s, alum has the potential for severe local and systemic complications including sterile abscesses, eosinophilia, and myofascitis, however, fortunately, most of the more serious side-effects are relatively rare [3]. Therefore, the study and development of new immunological materials with low toxicity, high efficacy, and vast resources are very important [6]. The demonstration that mycobacterial DNA had adjuvant activity, led to the exploration that the adjuvant activity was associated with a high content of CpG motifs present in bacterial nucleic acids. DNA containing CpG motifs is one of the main strong cellular adjuvants [3]. CpG oligodeoxynucleotides (ODNs) bind Toll-like receptor 9 (TLR9), causing the production of gamma interferon (IFN-γ), IL-6 and IL-10 by a natural killer (NK) cells and also produces of interleukin-1 (IL-1), IL-6, IL-12 and tumor necrosis factor-alpha by dendritic cells and macrophages[7]. During the progress of inflammation, cytokines play critical roles, essentially embodying as restriction and balance between proinflammatory and anti-inflammatory cytokines [2]. This adjuvant was specifically designed to drive immunity through cells and to stimulate a strong humoral and cell-mediated immune response in subunit vaccines. Bacterial DNA (bDNA) has been proposed as promising adjuvant for inactivated vaccines in mice [10,11] and poultry[12]. Our group has recently focused on the adjuvant qualities of some bDNAs. Inactivate P. multocida vaccines were formulated with P. multocida A, P. multocida B or Salmonella bacterial DNA adjuvants and inoculated into Balb/c mice.
The purpose of the present study was to assess the adjuvant effect of homologous and heterologous bDNAs associated with formalin or iron-inactivated vaccine against Pasteurella multocida by measuring IL-6 and IL-12 in vivo and in vitro.
Materials and Methods
Animals
In this experiment, six to eight-week-old, female Balb/c mice (weight 16-18 g) were chosen from the Laboratory Animal Center of Razi Vaccine and Serum Research Institute (RVSRI), Shiraz, Iran. The bacterial test explained that mice were free from Pasteurella infection. The mice were placed in the private cages and all experiments have been conducted by the guideline for care and use of experimental animal of the National Counsel of Animal Experimentation Control. A total of 56 mice were used in this study and animals were randomly divided into seven groups (8 cases per group).
Culture of bacteria
P. multocida serotype A sheep, P. multocida serotype B bovine and S. typhimurium isolate were used in the present study. Frozen bacteria were seeded on blood agar (BA) plates (containing 5% of sheep defibrinated blood) for isolation of single colonies. For broth cultures, Pasteurella strains were inoculated in brain heart infusion (BHI) broth (Merck) and Salmonella strain was inoculated in tryptic soy broth (TSB) (Merck) and incubated at 37°C for 24 h.
The purity of the isolate was checked by morphological characteristics on smears stained with Gram's staining and sterility of the vaccine preparations was confirmed by growth by inoculating TSB, BHI broth, nutrient agar (NA) and BA.
Preparation of formalin-inactivated antigen (FIA)
The formalin inactivation of P. multocida was performed according to the method of Kumar, Singh (2005). Typical colonies isolated on BA were seeded in BHI broth, and then 1 ml of the bacterial suspension inoculated into 50 ml of BHI broth and incubated at 37°C for 24 h. Finally, the bacterial culture was expanded by inoculating 20 ml into one liter of BHI broth at 37°C overnight. The bacteria were pelleted at 3500×g for 25 min at 4°C and washed twice with a normal saline solution (NSS). The pellet was re-suspended in NSS to a final volume of 20 ml. Formaldehyde (37%) (Equivalent to formalin 100%) was combined to a final concentration of 0.5% formalin in the suspension, mixed properly and incubated at 37°C for 24 h. The residual formaldehyde was eliminated by washing the bacteria twice in NSS. Finally, the bacterial suspension was prepared 20 ml in NSS and stored at 4-8°C in a refrigerator [11].
Preparation of the iron-inactivated antigen (IIA)
Bacterial growth and harvesting of P. multocida were carried out as mentioned in the previous section. The bacteria were pelleted and re-suspended in NSS to a final volume of 18 ml.
P. multocida was inactivated with 1 M FeCl3 in a final concentration of 10 mM. This bacterial suspension containing 10 mm FeCl3 was incubated at 37°C for 24 h. The bacteria were then pelleted by centrifugation and washed twice in NSS to remove the excess of FeCl3. Finally, the FeCl3-treated bacterial suspension was made 20 ml in NSS and stored at 4-8°C in a refrigerator [11,12].
Extraction of Pasteurella and Salmonella bDNA
Bacterial DNA was isolated by the extraction method was a refined method of the standard phenol/chloroform method [13]. In this method Tris-saturated phenol was added to tubes, followed by a vortex-mixing step, to lyse cells. RNA in DNA preparation was digested by RNase treatment in DNA preparation and DNA was further purified by repeated treatments of chloroform. Subsequently, the purified DNA was precipitated with 2 volumes of cold ethanol, pelleted, air dried, re-suspended in endotoxin-free water and stored at -20°C until use. The purity and yield of the DNA were appraised spectrophotometrically by calculating the A260/A280 ratios and the A260 for determining the impurities concentration of protein and DNA. P. multocida Agenomic DNA, P. multocida B genomic DNA, and S. typhimurium genomic DNA was designated as AbDNA, BbDNA, and SbDNA, respectively.
Purity test
To confirm that the vaccines were free from any bacterial and fungal contamination, vaccine mixtures were inoculated on NA, BA and in BHI broth and incubated at 37°C for 48-72 hours.
Safety/innocuity test
The safety of the vaccines was evaluated according to the general behavior of the mice after vaccination. The mice were observed for any symptoms of fatigue, mental disorders, depression, anorexia, the effect on skin conditions, bodied weight and temperature throughout the whole time of experiments.
Immunization of mice
To study the vaccine, mice were randomly divided into seven groups consisting of eight mice in each group (Table 1). Mice were inoculated twice by subcutaneously (s.c) route at days 0 and 14. The dose of inoculation/ mouse was 1.0 mg/0.2 ml of the IIA or FIA. IIA was adjuvanted with 5 μg of each bDNAs (IIA+bDNAs). FIA was adjuvanted with 0.5% aluminum potassium sulfate (FIA+ Alum). To prepare for FIA+bDNA, FIA was adjuvanted with AbDNA. Mice received only NSS or AbDNA adjuvant in the two control groups.
Table 1 Injectable immunogens into animal test and control groups; Formalin-inactivated antigen (FIA), iron-inactivated antigen (IIA).
| Group |
Antigen |
Adjuvant (concentration) |
Immunogen |
| 1 |
FIA |
Alum (0.5%) |
FIA+Alum |
| 2 |
FIA |
AbDNA (5 µg) |
FIA+AbDNA |
| 3 |
IIA |
AbDNA (5 µg) |
IIA+AbDNA |
| 4 |
IIA |
SbDNA (5 µg) |
IIA+SbDNA |
| 5 |
IIA |
BbDNA (5 µg) |
IIA+BbDNA |
| 6 |
No Antigen |
AbDNA (5 µg) |
AbDNA |
| 7 |
No Antigen |
No Adjuvant |
NSS |
Collection of serum
Twenty-eight days after the first immunization, blood was collected from all animals by heart puncture. Approximately 0.3 ml of blood was collected in a sterile micro-centrifuge tube without anticoagulant for serum separation. After centrifugation at 1500 g for 10 minutes, the serum supernatant was collected carefully. Serum samples were stored frozen at -20°C for serum cytokine assays (Figure 1).
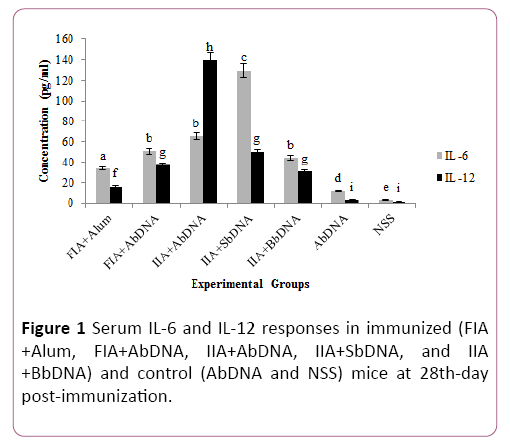
Figure 1: Serum IL-6 and IL-12 responses in immunized (FIA +Alum, FIA+AbDNA, IIA+AbDNA, IIA+SbDNA, and IIA +BbDNA) and control (AbDNA and NSS) mice at 28th-day post-immunization.
Evaluation of serum showed a significant increase in IL-12 titer in mice vaccinated with IIA+AbDNA (P=0.0001).
Spleen lymphocyte transformation test of mice
On the 28th day after the first immunization, splenocyte suspensions were obtained from the spleens of immunized mice. Mice were sacrificed and spleens were aseptically removed and placed in a tissue culture grade, sterile disposable 60 mm Petri dish containing 5-10 ml of incomplete Roswell Park Memorial Institute (RPMI) medium.
Following erythrocyte lysis by hypo-osmotic shock and the remaining cells were washed three times with RPMI medium, the supernatant fluid was discarded by centrifugation. Then, the supernatant fluid was removed and the pellet resuspended in 5-10 mL of completed medium (RPMI medium supplemented with 2% heat-inactivated fetal calf serum) per spleen (Figure 2).
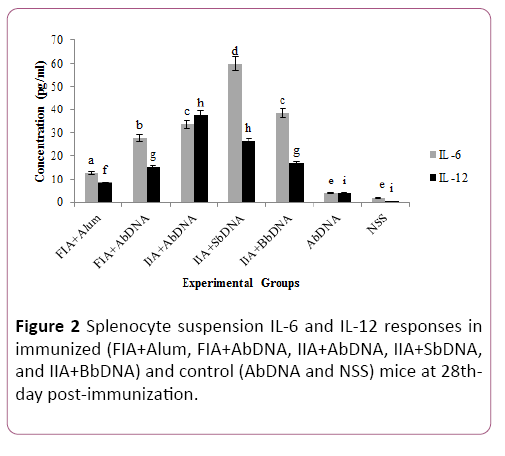
Figure 2: Splenocyte suspension IL-6 and IL-12 responses in immunized (FIA+Alum, FIA+AbDNA, IIA+AbDNA, IIA+SbDNA, and IIA+BbDNA) and control (AbDNA and NSS) mice at 28thday post-immunization.
Evaluation of splenocyte suspension was statistically significant increase in IL-12 titer in mice vaccinated with immunogenescompared to the control groups (P=0.0296).
The number of spleen cells was adjusted to 1×106 cells/ml. Each cell suspension of the spleen was divided into two wells and added in 24 wells culture plate with 1 ml per well. 100 μL P. multocida antigen was added in one well and another well was used as a control. After 48 h incubation period at 37°C in a 5% CO2 conditions, the supernatants were harvested, and stored at -20°C until analyzsed to measure cytokine production [14].
Quantitative expression of IL-6 and IL-12 in vivo and in vitro
Cytokine production (IL-6 and IL-12) was quantified in serum and splenocyte cultures by commercial ELISA kits (Eastbiopharm–USA) following the manufacturer’s instruction. Samples were read and 450 nm using spectrophotometer. Results were expressed as pg/ml.
Statistical analysis
Statistical analysis was performed using the Graph-Pad program PRISM 6.0. Interleukin titers were resembled by variance analysis (ANOVA) with repeated measurements. The significant differences between the means in a similar group the paired Student’s t-test were used. The P- value of <0.05 was taken as the level of significance.
Results
Vaccine purity, sterility, and safety
The content of bDNA was found to be 250 ng/μL for P. multocida (A), 210 ng/μL for P. multocida (B) and 320 ng/μL for S. typhimurium that were used as adjuvants. Bacterial DNAs extracted were found to be free from proteins and RNA as measured by the A260/A280 ratio (=1.8) (Figure 3).
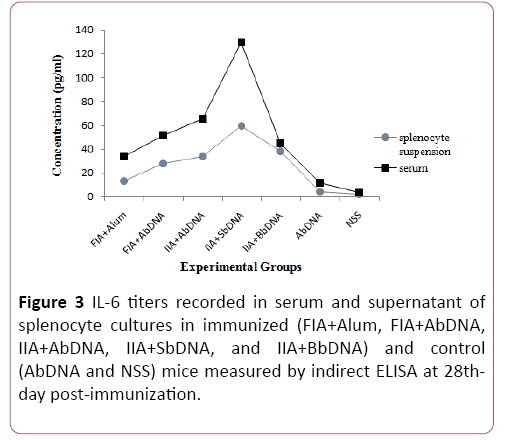
Figure 3: IL-6 titers recorded in serum and supernatant of splenocyte cultures in immunized (FIA+Alum, FIA+AbDNA, IIA+AbDNA, IIA+SbDNA, and IIA+BbDNA) and control (AbDNA and NSS) mice measured by indirect ELISA at 28thday post-immunization.
The instant inactivated vaccines of P. multocida were free from any bacterial and fungal contamination when grown on diverse media such as BA, NA, and BHI broth. The pure vaccine showed no growth on these media and confirmed to be safe after the inoculation of Balb/c mice without any morbidity or mortality.
The injection sites were monitored after injection during the trial. Any modification of animal behavior per group could be mentioned. No systemic or local (injection site) adverse reactions were recorded after vaccination in any group. IL-6 and IL-12 cytokine release induced by vaccine formulations
IL-6 and IL-12 cytokine titers measured by ELISA in serum and splenocyte cultures from mice immunized with different vaccine formulations are shown in Figures 1-4. Cytokine measurement of spleen culture supernatants demonstrated that animal vaccinated with vaccine formulation containing iron inactivated bacteria significantly enhanced cytokine titers (P=0.0206 for IL-6 and P=0.0245 for IL-12), in comparison to animals vaccinated with formulations containing formalininactivated bacteria and control groups (Figure 4).
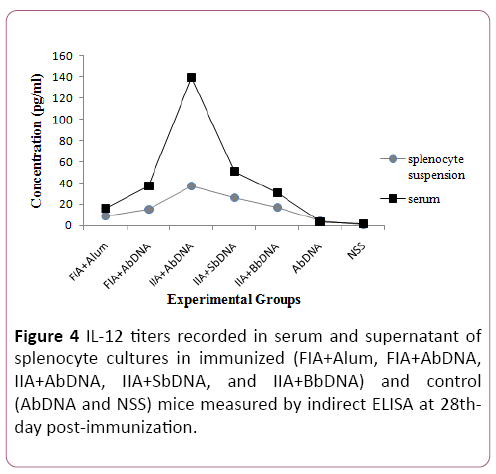
Figure 4: IL-12 titers recorded in serum and supernatant of splenocyte cultures in immunized (FIA+Alum, FIA+AbDNA, IIA+AbDNA, IIA+SbDNA, and IIA+BbDNA) and control (AbDNA and NSS) mice measured by indirect ELISA at 28thday post-immunization.
The maximum IL-6 titers were observed among the groups injected with bDNAs vaccines in the IIA+SbDNA group in both the serum and supernatants samples. These titers were 129.2 and 59.8 pg/ml for serum and splenocyte suspensions, respectively. The serum IL-12 levels in the IIA+AbDNA vaccine group were significantly higher than those in all other vaccine and control groups (P=0.0001).
Also, serum cytokine levels were significantly (p<0.01) higher than that of the immunized mice when compared to control mice. The peak of IL-12 in spleen culture supernatants (37.5 pg/ml) was reached in the IIA+AbDNA group. Control groups showed no or lower cytokine levels than the immunized groups of the vaccines for both serum and splenocyte suspensions.
Discussion
Bacterial DNA can stimulate macrophages, monocytes, and dendritic cells that then produce various cytokines, including the Th1 related cytokines such as IL-12, IFN-γ [15,16].
To understand the immune stimulatory effect of bDNA adjuvants, it is essential to consider
cytokine functions. The present study was conducted in mice to investigate the adjuvants effects of different groups of bDNA adjuvants included in diverse P. multocida serotype A formulations, by measuring two pro-inflammatory cytokines in serum and splenocyte culture supernatants.
Despite the advanced technological developments in vaccine production, most vaccines depend on the cooperation with adjuvant materials [5,17].
An important function of vaccine adjuvants is to modulate the type of immune response induced by the antigen, for example by enhancing the cell-mediated immunity conferred by the vaccine. The association of various adjuvant substances aims at combining their attributes responsible for stimulating the immune system [5]. It has been shown that bacterial DNA with CpG motifs is taken by cells and interacts with TLR9 in endosomal vesicles, triggering a signaling cascade that effects cellular activation [18]. CpG ODN has been used as a vaccine adjuvant in animal studies because it elicits Th1-type cellmediated immunity, which indicates an increase in IFN-γ by binding to TLR9 [4]. However, bacterial DNA can induce the production in vitro and in vivo of a diversity of proinflammatory cytokines [15]. Cytokines also have a key role in host advocacy responses against bacteria [2].
In the current study, we investigated the adjuvant effects of P. multocida A, P. multocida B and Salmonella bacterial DNA by my measuring cytokine release induced in vivo and in vitro following immunization of Balb/mice with different vaccine formulations. Furthermore, we compared the use of iron instead of formalin as an inactivating agent for the preparation of killed antigen. While the formalin reaction is reversible, there is always the amount of formalin remaining in vaccines prepared with formalin. However, at high concentrations, heavy metal ions (such as iron) react to create toxic compounds in bacterial cells. Iron can precipitate proteins in the cell walls of bacteria and destroy nucleic acids by free radical reactions [11,12]. We inactivated P. multocida by 10 mM FeCl3 that resulted in total inactivation of P. multocida within 24 h.
To determine whether bDNA is better than alum to induce immunity, we evaluated its adjuvant capacity of bDNA and alum in FIA+Alum, FIA+AbDNA, and IIA+AbDNA vaccine groups. Herath et al. (2010) vaccinated chickens against P. multocida with formalin and iron-inactivated vaccine (FIV and IIV). FIV was adjuvanted with 0.5% alum and IIV was adjuvanted with 5 μg of bDNA and 10 μg of bDNA. They advised thatIIV-10 μg bDNA vaccine protected 100%, IIV-5 μg bDNA vaccine protected 75% and FIV-Alum vaccine protected 62.5% of the chickens [12]. We found that iron inactivated vaccines were superior to formalin killed preparations, unexpectedly when the bacterins were injected with bDNA adjuvants. All iron inactivated vaccine groups showed higher than the titer of splenocyte suspension cytokines compared to the control and formalin-inactivated vaccine groups.
Also, the levels of serum IL-6 and IL-12 in the FIA+Alum group were lower than those of the other vaccinated groups post-immunization. As regards lymphocyte proliferation assay, a significant difference was observed in the stimulation index of bDNA adjuvant vaccinated groups as compared to the alum adjuvant group. Infection of P. multocida with intra tracheal inoculation induces the mRNA expressions of proinflammatory cytokines (TNF-α, IL-1, IL-6, IL-8, and IL-12), and increases the number of neutrophils in the lungs of cattle [2]. Praveena et al. [1] reported that in infected mice, serum cytokine profiles showed significantly (p<0.01) higher than those of pro-inflammatory cytokines (TNF-α, IL-1b, IL-6 and mouse KC) when compared to control mice. TNF-α level was found to be higher at 12 (0.15 ng/ml) and 24 (0.26 ng/ml) h after infection and IL-6 concentration was found to be increased at 36 h after infection (0.36 ng/ml) and remained higher until late hours of infection [1].
In the study of Krieg et al. (1998), mice that were injected with bacterial DNA or synthetic oligodeoxynucleotides (ODN) containing CpG motifs responded with rapid production of IFN- γ and IL-12. They reported that the serum IL-12 levels were increased for at least 8 days after a single injection of CpG ODN, but IFN-γ levels returned to baseline within 24 h [10]. Although the exact mechanism of action is still unclear, SbDNAmay stimulate the production of cytokines, in particular, IL-6. There are some similarities between the levels of the serum and splenocyte suspension cytokines. Till now, there has been no report on adjuvanticity of SbDNA on P. multocida infection. Our results indicate that SbDNA adjuvanted formulations significantly increased serum IL-6 titer after vaccinations, while the peak serum IL-12 titer wasfound in the AbDNA adjuvant group. Kumar et al. [11], also found that immunized mice with DNA adjuvanted vaccines produced a higher level of antibody and homologous bDNA can protect mice from Salmonella typhimurium [11]. We conclude that the homologous bDNA (AbDNA) is a potent adjuvant in the immune response with the production of higher serum and splenocyte suspension IL-12 levels.
Increasing the serum cytokine levels of pro-inflammatory cytokines is associated with the observation of cellular infiltrated in the lungs and liver. It has been reported that these cytokines increase transmigration of leukocyte the site of infection by favoring the expression of cell adhesion molecules and production of prostaglandins that perpetuated the inflammatory cascade in septicemia [1].
Conclusion
In conclusion, a mouse model of cell mediates immunity against P. multocida infection is successfully confirmed by P. multocida serogroup A strain vaccine in this study. The immune response in mice against P. multocida vaccine grown in the presence of serum was compared with splenocyte suspension using ELISA. Our observations suggest that the use of serum samples of mice stimulated with the P. multocida antigen to detect IL-6 and IL-12 levels would provide higher titers of these cytokines and are better than of splenocyte cultures. The higher IL-6 and IL-12 levels recorded in mice that received vaccine formulations compared to control
Groups showed that the increase in IL-6 and IL-12 titers was antigen-specific. Therefore, our study demonstrated that antigens with bDNA could significantly enhance the immune responses in mice.
The results showed some heterologous bDNA such as Salmonella sp. bDNA can be enhanced the immune response caused by the CpGmotif against P. multocida infections. The immunostimulatory effect of SbDNA on both serum and splenocyte culture supernatants observed in this study can be linked to its effects on cells such as macrophages that production of cytokines such as IL-6.
Finally, our findings suggest that the novel adjuvant bDNA is efficient adjuvants that have been shown to stimulate an immune response in mice. Pasteurella multocida bacterin plus bDNA adjuvanted formulations could induce the release of both IL-6 and IL-12 in a type-dependent manner. The use of other bacterial DNAs can contribute to the effect of inactivated vaccines, which act as an immune-stimulant.
Conflict of Interest
Authors declare no conflict of interest.
Acknowledgments
We thank Dr. M. Hayati (Molecular Biology Department), Dr. M. Namavari (Cell Culture Department) and Mr. S. Sadeghzadeh (Lab Animal Department) for their technical assistance. This study was supported by the Razi Vaccine and Serum Research Institute and in part by Grant number 12-18-18-9458-94014.
26521
References
- Praveena PE, Periasamy S, Kumar AA, Singh N (2010) Cytokine profiles, apoptosis and pathology of experimental Pasteurella multocida serotype A1 infection in mice. Research in Veterinary Science 89: 332-339.
- Wu C, Qin X, Li P, Pan T, Ren W, et al. (2017) Transcriptomic Analysis on Responses of Murine Lungs to Pasteurella multocida Infection. Front Cell Infect Microbiol 7: 251.
- Petrovsky N, Aguilar JC (2004) Vaccine adjuvants: Current state and future trends. Immunology and Cell Biology 82: 488-496.
- Okay S, Özcengiz E, Gürse I, Özcengiz G (2012) Immunogenicity and protective efficacy of the recombinant Pasteurella lipoprotein E and outer membrane protein H from Pasteurella multocida A:3 in mice. Research in Veterinary Science 93: 1261-1265.
- Fischer G, Cleff MB, Dummer LA, Paulino N, Paulino AS, et al. (2007) Adjuvant effect of green propolis on humoral immune response of bovines immunized with bovineherpesvirus type 5. Vet Immunol Immunopathol 116: 79-84.
- Freitas E, Marinho AC, Albuquerque D, Teles L, Sindeaux M, et al. (2013) Adjuvant activity of peanut, cottonseed and rice oils on cellular and humoral response. VacciMonitor 22: 4-9.
- Mapletoft JW, Oumouna M, Kovacs-Nolan J, Latimer L, Mutwiri G, et al. (2008) Intranasal immunization of mice with a formalin-inactivated bovine respiratory syncytial virus vaccine co-formulated with CpG oligodeoxynucleotides and polyphosphazenes results in enhanced protection. Journal of General Virology 89: 250-260.
- Tanwar H, Yadav AP, Brijbhushan Sh, Singh SB (2016) Immunity against Pasteurella Multocida in Animals Vaccinated with Inactivated Pasteurella Multocida and Herbal Adjuvant ‘DIP-HIP’. J Vaccines Immun 2: 010-014.
- Homayoon M, Tahamtan Y, Kargar M, Hosseini SMH, Akhavan SA (2018)Pasteurella multocida inactivated with ferric chloride and adjuvanted with bacterial DNA is a potent and efficacious vaccine in Balb/c mice. Journal of Medical Microbiology 67: 1383-1390.
- Krieg AM, Love-Homan L, Yi AK, Harty JT (1998) CpG DNA Induces Sustained IL-12 Expression In Vivo and Resistance to Listeria monocytogenes Challenge. The Journal of Immunology 161: 2428-2434.
- Kumar D, Singh A (2005) Salmonella typhimurium grown in iron-rich media, inactivated with ferric chloride and adjuvanted with homologous bacterial DNA is potent and efficacious vaccine in mice. Vaccine23: 5590-5598.
- Herath C, Kumar P, Singh M, Kumar D, Ramakrishnan S, et al. (2010) Experimental iron-inactivated Pasteurella multocida A:1 vaccine adjuvanted with bacterial DNA is safe and protects chickens from fowl cholera. Vaccine28: 2284-2289.
- Cheng HR, Jiang N (2006) Extremely rapid extraction of DNA from bacteria and yeasts. BiotechnologyLetters28: 55-59.
- Micallef M, Hosokawa M, Shibata T, Nakane A, Yang Z, et al. (1991) Immunoregulatory cytokine release in rat spleen cell cultures after treatment with bleomycin and its analogues in vivo. Cancer Immunol Immunother 33: 33-38.
- Weiner GJ, Liu HM, Woolderidge JE, Dahle CE, Krieg AM (1997) Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA 94: 10833–10837.
- Trevani AS, Chorny A, Salamone G, Vermeulen M, Gamberale R, et al. (2003)Bacterial DNA activates human neutrophils by a CpG-independent pathway. Eur J Immunol33: 3164-3174.
- Arous JB, Deville S, Pal JK, Baksi S, Bertrand F, et al. (2013) Reduction of Newcastle disease vaccine dose using a novel adjuvant for cellular immune response in poultry. Procedia in Vaccinology 7: 28-33.
- Ahmad AM (2014) Efforts towards the development of recombinant vaccines against Pasteurella multocida. Science World Journal 9: 1-7.









