Keywords
|
| Squamous cell carcinoma, HEP-2 cell line, Wharton’s jelly mesenchymal stem cells, Real-time PCR, p53, Bcl-2 |
Abbreviations
|
| cDNA: Complementary DNA; ct: Cycle Threshold; EGF: Epidermal Growth Factor; HNSCC: Head and Neck Squamous Cell Carcinoma; hWJSCs: Human Wharton’s Jelly Mesenchymal Stem Cells; MHC: Major Histocompatibility Complex; MMPs: Matrix Metalloproteinases; MSC-CD: Mesenchymal Stem Cell- Cluster of Differentiation; PI: Propidium Iodide; PCR: Polymerase Chain Reaction; RQ: Relative Quantification; SCID: Severe Combined Immunodeficiency; VEGF: Vascular Endothelial Growth Factor; WJMSCs: Wharton’s Jelly Mesenchymal Stem Cells; WJMSCs-CM: Wharton’s Jelly Mesenchymal Stem Cells-Conditioned Medium |
Introduction
|
| Head and neck squamous cell carcinoma (HNSCC) accounts for over 90% of all head and neck cancers [1]. Unfortunately, the mortality rates for this disease have remained unchanged over the past 40 years despite advances in the delivery of treatment and surgical reconstruction. Patients with HNSCC have a 60% mortality rate even with standard therapy, including radiotherapy, surgery, and/or chemotherapy [2]. |
| Cancer ranks among the main causes of death worldwide [3] despite advances in research and treatment options. Certain factors, including cancer aggressiveness and metastatic potential as well as defense host mechanism partly cause the poor outcome in the treatment of various types of cancers. Surgery, chemotherapy, and radiotherapy do not provide enough protection and, instead, affect normal as well as malignant cells [4]. |
| The search for a cancer cure from natural products (plants and animals) has been ongoing for over a century and purified chemicals are still used to treat cancer [5]. Researchers have developed new targeted therapies against cancer by understanding the molecular mechanisms and by identifying signaling pathways that drive oncogenesis [6,7]. Some of these anti-cancer therapies inhibit vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) receptors as well as matrix metalloproteinases (MMPs), whereas others promote cell death [8]. |
| The umbilical cord links the fetus to the placenta. It is typically composed of two arteries and a vein suspended in a hydrated extracellular matrix known as Wharton’s jelly. Two veins are originally present, but the second one usually atrophies during pregnancy [9]. Wharton’s jelly mesenchymal stem cells (WJMSCs) are more primitive than those isolated from other tissue sources and do not express the major histocompatibility complex (MHC) class II (HLA-DR) antigens [10]. A relatively large number of MSCs can be harvested, propagated without any feeder cells, and stored after birth without any risk to the donor [11]. Studies showed that WJMSCs are unique in that they have their own mesenchymal stem cell-cluster of differentiation (MSC-CD) signature. In addition, besides being hypo-immunogenic, these cells can maintain stemness characteristics for extended periods in vitro (up to 10 passages), can be converted into several desirable tissues, and do not cause tumorigenesis in immunocompromised mice [12-15]. |
| Wharton’s jelly mesenchymal stem cells may be more useful for cancer therapy than other adult stem cells since they are easily prepared in relatively large quantities from the umbilical cord after delivery. Moreover, the extracorporeal nature of this source facilitates isolation by eliminating invasive procedures and patient discomfort [16]. These mesenchymal cells are potentially quite applicable to human patients without a complete genetic match since they are unlikely to induce an acute inflammatory response [17]. Biochemical and immunohistochemical studies show that they possess some MSC markers that are highly expressed (CD105, CD90, CD44), whereas cell-surface markers for embryonic stem cells (Tra-1-60, Tra-1-81, and SSEA-4) are expressed at low levels [18]. Troyer and Weiss [19] studied the immune properties of human Wharton’s jelly mesenchymal stem cells (hWJSCs) using immunology protocols and concluded that these was no evidence to suggest the immune rejection of undifferentiated hWJSCs in vivo and that these cells would be tolerated well in allogeneic transplantation settings. Further, injection of WJMSCs into immune-deficient mice did not produce teratomas [18]. The versatility and availability of umbilical cord stem cells make them a potent resource for transplant therapies for various diseases, including cancer [20]. |
| |
| A recent in vivo study [21] demonstrated that mesenchymal stem cells are capable of migrating to sites of tissue injury and inflammation. This characteristic has permitted researchers to use MSCs in gene therapy and to deliver antineoplastic drugs, including apoptotic inducers, cytokines, and interferons to cancer sites to inhibit cell proliferation [22]. Besides acting as vehicles for the delivery of anticancer agents, it has been demonstrated that MSCs interact with tumors and inhibit cell proliferation and cause cell death, depending upon the type of tumors they encounter [23]. |
| Ganta et al., [20] observed that rats with mammary carcinoma showed complete regression of the lesion with no evidence of metastases following intra-tumoral injection of rat umbilical cord matrix-derived stem cells. Similarly, intravenous injection of unengineered human MSCs in a xenotransplant rat model with induced human breast carcinoma led to a decrease in cancer burden as a result of the human MSCs homing to pulmonary metastases [24]. According to a recent report [25], human umbilical cord MSCs inhibited the growth of breast cancer cells in a severe combined immunodeficiency (SCID) mouse model by secreting dickkopf and suppressing the WNT signaling pathway. In a more recent report, Gauthaman et al., [26] examined the effect of WJMSC extracts (conditioned medium) on three cancer cell lines: breast adenocarcinoma (MDA-MB-231), ovarian carcinoma (TOV-112D), and osteosarcoma (MG-63). They found that all three cancer cell lines exhibited cell shrinkage, blebbing, and vacuolation suggestive of apoptosis. They suggested that WJMSCs possess tumor inhibitory properties—mediated via agents in its extract—that were not specific for mammary carcinoma alone. |
| This study aims to determine the anticancer effect of WJMSCs conditioned medium (WJMSCs-CM) on HEP-2 cell line after 24, 48 and 72 hours using flow cytometry and real time PCR. |
Materials and Methods
|
| Squamous cell carcinoma cell line (HEP-2) and hWJMSCs were supplied from the Cell Culture Department (VACSERA®, Egypt). |
|
Propagation of HEP-2 cell line
|
| Human laryngeal squamous cell carcinoma (HEP-2) cells were obtained from VACSERA® and grown in a sterile tissue 50 cm3 flask in a complete medium containing Dulbecco’s modified Eagle’s medium enriched with 10% fetal bovine serum and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) in 95% air 5% CO2 at 37°C (Figure 1). |
| The cell line was propagated and subdivided into three groups corresponding to the three growth time intervals of 24, 48, and 72 hours. |
|
Propagation of Wharton’s jelly mesenchymal cells
|
| Wharton’s jelly mesenchymal stem cells were obtained from VACSERA® and grown in a sterile tissue 50 cm3 flask in a complete medium containing Dulbecco’s modified Eagle’s medium enriched with 10% fetal bovine serum and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) in 95% air and 5% CO2 at 37°C (Figure 2). The conditioned medium was obtained during the propagation of WJMSCs and stored at 4°C to be used in treating the HEP-2 cell line. |
|
Treatment of HEP-2 cell line with Wharton’s jelly mesenchymal cell-conditioned medium
|
| Wharton’s jelly mesenchymal stem cells were cultured as described above and the WJMSC-conditioned medium extracted. HEP-2 cells were pretreated with a mixture of complete medium containing Dulbecco’s modified Eagle’s medium enriched with 10% fetal bovine serum, antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin), and WJMSC-conditioned medium (1:1) for 24, 48, and 72 hours. The culture medium was replaced every 24 hours. |
| After treatment, the HEP-2 cells were scraped and centrifuged, the supernatant was discarded, and the cell pellet was stored for flow cytometric analysis and real-time polymerase chain reaction (PCR) assessment. |
|
Flow cytometry technique
|
| The ANNEXIN V-FITC kit is an apoptosis detection kit based on the binding properties of annexin V to phosphatidylserine and on the DNA-intercalating capabilities of propidium iodide (PI). |
| • Cell samples were washed with PBS and centrifuged for 5 minutes at 500x at 4°C. |
| • The supernatant was discarded and the cell pellet was suspended in binding buffer |
| • 1 μL of Annexin V-FITC solution and 5 μL of dissolved PI were added to the cell suspension and mixed gently. |
| • The tubes were kept on ice and stored in the dark for 15 minutes. |
| • 400 μL of binding buffer was added and mixed gently. |
| • The cell preparation was analyzed by flow cytometry. |
|
Real-time polymerase chain reaction
|
| • Extraction of RNA |
| This was carried out using the GF-1 Total RNA Extraction Kit (Vivantis Technologies, USA). The extracted RNA was stored at −20°C. |
| • Synthesis of complementary DNA (cDNA) by reverse transcriptase |
| This was carried out using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA). The converted cDNA was stored at −20°C. |
| • Real-time PCR was carried out using the Maxima SYBR Green qPCR Master Mix (Thermo Scientific, USA). The samples were placed inside the machine (StepOneTM Real-Time PCR System) and the thermal cycle was adjusted as below: |
| - One cycle of initial denaturation at 95°C for 10 minutes. |
| - Forty cycles of: |
| 1. Denaturation at 95°C for 15 seconds. |
| 2. Annealing at 60°C for 30 seconds. |
| 3. Extension at 72°C for 30 seconds. |
| • Relative Quantification (RQ) (relative expression) |
| The PCR data obtained in this study included cycle threshold (ct) values of the assessed genes (P53 gene and BcL-2 gene) and the housekeeping (reference) gene. Beta actin is the housekeeping gene used as it is continuously and normally expressed in the cells. In order to measure the expression of a certain gene, a negative control sample was used, and the expression of the target gene was assessed and related to the reference (internal control) gene according to the following equations: |
| 1. Δ Ct sample = Ct assessed gene – Ct reference gene |
| 2. Δ Δ Ct = Δ Ct sample – Ct control gene |
| 3. RQ = 2 – (Δ Δ Ct) |
Results
|
|
Flow Cytometry Results
|
| Control group |
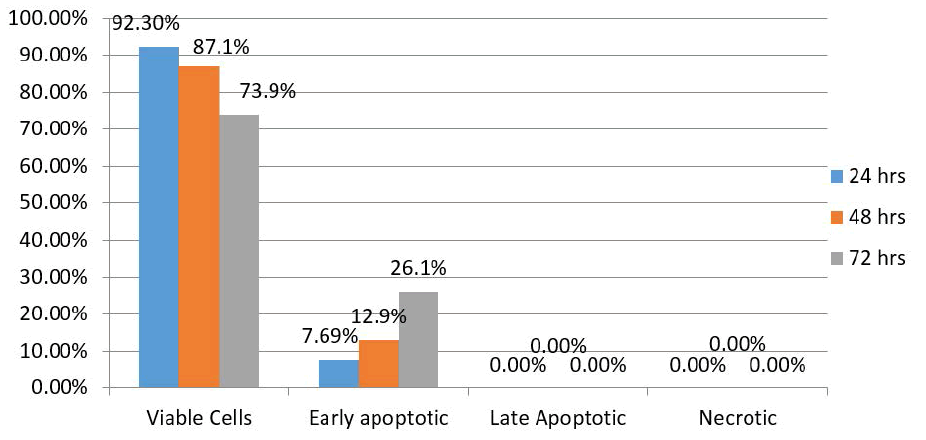
| Results of the control group showed 92.3% of HEP-2 cells were viable 24 hours after treatment, with 7.69% exhibiting early apoptotic changes. There were no late apoptotic changes or necrotic cells. After 48 hours, the proportion of viable cells and those exhibiting apoptotic changes were 87.1% and 12.9%, respectively. In addition, there were no late apoptotic changes or necrotic cells. After 72 hours, however, 73.9% of the HEP-2 cells were viable, while 26.1% exhibited early apoptotic changes. There were no late apoptotic changes or necrotic cells (Figure 3). |
|
HEP-2 treated with Wharton’s jelly mesenchymal cell-conditioned medium
|
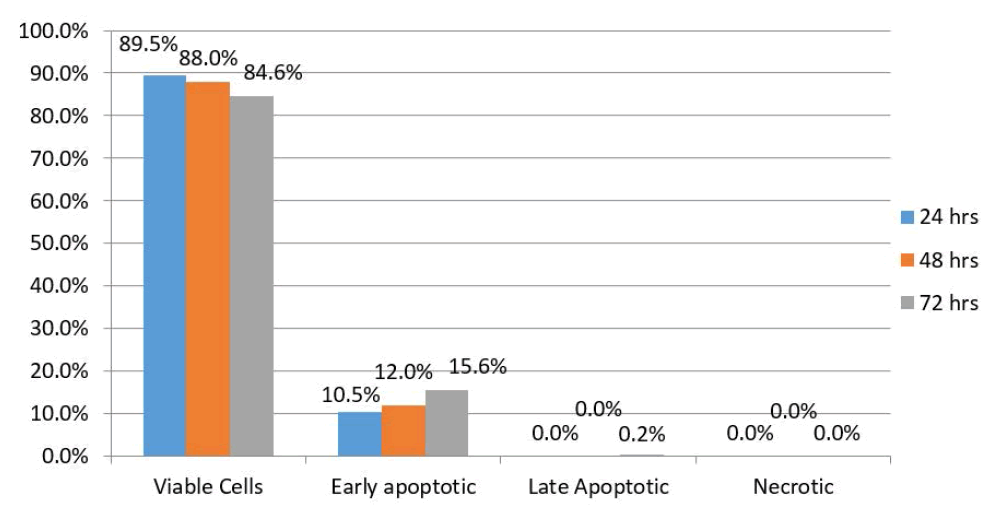
| Results of HEP-2 cells treated with WJMSC-conditioned medium showed that 89.5% were viable 24 hours after treatment while 10.5% exhibited early apoptotic changes. There were no late apoptotic changed or necrotic cells. After 48 hours, this proportion had changed to 88% and 12%, respectively. In addition, there were no late apoptotic changes or necrotic cells. However, after 72 hours, 84.6% of the HEP-2 cells were viable while 15.6% exhibited early apoptotic changes. Furthermore, there were no late apoptotic changes or necrotic cells (Figure 4). |
Polymerase Chain Reaction Results
|
|
Expression of p53
|
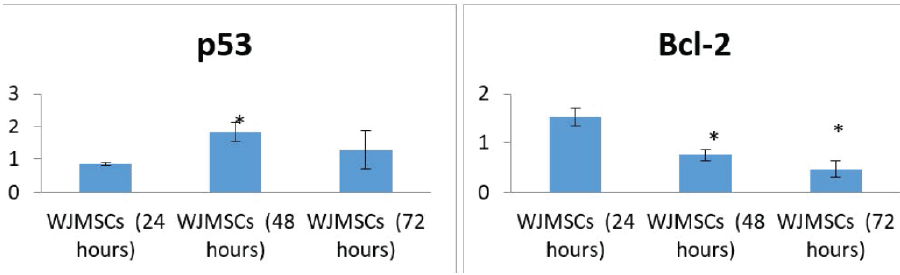
| The expression of p53 was markedly increased after treatment of the cell line with WJMSC-conditioned medium compared with the control group. |
|
Expression of Bcl-2
|
| The expression of Bcl-2 was markedly decreased after treatment of the cell line with WJMSC-conditioned medium compared with the control group. |
Statistical Results
|
| The expression of p53 and Bcl-2 in HEP-2 cells was statistically significant after 48 hours of treatment with WJMSC-conditioned medium. The expression of Bcl-2 was also significant after 72 hours (Figure 5). |
Discussion
|
| Head and neck squamous cell carcinoma (HNSCC) is classified as the sixth most common cancer in the world, with an incidence of 600,000 cases per year and a mortality rate of 50% [3]. The major risk factors for HNSCC are tobacco use, alcohol consumption, and human papilloma virus infection [27]. |
| New insight in the apoptotic process has resulted in the creation of new parameters that can be used to detect and measure apoptosis. One of these parameters is the appearance of phosphatidylserine on cell surfaces. In the early phase of apoptosis, the integrity of the cell membrane is maintained, but the cells lose the asymmetry of their membrane phospholipids [28]. Phosphatidylserine becomes exposed at the cell surface and signals for the recognition and removal of apoptotic cells by macrophages [29]. |
| Loss of plasma membrane asymmetry is one of the earliest features of apoptosis. In apoptotic cells, the membrane phospholipid phosphatidylserine is translocated from the inner to the outer leaflet of the plasma membrane, thereby exposing phosphatidylserine to the external cellular environment [30]. |
| Due to the high sensitivity of this technique, this study used the Annexin-V FITC kit to detect the percentage of apoptosis in the HEP-2 cell line. Moreover, WJMSC-conditioned medium was used for treating the HEP-2 cell line rather than cell co-culturing, which is sometimes unreliable owing to the likelihood of one cell type showing increased growth than the other caused by the culture medium instead of a true anticancer effect [26]. To exclude this possibility, the present study focused on the role of WJMSCconditioned medium rather than the cell itself. |
| Gauthman et al., [26] examined the effect of umbilical cord WJMSC extract on three cancer cell lines: breast adenocarcinoma, ovarian carcinoma, and osteosarcoma. They found that the three cancer cell lines showed different levels of anticancer activity; the effects were most severe on osteosarcoma and least on ovarian carcinoma. The likelihood of each cancer cell type to be inhibited, either through one or several mechanisms, may explain this observation given that the cancer types showed dissimilar phenotypic changes, including shrinking of the cell, fragmentation, blebbing, vacuolation, and incapacity of the cell membrane to acquire many granular particles. |
| Using WJMSC-conditioned medium to treat a HEP-2 cell line resulted in apoptosis at a rate of 10.5%, 12%, and 15.6% after 24, 48, and 72 hours, respectively, as shown in the flow cytometry results. According to the literature, regulatory components in cell extracts affect several signaling mechanisms. These include induction of transcriptional activity, distinctive expression of functional genes, and reprogramming of distinct cell types [31]. Nevertheless, the molecules in the extracts that are assumed to cause cell changes have not been identified [26]. |
| Our results revealed an increased expression of p53 in HEP- 2 cells after 24 hours of treatment. After 48 hours, there was a statistically significant increase in p53 expression, which decreased after 72 hours. Bcl-2 expression also decreased with increasing treatment time, with the greatest decrease noted after 72 hours. The decrease in expression for both the p53 and Bcl-2 genes after 72 hours may be due to apoptosis of the cells (due to cellular aging), as the control group (untreated HEP-2 cells) showed 26% apoptosis after 72 hours. |
| Overall, the findings of this study demonstrate that the apoptotic effect of WJMSC-conditioned medium on the HEP-2 cell line was minimal after 24 hours, but gradually increased after 48 and 72 hours. |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |
| |










