggan Sargowo, SPPD, SpJP (K), Prof. Dr. Handono Kalim, SpPD , Prof. Mulyohadi Ali, Ketut Muliartha, Sumarno directorate general of higher education of the republic of Indonesia for financial support.
6537
References
- File TM Jr, Tan JS (2000) Chlamydia pneumoniae pneumonia. Semin Respir Crit Care Med 21: 285-294.
- Kol A, Sukhova GK, Lichtman AH, Libby P (1998) Chlamydial Heat Shock Protein 60 Localizes in Human Atheroma and Regulates Macrophage Tumor Necrosis Factor-a and Matrix Metalloproteinase Expressin. Circulation 98:300-307.
- Kaukoranta-Tolvanen SS, Ronni T, Leinonen M, Saikku P, Laitinen K (1996) Expression of adhesion molecules on endothelial cells stimulated by Chlamydia pneumoniae. Microb Pathog 21: 407-411.
- Quinn TC, Gaydos CA (1999) In vitro infection and pathogenesis of Chlamydia pneumoniae in endovascular cells. Am Heart J 138: S507-511.
- Sherer Y, Shoenfeld Y(2001) Archieved Reports. Report on the Europen Atherosclerosis Society Workshop on Immune System in Atherosclerosis.
- Wizemann TM, Adamou JE, Langermann S (1999) Adhesins as targets for vaccine development. Emerg Infect Dis 5: 395-403.
- Hassan NF, Campbell DE, Douglas SD (1997) Purification of human monocytes on gelatin-coated surface. J Immun Methods 24: 273-296.
- Orford JL, Selwyn AP (2005) Atherosclerosis. Instant Access to the Mind of Medicine. Department of Internal Medicine, Brigham an Women’s Hospital, Haevard Medical School.
- Murwani S, Ali M, Muliartha K, Purwanto, Susilawati I, et al.(2006) Studi Seroepidemiologi Infark Miokardial Akut (IMA) yang Terkait Infeksi Chlamydia pneumoniae dan Adanya Infeksi Multiple dengan Mikroorganisme lain yang Diduga dapat Menyebabkan IMA. Jurnal Kedokteran YARSI 15: 22-26
- Yamaguchi H, Haranaga S, Widen R, Friedman H, Yamamoto Y (2002) Chlamydia pneumoniae infection induces differentiation of monocytes into macrophages. Infect Immun 70: 2392-2398.
- Krüll M1, Klucken AC, Wuppermann FN, Fuhrmann O, Magerl C, et al.(1999) Signal Transduction Pathway Activated in Endothelial Cells Following Infection with Chlamydia pneumoniae. J Immunol 162: 4834-4841.
- Ngeh J, Gupta S (2000) Chlamydia pneumoniae and Atherosclerosis: Causal or Coincidental Link?. ASM News 66: 732
- Mayr M, Metzler B, Kiechl S, Willeit J, Schett G,(2003) Endothelial Cytotoxicity Mediated by Serum Antibodies to Heat Shock Proteins of Escherichia coli and Chlamydia pneumonia: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation. 99: 1560-1566.
- Vehmaan-Kreula P, Puolakkainen M, Sarvas M, Welgus HG, Kovanen PT (2001) Chlamydia pneumoniae Protein Induce Secretion of the 92-kDa Gelatinase by Human Monocyte-Derived Macrophages. Atherioscler Thromb Vasc Biol 21:ie.
- Falk E, Shah PK, Fuster V (1995) Coronary plaque disruption. Circulation 92: 657-671.
- Bellosta S (2000) Plaque Instability and Acute Coronary Synsrome. XIIth International Symposium on Atherosclerosis. June 25-29 2000, Stockhlom. Sweden
- Worthley SG, Osende JI, Helft G, Badimon JJ, Fuster V (2001) Coronary artery disease: pathogenesis and acute coronary syndromes. Mt Sinai J Med 68: 167-181.
- Galis ZS, Khatri JJ (2002) Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 90: 251-262.
- Kalela A (2002) Factor Affecting Serum Matrix Metalloproteinase-9 with Special Reference to Atherosclerosis. Academic Dissertation. Acta Universitasis Tamperensis 886, University of Tampere, Tampere.
- Loftus IM, Naylor AR, Goodall S, Crowther M, Jones L, et al. (2000) Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke 31: 40-47.
- Boyle JJ1 (2005) Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol 3: 63-68.
- Ortega N, Werb Z (2002) New functional roles for non-collagenous domains of basement membrane collagens. J Cell Sci 115: 4201-4214.
- Ridker PM1 (2003) Cardiology Patient Page. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation 108: e81-85.
- American Type Culture Collection (2006). Culture Method of Chlamydia pneumoniae.
- Barenkamp S, Munson RSJr, Granoff DM (1981) Subtyping Isolates of Haemophillus influenzae Type b by Outer Membrane Protein Profiles. In Characterization of Outer Membrane Protein-Enriched from Pasteurella multocida Isolated From Turkey, (eds) K.H. Coi, S.K. Maheswaran, L.J. Felice. Am J Vet Res. 50: 676-682.
- Murwani S, Hidayati DYN (2007) Identification of immunogenic protein of chlamydia pneumoniae to acute myocardial infarction patients serum. Medical Journal of Brawijaya 23: 100-105
- Brandes RP, Janiszewski M (2005) Direct detection of reactive oxygen species ex vivo. Kidney Int 67: 1662-1664.
- Tjahajati I, Prodjoharjono S, Subono H, Asmawa W, Harada N (2004) Peningkatan Aktivitas Fagositosis Makrofag Peritoneum Kuciny yang Diinfeksi dengan M. tuberculosis. J. Sain. Vet. XXII.
- Mahmood T, Yang PC (2012) Western blot: technique, theory, and trouble shooting. N Am J Med Sci 4: 429-434.
- Laochumroonvorapong P, Paul S, Elkon KB, Kaplan G (1996) H2O2 Induces Monocyte Apoptosis and Reduces Viability of Mycobacterium avium-M. intracellulare within Cultured Human Monocytes. Infect Immun 64: 452-459.
- Romanelli R, Mancini S, Laschinger C, Overall CM, Sodek J, et al. (1999) Activation of neutrophil collagenase in periodontitis. Infect Immun 67: 2319-2326.
- Liao JK1 (1998) Endothelium and acute coronary syndromes. Clin Chem 44: 1799-1808.
- Geng Y, Shane RB, Berencsi K, Gonczol E, Zaki MH, et al. (2000) Chlamydia pneumoniae Inhibits Apoptosis in Human Peripheral Blood Mononuclear Cells Through Induction of IL-10. J Immunol 164: 5522-5529.
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE (1991) Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174: 1209-1220.












