Keywords
Botulinum toxin-A; Gummy smile; Lip-drop
Introduction
The most complicated and most pleasant facial expression is the smile among all of human facial expressions [1]. The smile is the principal facial expression of good mood, pleasure and happiness [2]. The contraction of the muscles in the lower and middle parts of the face take place during smiling, resulting in the display of teeth and gums [3]. During smiling, the lips, teeth and gingiva are all in harmony and contribute to the aesthetic appearance of the individual. Disturbances in this harmony affect the external appearance of the individual, reducing his or her self-confidence, which affects the individual's social life and psychology. A smile is not attractive when the mouth is closed by the hands, or when an individual makes an effort to smile too much. An aesthetic smile can only be achieved if many factors are aligned with each other. The forms of the lips, gingival factors, negative spaces and smile symmetry are among these factors involved with an aesthetic smile [4]. Extreme gingival display or gummy smile (GS) is defined in the literature as a 2 mm appearance of the gingiva above the teeth during smile [5]. In 1974, Rubin classified the types of smiles into 3 categories. A Mona Lisa smile is dominated by the zygomaticus major (ZM) muscle and is defined by sharply elevated corners. A “canine smile” is characterized by the elevation of the medial portion of the upper lip by a prominent activity of the levator labii superioris muscle (LLS). The latter, a “full denture” smile is dominated by all of the upper retractor muscles in addition to the lower depressors, resulting in a smile that exposes all teeth [6]. Etiological factors involved in the formation of GS is the short lip length, extreme lip activity, short clinical crowns, altered passive eruption, dento-alveolar extrusion, and excessive growth of the maxilla in the vertical direction [7]. Gingivoplasty, orthodontic treatment, orthognathic surgery and bone resection are applied treatments for the GS [1]. These highly complex methods are expensive, associated with long-term demands and may lead to moderate-serious side effects [8]. On the other hand, aesthetic correction of GS with OnabotulinumtoxinA (ONA) is a simple, rapid and effective method [9]. The vectors of the Levator labii superior (LLS), Levator labii superior alaque nasi (LLSAN) and zygomaticus minor (ZMi) muscles form a triangle. In females, the mean horizontal distance from the center of the triangle to the nasal ala is 10.5 mm; and the mean vertical distance from the center to the upper lip corner is 32.1 mm. In males, the mean horizontal distance from the center of the triangle to the nasal ala is 10.2 mm, and the mean vertical distance from the center to the upper lip corner is 32.1 mm. These points in females and males are used to determine the injection site. These points provide safe and effective indicators to determine the injection site for patients with excessive gingival display [10]. Botulinum toxin (BoNT) has been used since 1970 for medical conditions including pain and excessive muscle contraction. BoNT is produced by Clostridium botulinum, an anaerobic bacterium. BoNT has 8 serotypes. Among them, the most effective and most widely used one is Type-A (BTXA) [11]. BoNT inhibits acetylcholine release from presynaptic vesicles at the neuromuscular junction. Thus, it leads to muscle paralysis, which is depends on the injected dose and is reversible. Following the 2 weeks from the day of injection, the effectiveness of BoNT reaches its maximum. The effect of the toxin is terminated by the development of axonal sprouting [12]. The purpose of this study was to evaluate to change of the gingival display and to evaluate the lip-drop following 2.5 U BTXA injection in 8 female patients with GS.
Materials and Methods
This study was performed on 8 female patients with GS due to different etiologies. All patients had complaints of excessive gingival display on smiling. As measured in the photographs of the patients taken for a preliminary diagnosis, all patients had more than 3 mm gingival appearance on smiling. The patients were informed about BoNT and its potential complications. Patients reported no drug use, such as albumin, BoNT, antibiotics, or any other anti-allergy medicine. The patients had no neuromuscular problems and no peripheral motor neuropathies. Patients were not pregnant or nursing. In addition, the distance of the upper lip downwards to the border of the incisal and coronal edges of the central incisor during maximum smiling before the injection was measured with a periodontal probe and it was photographed (Figure 1).
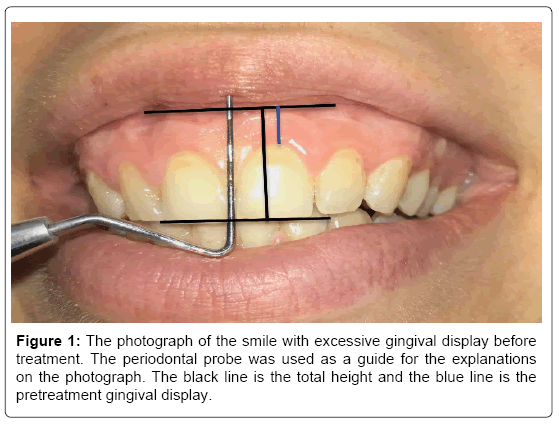
Figure 1: The photograph of the smile with excessive gingival display before treatment. The periodontal probe was used as a guide for the explanations on the photograph. The black line is the total height and the blue line is the pretreatment gingival display.
Patients were informed about the harms and benefits of BTX-A (Botox; Allergan Inc, Westport, Ireland). BTX-A is dispensed as a freeze-dried powder of 100 U. It was reconstituted with 2.5 cc saline (% 0.09) solution to make 4.0 U/0.1 cc dose according to manufacturer’s instructions. Injection sites were determined by palpation, identifying the location of the muscles precisely. All of the patient injections were made in the middle of the triangle formed by LLS, LLSAN, and Zmi bilaterally with a dose of BTX-A of 2.5 U. The patients were advised not to lie down, massage or touch the injection area and not to exercise in the 5 hours following the procedure. The patients were called for the follow-up visits on the 15th day after injection to assess any postinjection changes. 15 days later following the procedure, the patients were examined in the visits and post-injection photos were taken at the same position which the pre-injection photos were taken (Figure 2).
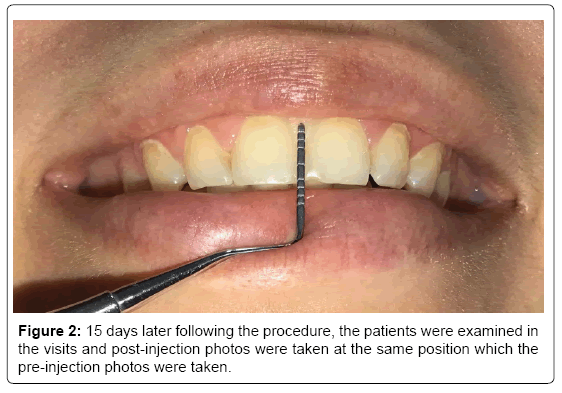
Figure 2: 15 days later following the procedure, the patients were examined in the visits and post-injection photos were taken at the same position which the pre-injection photos were taken.
In addition, the distance between the lower border of the upper lip and the incisal border of central incisor was measured by a periodontal probe during maximum smiling to determine the amount of change in the gingival display after the injections. The measurements were made by using mac os photo editing tool on the photographs taken before and after the procedure. To determine the total length of the gingival display, a vertical line was drawn on the central incisor from the incisal border, passing through the zenith, then upwards to the inferior border of the lip during smiling. Then, the central incisor crown height values were extracted from the total lengths by using the before and after photos to calculate the amount of gingival display during the smile. In addition, in order to calculate the amount of the lip drop, the apparent length of the central crown height was extracted from the total height by using after photos. Our aim in this study was to collect information about the responses of the muscles to the same dose of BTX-A and to assess whether there is a correlation between the amount of the upper lip drop and the amount of gum displayed during smiling.
Statistical analysis
Data were collected and analyzed using Statistical Package for the Social Sciences software (version 24, Chicago, Illinois, USA). The normality test was performed with the Shapiro-Wilk test since the groups had means of descriptive statistics and the number of patients in the study was low. Descriptive statistics were used to describe the mean percentage of the improvement in the gummy smile, the amount of the lip drop and total heights. Correlation between the two groups was evaluated by the Pearson correlation coefficient as the groups showed normal distribution. Pearson correlation analysis was considered to be statistically significant at p<0.05.
Results
The mean age of the total of 8 female patients in the study was calculated as 25.87 ± 3.97, in a range from 22 to 34 years of age. For the gummy smile treatment, 2.5 U BTX-A was injected bilaterally in the middle of the triangle where the LLSAN, LLS, and Zmi muscles were intersected. Patients did not develop any abnormal findings such as swelling, redness, bruise or infection after injection. Patients were called for the follow-up visits 15 days later following the injection. The mean pre-treatment and post-treatment measurements of gingival display, upper lip drop length and total length are listed in Table 1.
| |
N |
Minimum |
Maximum |
Mean |
Std. Deviation |
| Pretreatment Gingival Display |
8 |
3.23 |
6.80 |
4.6275 |
1.17956 |
| Posttreatment Gingival Display |
8 |
-1.17 |
-0.09 |
-0.6775 |
0.37232 |
| Lip Drop Length |
8 |
4.18 |
7.04 |
5.1750 |
0.88335 |
| Total Length |
8 |
7.92 |
14.20 |
11.0788 |
1.88003 |
Table 1: The mean pre-treatment and post-treatment measurements of gingival display, upper lip drop length and total length.
The mean amount of gingival display before the treatment was 4.62 1.17 mm during maximum smiling. As observed in the followup visits, the gingival display disappeared during maximum smiling in all patients with a mean amount of lip drop of 5.17 ± 0.88 mm. A statistically significant correlation was found between the amount of the pre-treatment gingival display during maximum smiling and the amount of lip drop (Table 2). Therefore, we can argue that injection of 2.5 U BTX-A to 1 cm lateral of LLSAN is enough to correct the gingival display in a range from 5 to 6.5 mm, and the amount of the lip drop is correlated with the amount of the pre-treatment gingival display in gummy smile patients.
| Lip Drop (mm) |
Pearson Correlation |
1 |
0.888** |
| Sig. (2-tailed) |
|
0.003 |
| N |
8 |
8 |
| Pre-Treatment Gingival Display (mm) |
Pearson Correlation |
0.888** |
1 |
| Sig. (2-tailed) |
0.003 |
|
| N |
8 |
8 |
| **Correlation is significant at the 0.01 level (2-tailed) |
Table 2: Correlation of between the amount of the pre-treatment gingival display during maximum smiling and the amount of lip drop.
Discussion
When Botulinum toxin type-A is injected into the muscle, it is taken up by the presynaptic nerve terminals by endocytosis in the neuromuscular joint, leading to an inhibition of the release of acetylcholine. The fusion proteins are affected, and it causes chemical denervation intrinsically, leading to a temporary paralysis [13]. Generally, the effect reaches its maximum level at two weeks and lasts for approximately 3 months. The complete recovery with complete re-innervation develops in the 6th month following the injection [13]. As the maximum effect develops two weeks later following the injection, we have invited the patients for the follow-up visits two weeks later than injection. Although BoNT has been reported to have a high tolerability; allergic, immunologic and local complications can be observed. No allergic reactions were encountered until this time, although theoretically it was thought that allergic reactions could occur in patients because albumin was used in the preparation of BTX-A [14]. Complications and side effects; may be classified as either injectionrelated or associated with BTX-A. Injection-related complications may include pain, bruising, erythema, edema, tenderness, headache, infection, numbness, vasovagal attack and loss of consciousness. Complications related to BoNT in therapeutic doses are asphyxia, weakness in facial muscles, asymmetry in limb movements, xerostomia, affected smile and mimic movements, limited mouth opening, double vision, weakness in swallowing, jaw dislocation and voice changes [14,15]. In our study, we did not observe any of the complications mentioned in our study, following the injections in any of the 8 patients. The facial muscles responsible for the up and lateral movement of the lips are the LLSAN, ZM, ZMi, risorius muscles. The muscle, which is associated less is the depressor septi nasi. All of these muscles are associated with orbicularis oris, which create the smile [6]. Rubin et al. described the mechanism of the smile in their cadaver studies [8]. Polo recommends botulinum toxin-A injection for every elevator muscle at doses ranging from 0.625 to 2.5 IU [16]. Kane recommends a total of 5 IU BTX-A injections for gummy smile treatments on each side of the LLSAN muscle, with a starting dose of 1 IU, followed by subsequent doses in 2-3 weeks. Recently Hwang et al. have proposed an injection point for botulinum toxin-A and named it as YONSEI POINT and they recommended a dose of 3 U BTX-A injection at each Yonsei point. They reported that this was the sufficient dose for GS treatment [17]. Referencing Hwang et al., we injected 2.5 U BTX-A bilaterally on the face on Yonsei points.
In a study of 30 patients diagnosed with GS, BTX-A injection into the upper lip elevator muscles was performed and patients were followed up for 2 to 24 weeks. Patient satisfaction was questioned in the follow-up visits and smile profile was checked with standard photography. The gingival display before injection was 5.2 mm, while at the end of 2 weeks it decreased to 0.9 mm. The values did not reverse at baseline levels in 24 weeks, but BTX-A activity was lost at the end of 30-32 weeks [8]. Also, another study performed on 14 patients diagnosed with gummy smile reported that the display of gingiva was decreased to 4.14 mm at end of 2 weeks [18]. The recommended dosage of BTX-A is 1.95 U for a mean gingival display of 3.62 mm, whereas the recommended dosage of BTX-A is 5 U for gummy smile patients with 7 mm gingival displays [19,20]. Rosemarie et al. performed a study on 16 patients with gummy smiles, and they reported that an injection of BTX-A into the elevator muscles of the lip lead to a reduction of approximately 75.09% in the amount of the gingival display [21]. Sathyanarayanan et al. reported that performing a 2.5 U BTX-A injection into the LLSAN, decreased the gingival display by 3-8 mm in 5 patients with gummy smiles [22]. In our study, we observed a mean reduction of 5.17 ± 0.88 mm in the gingival display after injection of BTX-A in the Yonsei points of 8 patients with gummy smiles. These amounts found in our study correspond to the amounts of lip-drop. Application of BTX-A for treatment of GS is effective, safe, easy to apply and with a low-risk profile. The effect is temporary. Probably the most remarkable characteristic of this method, among all, is the rapid cosmetic effect obtained after the procedure. Therefore, application of BTX-A is considered to be the first treatment option in GS [20]. There are various options in the treatment of the gummy smile, which is caused by an overactive upper lip. Most of the treatment options for GS are aggressive surgical procedures. BTX-A is used as a non-surgical method, which is highly effective in the treatment of GS caused by overactive upper lips. Moreover, the possible complications of this method are less than the other surgical methods. The results are very satisfactory for patients, too. The main disadvantage of this method is that the process is repeated at regular intervals. The use of botulinum toxin is much less invasive with less frequent complications, and the result is quite satisfactory for patients when compared to other methods used in the treatment of gummy smile.
Conclusion
Most of the treatment options for GS are aggressive surgical procedures. BTX-A is an effective and safe non-surgical method for the correction of the GS, when the correct injection points, and the appropriate doses are respected. The results are very satisfactory for patients, too. The main disadvantage of the BTX-A treatment for GS is it's being temporary. It takes a short time to perform and the process is repeated at regular intervals. The use of botulinum toxin is much less invasive, and the development of complications is less frequent with quite satisfactory results for patients compared to the other methods used in the treatment of GS. The authors conclude that in the cosmetic correction of GS, the amount of the mean lip-drop is 5-6 mm and there is a positive correlation between the lip-drop and pretreatment gingival display. Clinical trials with more patients would be useful to confirm the results presented in this article.
21814
References
- Garber DA, Salama MA (1996) The aesthetic smile: diagnosis and treatment. Periodontol11:18-28.
- Peck S, Peck L, Kataja M (1992) The gingival smile line. Angle Orthod62:91-100.
- Baiju CS,Khashu H, Garg A (2010) Smile design- periodontal out look of basics. Journal of Oral Health& Community Dentistry4: 1-3.
- Khanna B (2007) Lip stabilisation with botulinum toxin. Aesthet Dent Today1:54-59.
- Rubin LR (1974) The anatomy of a smile: its importance in the treatment of facial paralysis. PlastReconstrSurg53:384-387.
- Robbins W (1999) Differential diagnosis and treatment is excess gingival display. PractPeriodontAesthet Dent11:265-272.
- Polo M (2008) Botulinum toxin type A (Botox) for the neuromuscular correction of excessive gingival display on smiling (gummy smile). Am J Orthod Dentofacial Orthop133:195-203.
- Carruthers A, Carruthers J (1998) Cosmetic uses of botulinum A exotoxin. In: Klein AW (ed.), Tissue augmentation in clinical practice: Procedures and techniques. Marcel Dekker, New York, USA, pp: 207-236.
- Priya PR, Megha AD, Pratibha PP, Ashwini D (2015)To clinch a point for injection of a botulinum toxin for aesthetic smile. Research Journal of Pharmaceutical, Biological and Chemical Sciences6:1515.
- Dolly PP, Sandip AT, Jaymin RS (2012) Adjunctive Treatment of Gummy Smile Using Botulinum Toxin Type-A. Int J Med Dent Sci3: 22-29.
- Dolly O (2003) Synaptic transmission: inhibition of neurotransmitter release by botulinum toxins. Headache43:16-24.
- Rossetto O, Seveso M, Caccin P (2001) Tetanus and botulinum neurotoxins: turning bad guys into good by research. Toxicon39:27-41.
- Majid OW (2010) Clinical use of botulinum toxins in oral and maxillofacial surgery. International Journal of Oral and Maxillofacial Surgery39:197-207.
- Small R, Hoang D (2015) A Practical Guide to Botulinum Toxin Procedures. In: Gül Ü (ed.), Botulinum ToksinUygulamalari. 1 Baski Ankara: DünyaTipKitapevi, pp: 20-21.
- Kane MA (2003) The effect of botulinum toxin injections on thenasolabial fold. PlastReconstrSurg112:66S-72S.
- Hwang (2009) Surface anatomy of the lip elevator muscles for the treatment of gummy smile using botulinum toxin. Angle Orthod79:70-77.
- Jessica SS, Trish PD, Melanie DP,Paul D, Smith MD (2014) OnabotulinumtoxinA for the Treatment of a “Gummy Smile”. Aesthetic Surgery Journal34: 432-437.
- Sucupira E, Abramovitz A (2012) A simplified method for smile enhancement: botulinum toxin injection for gummy smile. PlastReconstrSurg130:726-728.
- Polo MA (2013) Simplifiedmethod for smile enhancement: botulinum toxin injection for gummy smile. PlastReconstrSurg131:934e-935e.
- Mazzuco R, Hexsel D, Carazinho C, Allegre P (2010) Gummy smile and botulinum toxin: A new approach based on gingival exposure area. J Am AcadDermatol 63:1042-1051.
- Sathyanarayanan R, Karthikeyan R (2012) Role of Botulinum toxin in the management of vertical maxillary excess. Pakistan Oral & Dental Journal32: 1.








