Keywords
Neuroinflammation; Postoperative cognitive dysfunction (POCD) ; Anti-inflammatory agents; Metaanalysis
Introduction
With an aging population and advanced surgical techniques, more patients will undergo surgery at advanced age, with the risk of postoperative cognitive decline (POCD) [1]. POCD is a surgical complication involving chronic impairments in different cognitive domains, including memory, attention, learning, information processing and executive functions [2]. These impairments often become apparent in days to weeks after surgery. POCD can have serious consequences as it adversely affects independent living, quality of life and mortality [3]. Risk factors for POCD include advanced age, major surgery and pre-existing cognitive impairment [4]. POCD can be distinguished from postoperative delirium (POD), which is defined as an acute fluctuating and transient disturbance of perception and cognition that cannot be attributed to a pre-existing neurocognitive disorder [5] and usually occurs within the first three days after surgery [6]. POCD can best be detected with a set of neuropsychological tests, applied before and after surgery. Usually, these are more extensive tests than delirium screening tools [7].
Neuroinflammation
Although the exact mechanisms behind POCD are still unknown, there is increasing evidence for a key role of neuroinflammation [8]. Tissue damage during surgery activates damage-associated molecular pattern proteins, necessary for wound healing. These proteins evoke an inflammatory response [9], also affecting the brain and ultimately resulting in microglia activation [10]. Microglia are the brain’s resident immune cells. In response to extensive tissue damage and subsequent immune activation, microglia can become activated, resulting in reduced production of growth factors, more extensive synaptic pruning [11] and increased production of pro-inflammatory products (cytokines). Cytokines, such as brain-derived neurotrophic factor (BDNF), tumor necrosis factor alfa (TNFα), interleukin 1 (IL-1) and interleukin 6 (IL-6), further activate the immune system, having a detrimental effect on memory, neuroplasticity and neurogenesis [12-14]. They may also negatively affect neuronal functioning through the modulation of intraneuronal pathways [15].
In particular BDNF and its intraneuronal pathway have been implicated as mediators between neuro-inflammation and cognitive impairment [16]. Hence, multiple processes may cause cognitive impairment, including neuronal loss and decreases in neurogenesis [17], synaptic plasticity [18] and long-term potentiation [19]. A meta-analysis of clinical data showed that elevated peripheral inflammatory markers are indeed associated with POCD, with the strongest relationship for IL-6 [20]. In POCD, the inflammatory response seems to be derailed, with elevated peripheral inflammatory factors, inducing lasting cognitive deficits [20]. As a consequence, interventions to moderate the immune response during and after surgery may help to rescue cognition.
Anti-inflammatory medication
Medical interventions that slow down or stop microglial activation may consequently prevent or reverse cognitive deterioration. Therefore, several studies have investigated the effect of anti-inflammatory medication on POCD. Recent studies on the effects of individual anti-inflammatory agents on POCD have provided promising results [21-24]. However, for a large part of these studies, POCD diagnosis was based on only one cognitive screening instrument (Mini Mental State Examination), which is not appropriate for a proper diagnosis [7]. To the best of our knowledge this is the first meta-analysis to provide an overview of the effects of different antiinflammatory agents on POCD that used a battery of neuropsychological tests for POCD diagnosis. Consequently, this overview may provide a rational for future clinical studies and hospital strategies.
Research Methodology
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta- Analyses (PRISMA) guidelines [25]. An electronic search was performed using PubMed, Psychinfo, EmBase, Cochrane Database of Systematic Reviews and clinicaltrial.gov. The basic search terms that were used are: postoperative cognitive dysfunction OR postoperative cognitive decline OR POCD AND the specific pharmacological components. Besides the wellknown NSAIDs (aspirin, celecoxib, ibuprofen, diclofenac, and naproxen), and corticosteroids (prednisone, prednisolone, hydrocortisone, methylprednisolone, dexamethasone, cortisone, triamcinolone, and betamethasone), many pharmacological agents have some degree of antiinflammatory action. For some, this is their predominant function, for others this is just a pleiotropic effect.
Therefore, we selected all agents that have some antiinflammatory aspects, as demonstrated in (rodent) experimental settings, resulting in a very broad range, including: davunetide, EPA and DHA fatty acids, estrogens (including also selective estrogen receptor modulators), specific antibiotics (e.g., minocycline), n-acetyl cysteine (NAC) and transplantation adjuncts (tacrolimus, cyclosporine, everolimus, serolimus, mycophenolate mofetil), cytostatics (bexarotene, bone marrow irradiation/transplantation, methotrexate, cyclophosphamide), melatonin, ketamine, parecoxib, erythromycin, dexmedetomidine, piracetam, statins, pregabalin, lidocaine, erythropoietin, propofol, thiopental, xenon and magnesium. The search dates ranged from the establishment of each database to November 2019.
Inclusion criteria
Studies are included based on the following criteria:
• Only randomized, double-blind, placebo-controlled studies that investigated clinical outcome are included.
• Studies include postoperative patients of all ages.
• Studies include patients with surgery under general anaesthesia, except brain surgery.
• Post-operative cognitive functioning is measured using a battery of neuropsychological tests.
• Studies included report sufficient information to compute common effect size statistics of change scores (i.e., means and SDs, exact P-, t- or z-values).
Cross-over, preventive intervention and treatment studies are also included. No year or language restrictions are applied.
Outcome measures
The primary outcome measure is the difference in incidence of POCD between treatment group and control group. POCD was measured as the post-operative change in scores on neuropsychological tests compared to pre-operative measurement. Different diagnostic criteria were used to define POCD (Table 1). The criterion used for each study can be found in Table 2.
Table 1: De?nitions of post-operative cognitive dysfunction.
| Number |
Criterion |
| 1 |
A decline of 1 SD in at least 1 test |
| 2 |
A decline of 1 SD in at least 2 tests |
| 3 |
A decline of 1 SD in at least 1 domain (consisting of multiple tests) |
| 4 |
A decline of 1 SD in at least 2 subtests (from different tests) |
| 5 |
A decline of 2 SD in at least 1 test |
| 6 |
A decline of 2 SD in at least 2 tests or the composite test score |
| 7 |
A 20% reduction in 20% of tests |
| 8 |
Reliable Change Index (RCI) equal to or less than -1.96 on at least 1 test |
| 9 |
Reliable Change Index (RCI) equal to or less than -1.96 on at least 2 tests |
Table 2: Characteristics of the included studies.
| Author (year) |
Type of surgery |
Anti-inflammatory agent |
Dose of treatment (duration) |
Time point |
Patients (N) |
POCD criterion |
| Mitchell (1999) |
Cardiac surgery |
Lidocaine |
1 mg/kg bolus + 240 mg/h (1 h), 120 mg/h (2 h) and 60 mg/h (2 days) |
10 Weeks |
26 |
1 |
| Wang (2002) |
Cardiac surgery |
Lidocaine |
1.5 mg/kg bolus + 4 mg/min intraoperative infusion |
9 Days |
43 |
4 |
| Nagels (2004) |
Cardiac surgery |
(S+) ketamine |
2.5 mg/kg bolus + 125 μg/kg/min intraoperative infusion |
10 Weeks |
50 |
2 |
| Hogue (2007) |
Cardiac surgery |
17β-estradiol |
0.01 ng/kg/min from 1 day preoperatively (6 days) |
4-6 Weeks |
86 |
2 |
| Haljan (2009) |
Cardiac surgery |
Erythropoietin |
375, 750 or 1500 U/kg bolus 1 day before, during and 1 day after surgery |
2 Months |
24 |
7 |
| Hudetz (2009) |
Cardiac surgery |
Ketamine |
0.5 mg/kg bolus intraoperative |
1 Week |
26 |
6 |
| Mathew (2009) |
Cardiac surgery |
Lidocaine |
1 mg/kg bolus + infusion (2 days) |
6 Weeks |
88 |
3 |
| Mitchell (2009) |
Cardiac surgery |
Lidocaine |
1 mg/kg bolus + 2 mg/min (2 h) and 1 mg/min (12 h) |
10 Weeks |
59 |
1 |
| Mathew (2013) |
Cardiac surgery |
Magnesium |
50 mg/kg bolus + 50 mg/kg intraoperative infusion |
6 Weeks |
168 |
3 |
| Fang (2014a) |
Microvascular decompression |
Dexamethasone |
0.1 mg/kg intraoperative bolus |
5 Days |
320 |
2 |
| Fang (2014b) |
Microvascular decompression |
Dexamethasone |
0.2 mg/kg intraoperative bolus |
5 Days |
315 |
2 |
| Hansen (2014) |
Lumpectomy or mastectomy |
Melatonin |
6 mg bolus daily (3 months) |
3 Months |
27 |
6 |
| Ottens (2014) |
Cardiac surgery |
Dexamethasone |
1 mg/kg intraoperative bolus |
1 Month |
140 |
8 |
| Lee (2015) |
Orthopedic surgery |
Ketamine |
0.5 mg/kg intraoperative bolus |
6 Days |
25 |
6 |
| Zhu (2016) |
Total knee arthroplasty |
Parecoxib |
40 mg bolus intraoperative and 12 h postoperative |
1 Week |
60 |
2 |
| Glumac (2017) |
Cardiac surgery |
Dexamethasone |
0.1 mg/kg bolus 10 h preoperative |
6 Days |
80 |
8 |
| Thomaidou (2017) |
Cardiac surgery |
Erythromycin |
25 mg/kg bolus 12 h preoperative + 12 h postoperative |
3 Months |
19 |
1 |
| Zhu (2018) |
Total knee arthroplasty |
Celecoxib |
200 mg every 12 h for 7 days starting the day before surgery |
1 Week |
81 |
2 |
| Cheng (2019) |
Gastro-intestinal laparotomy |
Dexmedetomidine |
0.5 mg/kg bolus followed by 0.4 mg.kg.hr infusion |
1 Week |
269 |
9 |
| Klinger (2019) |
Cardiac surgery |
Lidocaine |
1 mg/kg bolus followed by a continuous infusion |
6 Weeks |
211 |
3 |
Statistical analyses
The meta-analysis was conducted using Review Manager Version 5.3 (Review Manager, 2014). The odds ratios (ORs) with 95% confidence intervals (CIs) were used to express the effect-size. The odds ratio for each study was calculated using the available data provided in the paper. This included the incidence of POCD per group and the sample sizes of the treatment group and control group. A random effects model was applied because of functional differences between included studies [26].
To measure the degree of heterogeneity between studies, I2 was calculated. This measure describes the percentage of total variation across studies that are caused by heterogeneity rather than chance [27].
Values of I2 can range from 0% to 100%, whereby the following interpretations for heterogeneity are suggested by the Cochrane Collaboration: low (0-40%), moderate (30-60%), substantial (50-90%) and high (75-100%) [25].
Meta-regression of categorical moderators was performed when at least four studies were available [28]. Following this rule, the effects of type of surgery (cardiac or non-cardiac) and time of measurement (>1 month) were assessed using StataVersion 15 (Statacorp., 2017). All results with a p-value <0.05 were considered significant.
Results
The search yielded 574 papers, of which 133 remained after removal of duplications and subsequent screening on title and abstract. Full-text reading resulted in nineteen papers that fulfilled the inclusion criteri (Figure 1). The current metaanalysis included ten different anti-inflammatory agents and a total of 2117 patients and 2097 healthy controls.
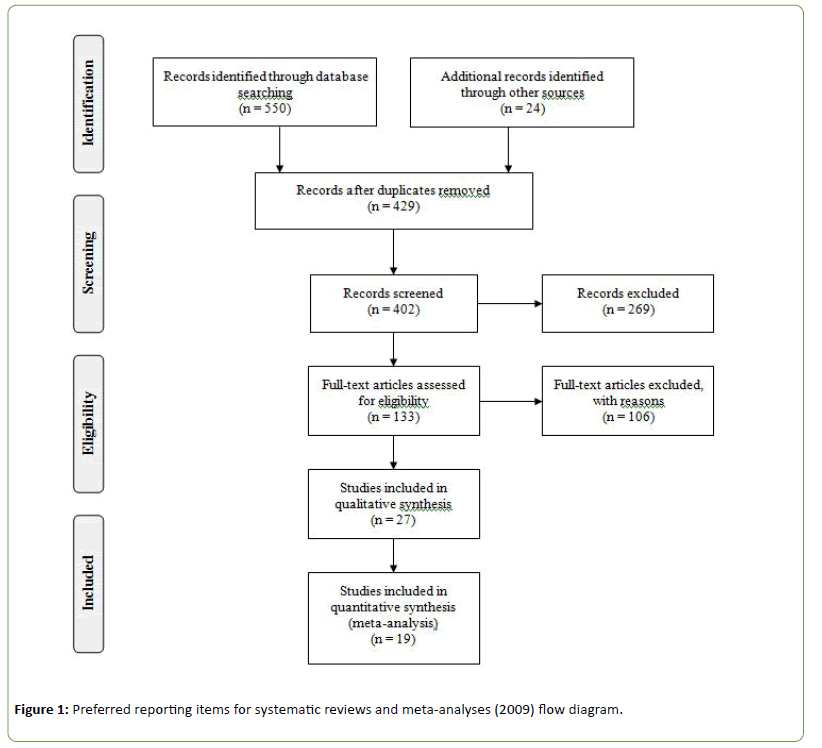
Figure 1: Preferred reporting items for systematic reviews and meta-analyses (2009) flow diagram.
Characteristics of the included studies are shown in Table 2. Some studies included measurements at different time points. In this case only the first measurement was selected to avoid practice effects.
Figure 2 shows the effect of different anti-inflammatory agents on POCD. An odds ratio of 0.67 was found, indicating a significant effect of anti-inflammatory agents on the incidence of POCD (Z=2.59, p=0.010). Heterogeneity was substantial to high (I2=71%). Using the arbitrary cut-off of a sample size of less than 1000 patients for a lack of power, this meta-analysis has sufficient power. Sub analyses on individual antiflammatory agents are presented below.
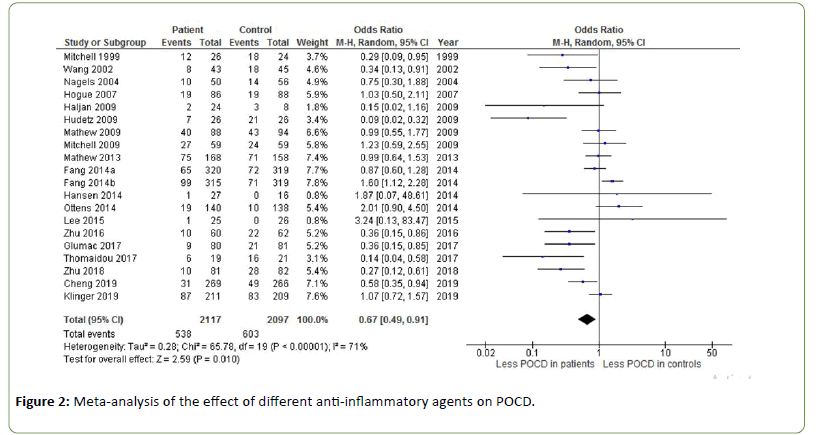
Figure 2: Meta-analysis of the effect of different anti-inflammatory agents on POCD.
Erythromycin
Thomaidou et al. found an odds ratio of 0.14 for the effect of erythromycin on POCD (Z=2.72, p=0.006) [29].
Erythropoietin
Haljan et al. investigated the effect of three doses of recombinant human erythropoietin (rHuEpo) given the day before, during, and the day after coronary artery bypass grafting (CABG) surgery [30]. Two months after surgery, POCD occurred in 8.3% of patients in the treatment group and in 38.0% of patients in the placebo group (OR=0.15, Z=1.82, p=0.07).
COX-2 inhibitors
One week post-surgery, patients receiving COX-2 inhibitors during total knee arthroplasty experienced significantly less POCD than healthy controls (OR=0.31, Z=3.90, p<0.0001 [31,32]. There was no heterogeneity between studies (Figure 3).
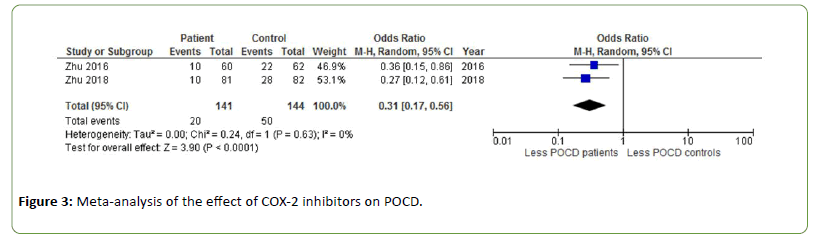
Figure 3: Meta-analysis of the effect of COX-2 inhibitors on POCD.
Ketamine
The effect of ketamine on POCD was investigated in three different studies showing inconsistent results with an odds ratio of 0.44 (Z=0.88, p=0.38) [33-35]. Heterogeneity was substantial to high (I2=77%) (Figure 4).
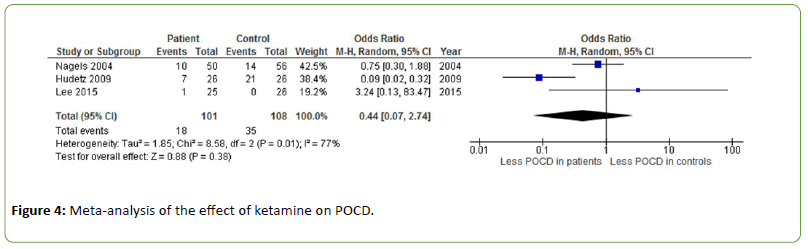
Figure 4: Meta-analysis of the effect of ketamine on POCD.
Dexmedetomidine
Intra-operative dexmedetomidine was found to reduce POCD in elderly patients undergoing laparotomy (OR=0.58, Z=2.22, p=0.03) [36].
Lidocaine
Five different studies measured the effect of lidocaine on POCD after cardiac surgery, with an overall odds ratio of 0.79 (Z=0.98, p=0.33) [37-41]. Heterogeneity was moderate to substantial (I2=55%) (Figure 5).
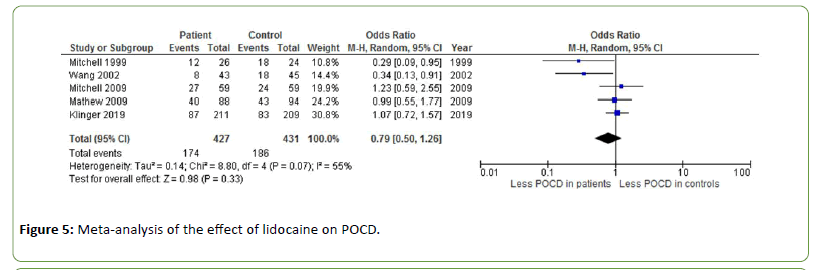
Figure 5: Meta-analysis of the effect of lidocaine on POCD.
Magnesium
Mathew et al. found no effect of the administration of magnesium on POCD six weeks after surgery (OR=0.99, Z=0.05, p=0.96) [42].
17β-estradiol
Hogue et al. found no significant effect of 17β-estradiol on POCD at four to six weeks postoperatively (OR=1.03, Z=0.08, p=0.94) [43].
Dexamethasone
The effect of dexamethasone on POCD is investigated by three studies (OR=1.05, Z=0.16, p=0.88), showing contrasting results (Figure 6) [44-46]. Fang et al. administrated dexamethasone at doses of 0.1 mg/kg (a) and 0.2 mg/kg (b) [44]. The incidence of POCD was 22.3% in the placebo group, 20.6% in the low-dose dexamethasone group and 31.4% in the high-dose dexamethasone group. Heterogeneity between the studies was substantial to high (I2=79%).
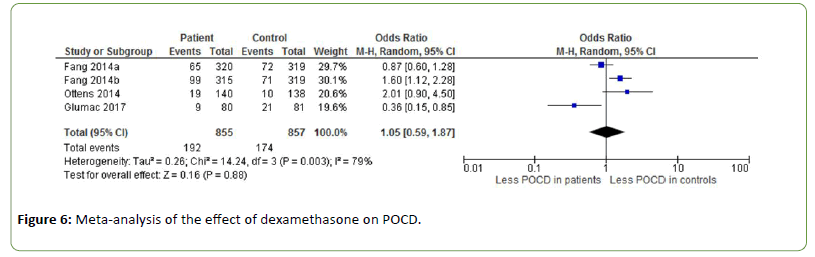
Figure 6: Meta-analysis of the effect of dexamethasone on POCD.
Melatonin
Hansen et al. investigated the effect of melatonin on POCD. However, there was only one patient with POCD at three months post-operatively (OR=1.87, Z=0.38, p=0.71) [47].
Effect of moderators
Meta-regression analysis showed that type of surgery (p=0.863) and time of measurement (p=0.201) were insignificant predictors for the effect of anti-inflammatory medication on POCD.
Discussion
The current meta-analysis provides a quantitative systematic overview of literature regarding the efficacy of agents with anti-inflammatory properties on the incidence of POCD. A total of nineteen randomized controlled trials, using ten different anti-inflammatory agents were included. Overall, the administration of different anti-inflammatory agents was found to significantly reduce the incidence of POCD in patients. Administration of COX-2 inhibitors, ketamine and lidocaine showed better results than placebo in a metaanalysis of at least two studies. Erythromycin, recombinant human erythropoietin (rHuEpo) and dexmedetomidine were significant in single studies. No beneficial effects were found for magnesium, 17β-estradiol, dexamethasone and melatonin.
Anti-inflammatory agents that showed replicated significant result
COX-2 inhibitors, ketamine and lidocaine were found to be effective anti-inflammatory agents in reducing incidence of POCD. COX inhibitors reduce the activation of microglia and subsequent neuroinflammation [48]. This meta-analysis showed that cognitive performance one week post-surgery was significantly better in the COX-2 inhibitor group compared to controls [31-32].
Ketamine is a common anesthetic agent with a range of pharmacological effects that are potentially neuroprotective [49]. It prevents the extension of local inflammation without blunting the local process and delaying inflammatory resolution [50]. In the current meta-analysis, ketamine reduced POCD incidence in cardiac surgery, but this finding could not be replicated in non-cardiac surgery [33-35].
Lidocaine is a commonly used local anaesthetic and antiarrhythmic drug targeting specific voltage-gated sodium channels associated with neuropathic and inflammatory pain. Due to its capacity to suppress pain, it has been proposed that lidocaine may likewise reduce inflammatory responses [51]. Five studies measured the effect of different doses of lidocaine on POCD, showing an overall cognitive improvement across studies [37-41].
Effective anti-inflammatory agents in single studies
Other anti-inflammatory agents that may be effective are erythromycin, recombinant human erythropoietin (rHuEpo) and dexmedetomidine, of which administration resulted in a significant reduction of POCD in single studies. Macrolide antibiotics, such as erythromycin, are popular due to their spectrum of activity and their relative safety [52]. The effects of macrolides are not restricted to antibacterial properties, but also involve modulation of a variety of inflammatory processes [53]. Thomaidou et al. administrated a 25 mg/kg bolus of erythromycin twelve hours before and twelve hours after cardiac surgery and found a lower incidence of POCD at three months post-surgery [29].
Erythropoietin (EPO) directly affects cells of the nervous system, which makes it an attractive candidate drug for neuroprotection [54]. RHuEpo can attenuate the production of proinflammatory cytokines and reduce the influx of inflammatory cells into the damaged region [55]. Haljan et al. demonstrated the feasibility and safety of rHuEpo as a neuroprotective agent [30].
Dexmedetomidine is an α2 receptor agonist, used as a sedative agent with analgesic properties [56]. A number of mechanisms of dexmedetomidine have been presumed, including the modulation of cytokine production, inhibition of apoptosis and interactions between pain and immune factors such as proinflammatory cytokines [57]. Cheng et al. found a lower incidence of POCD in elderly patients undergoing laparotomy after the intra-operative administration of dexmedetomidine [36].
Other anti-inflammatory agents
Four of the investigated agents were not found to be effective in reducing the incidence of POCD: magnesium, 17β- estradiol, dexamethasone and melatonin. Magnesium sulfate is an anti-convulsant that possesses anti-inflammatory capacity, as it can inhibit endotoxin-induced upregulation of inflammatory molecules [58]. However, no effect of magnesium on POCD was found [42].
Estrogens, especially 17β-estradiol, also have mild antiinflammatory properties. 17β-estradiol reduces TNF-α and NO25 in addition to actions such as reducing anti-oxidative stress, influencing dopaminergic neurotransmission and controlling energy balance and glucose homeostasis [59]. A potential explanation for the failure of 17β-estradiol to decrease the incidence of POCD might be inappropriate dosing or duration of 17β-estradiol administration. It is possible that early benefits were masked by ongoing neurological injury that may have occurred after the short period of treatment [43].
Corticosteroids such as dexamethasone are often used as additional drugs in anaesthesia to alleviate side effects such as pain, fatigue and vomiting [60,61]. Dexamethasone may also suppress the inflammatory reaction in the postoperative period through inhibition of inflammatory cells and suppression of inflammatory mediators [62,63]. However, no effect of dexamethasone on POCD was found in this metaanalysis [44-46].
Melatoninis a neurohormone and a broad-spectrum antioxidant [64]. The antioxidant activity of melatonin includes the production of reactive oxygen and activation of prooxidant enzyme, processes that fight the onset and progression of inflammation [65]. The results of Hansen et al. were difficult to interpret because of the small sample size and small number of people with POCD [47].
Difficulties in POCD research
There are three major methodological difficulties when evaluating different studies on POCD. First, there is no standardized internationally accepted definition of POCD and diagnostic criteria are lacking [8]. The most stringent criterion for the diagnosis of POCD is a decline of at least two standard deviations in two cognitive domains or in a single composite cognitive score [66,67]. A more liberal criterion was proposed, being a decline of at least one standard deviation in any cognitive domain tested or in a composite cognitive score compared to pre-operative performances [68]. Secondly, diagnosing POCD is only possible with neuropsychological testing [66]. However, there is no consensus on which tests and test measures to use [69]. According to Krenk and Rasmussen, test batteries should focus on different cognitive domains and their sensitivity should allow the diagnosis of mild deficits [70]. Finally, there is no consensus regarding the optimal timing of neuropsychological testing after surgery [35].
Limitations
In the current meta-analysis, nine different definitions for POCD were used (Table 1). It is important to consider that the probability of identifying POCD in a patient depends on the number of test measures used for analysis, which was shown in the ISPOCD normative population. A decline of one standard deviation was found in only 0.6% of the elderly volunteers when one test measure was considered. This proportion increased to 29% with the use of five test measures. When all 32 test measures were considered, 93% of the volunteers showed a decline of one standard deviation in at least one subtest [66]. Thus, on the one hand it is important to use a sensitive test battery including several cognitive domains. On the other hand, the number of tests included in the statistical analysis should be limited in order to avoid large variability and capitalization on chance. As an alternative, the results of different neuropsychological tests can be combined and expressed in a composite score. However, in doing so, information on specific cognitive functions is lost. In trying to deal with these issues, the current study compared incidences of POCD by any used definition instead of using group means. The disadvantage of this method is that a larger number of patients needs to be included to achieve sufficient statistical power [66]. In this study, the sub-analyses were seriously underpowered.
In addition, the substantial inconsistency between studies made it necessary to use random effect models that produced wider confidence intervals. Differences exist in type of surgery, anaesthesia and dose and duration of treatment. The type of surgery may have a considerable effect on the incidence of POCD, because cognitive impairments are more common after cardiac surgery than after non-cardiac surgery [1]. Besides, POCD is more common in major surgery than in minor surgery [71]. Furthermore, even though all included studies measured POCD in patients undergoing surgery under general anaesthesia, the duration of surgery and the depth of anaesthesia was variable. This is important to take into account because Ballard et al. found that optimization of anaesthetic depth led to a significant reduction in POCD at one week post-surgery, with sustained benefits up to one year post-surgery [72]. Furthermore, anaesthetics on their own may have profound and long-lasting effects on the brain [73]. Finally, other individual factors such as attention deficits, depression and alcohol and tobacco use also impact the incidence of POCD [4].
Another limitation of the current study is the large dropout of patients at follow-up. This dropout is likely confounded, since patients who experience substantial decline are more likely to be lost to follow-up. This may result in an underestimation of the incidence of POCD. Finally, it is important to note that the different agents that seem to decrease the incidence of POCD are broadly active substances, and it is unsure whether the beneficial effects are caused exclusively by their anti-inflammatory actions.
Altogether, research into the use of different antiinflammatory agents to reduce POCD is still in its infancy. Only few studies have been performed and many components with strong anti-inflammatory potential, such as many NSAIDs, specific antibiotics and corticosteroids, have not yet been investigated. For future research into POCD, consistent diagnostic criteria need to be developed along with a generally accepted battery of neuropsychological tests to measure POCD. Besides, decisions need to be made regarding the optimal timing of measurement.
Conclusion
The current meta-analysis shows that anti-inflammatory effects of agents may positively affect cognitive functioning after surgery, as overall the administration of different antiinflammatory agents was found to significantly reduce the incidence of POCD in patients. In addition to known-antiinflammatory drugs, many other substances with antiinflammatory properties were shown to improve POCD as well. Although POCD as a surgical complication has only recently been recognized, and a clear consensus about diagnostics is not yet available, the present meta-analysis indicates that an anti-inflammatory strategy may provide a promissing route to proceed in the development of treatment for POCD. Further research is necessary to determine which agents are most appropriate for clinical application.
28261
References
- Rasmussen LS (2006) Postoperative cognitive dysfunction: incidence and prevention. Best Pract Res Clin Anaesthesiol 20: 315-330.
- Hovens IB, Schoemaker RG, Van der Zee EA, Heineman E, Izaks GJ, et al. (2009) Thinking through postoperative cognitive dysfunction: how to bridge the gap between clinical and pre-clinical perspectives. Brain Behav Immun 26: 1169-1179.
- Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS (2009) Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 110: 548-555.
- Deiner S, Silverstein JH (2009) Postoperative delirium and cognitive dysfunction. Br J Anaesthesia 103: 41-46.
- European Delirium Association and American Delirium Society (2014) The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med 12: 141.
- Hove LD, Steinmetz J (2013) Inadequate recovery: when emergence from anesthesia is not really smooth. Minerva Anestesiol 76: 385-386.
- Steinmetz J, Rasmussen LS (2016) Peri-operative cognitive dysfunction and protection. Anaesthesia 71: 58-63.
- Krenk L, Rasmussen LS, Kehlet H (2010) New insights into the pathophysiology of post-operative cognitive dysfunction. Acta Anaesthesiol Scand 54: 951-956.
- Pandolfi F, Altamura S, Frosali S (2016) Key Role of DAMP in Inflammation, Cancer, and Tissue Repair. Clin Therap 38: 1017-1028.
- Hu X, Liou AK, Leak RK (2014) Neurobiology of microglial action in CNS injuries: receptor- mediated signaling mechanisms and functional roles. Prog Neurobiol 119: 60-84.
- York EM, Bernier LP, Mac Vicar BA (2018) Microglial modulation of neuronal activity in the healthy brain. Develop Neurobiol 78: 593-603.
- Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, et al. (2011) Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLC gamma 1, and ERK in hippocampal synapto neurosomes. J Neurosc 31: 4274–4279.
- Ji MH, Yuan HM, Zhang GF, Li XM, Dong L, et al. (2013) Changes in plasma and cerebrospinal fluid biomarkers in aged patients with early postoperative cognitive dysfunction following total hip- replacement surgery. J Anesthesia 27: 236–242.
- Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, et al. (2007) Postoperative impairment of cognitive function in rats: A possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology 106: 436–443.
- Yirmiya R, Goshen I (2011) Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25: 181-213.
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, et al. (2004) BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intra hippocampal IL-1beta administration. J Neuroimmunol 155: 119-126.
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, et al. (2008) TrkB regulates hippocampal neurogenesis and governs sensitivity to anti-depressive treatment. Neuron 59: 399–412.
- Tong L, Prieto GA, Kramár EA, Smith ED, Cribbs DH, et al. (2012) Brain derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1β via p38 mitogen-activated protein kinase. J Neurosc 32: 17714–17724.
- Lu Y, Christian K, Lu B (2008) BDNF: a key regulator for protein synthesis-dependent LTP and longa. term memory? Neurobiol Learn Mem 89: 312–323.
- Peng L, Xu L, Ouyang W (2013) Role of peripheral inflammatory markers in postoperative cognitive. dysfunction (POCD): A meta-analysis. PLoS One 8.
- Habibie MR, Habibie V, Habibie A, Suleiman A (2018) Lidocaine dose-response effect on postoperative cognitive deficit: meta-analysis and meta-regression. Expert Rev Clin Pharmacol 11: 361-371.
- Huang S, Hu H, Cai YH, Hua F (2019) Effect of parecoxib in the treatment of postoperative cognitive dysfunction. Medicine 98: e13812.
- Hovaguimian F, Tschopp C, Beck-Schimmer B, Puhan M (2018) Intraoperative ketamine administration to prevent delirium or postoperative cognitive dysfunction: A systematic review and meta-analysis. Acta Anaesthesiolo Scand 62: 1182-1193.
- Zhou C, Zhu Y, Liu Z, Ruan L (2016) Effect of dexmedetomidine on postoperative cognitive dysfunction in elderly patients after general anaesthesia: A meta-analysis. J Int Med Res 44: 1182–1190.
- Higgins JPT, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0.
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR (2010) A basic introduction to fixed-effect a. and random-effects models for meta-analysis. Res Syn Meth 1: 97-111.
- Higgins JPT, Thompson SG, DeeksJJ, Altman DG (2003) Measuring inconsistency in meta- analyses. BMJ 327: 557-560.
- Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, et al. (2011) Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol 64: 1187-1197.
- Thomaidou E, Argiriadou H, Vretzakis G, Megari K, Taskos N, et al. (2017) Perioperative use of erythromycin reduces cognitive decline after coronary artery bypass grafting surgery: A pilot study. Clin Neuro Pharmacol 40: 195-200.
- Haljan G, Maitland A, Buchan A, Arora RC, King M, et al. (2009) The erythropoietin neuroprotective effect: Assessment in CABG surgery (TEN PEAKS). Stroke 40: 2769-2775.
- Zhu Y, Yao R, Zhang Z, Xu H, Wang LW (2016) Parecoxib prevents early postoperative cognitive dysfunction in elderly patients undergoing total knee arthroplasty. Medicine 95: e4082.
- Zhu Y, Yao R, Li Y, Wu C, Heng L, et al. (2018) Protective effect of celecoxib on early postoperative cognitive dysfunction in geriatric patients. Front Neurol 9: 633.
- Nagels W, Demeyere R, Van Hemelrijck, Vandenbussche E, Gijbels K, et al. (2004) Evaluation of the neuroprotective effects of S (+) -ketamine during open-heart surgery. Anesth Analg 98: 1595-1603.
- Hudetz JA, Iqbal Z, Gandhi SD, Patterson KM, Byrne AJ, et al. (2009) Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol Scand 53: 864-872.
- Lee KH, Kim JY, Kim JW, Park JS, Jeon SY (2015) Influence of ketamine on early postoperative cognitive function after orthopedic surgery in elderly patients. Anesthesiol Pain Med 5: e28844.
- Cheng XQ, Mei B, ZuoYM, Wu H, PengXH, et al. (2019) A multicentre randomised controlled trial of the effect of intra-operative dexmedetomidine on cognitive decline after surgery. Anaesthesia 74: 741-750.
- Mitchell SJ, Pellett O, Gorman DF (1999) Cerebral protection by lidocaine during cardiac operations. Ann Thoracic Surg 67: 1117-1124.
- Wang D, Wu X, Li J, Xiao F, Liu X, et al. (2002) The effect of lidocaine on early post-operative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg 95: 1134-1141.
- Mitchell SJ, Merry AF, Frampton C, Davies E, Grieve D, et al. (2009) Cerebral protection by lidocaine during cardiac operations: A follow-up study. Ann Thoracic Surg 87: 820-82
- Mathew JP, Mackensen GB, Phillips-Bute B, Grocott HP, Glower DD, et al. (2009) Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke 40: 880-887.
- Klinger RY, Cooter M, Bisanar T, Terrando N, Berger M, et al. (2019) Intravenous lidocaine does not improve neurologic outcomes after cardiac surgery: A randomized controlled trial. Anesthesiology 130: 958-970.
- Mathew JP, White WD, Schinderle DB, Podgoreanu MV, Berger M, et al. (2013) Intraoperative magnesium administration does not improve neurocognitive a. function after cardiac surgery. Stroke 44: 3407- 3413.
- Hogue Jr CW, Freedland K, Hershey T, Fucetola R, Nassief A, et al. (2007) Neurocognitive outcomes are not improved by 17β-estradiol in postmenopausal women undergoing cardiac surgery. Stroke 38: 2048-2054.
- Fang Q, Qian X, An J, Wen H, Cope DK, et al. (2014) Higher dose dexamethasone increases early postoperative cognitive dysfunction. J Neurosurg Anesthesiol 26: 220-225.
- Ottens TH, Dieleman JM, Sauër AM, Peelen LM, Nierich AP, et al. (2014) Effects of dexamethasone on cognitive decline after cardiac surgery. Anesthesiology 121: 492-500.
- Glumac S, Kardum G, Sodic L, Supe-Domic D, Karanovic N (2017) Effects of dexamethasone on early cognitive decline after cardiac surgery. Eur J Anaesthesiol 34: 776- 784.
- Hansen MV, Madsen MT, Andersen LT, Hageman I, Rasmussen LS, et al. (2014) Effect of melatonin on cognitive function and sleep in relation to breast cancer surgery: A randomized, double-blind, placebo-controlled trial. Int J Breast Cancer 416531.
- Choi SH, Aid S, Bosetti F (2009) The distinct roles of cyclooxygenase-1 and -2 in neuro inflammation: implications for translational research. Trends Pharmacol Sci 30: 174-181.
- Hudetz JA, Pagel PS (2010) Neuroprotection by ketamine: a review of the experimental and clinical evidence. J Cardiothorac Vasc Anesth 24: 131-142.
- De Kock M, Loix S, Lavand’homme P (2013) Ketamine and peripheral inflammation. CNS Neurosci Ther 19: 403-410.
- Papich MG (2007) Saunders handbook of veterinary drugs. St. Louis, MO: Saunders Elsevier.
- Kaneko T, Dougherty TJ, Magee TV (2007) Macrolide Antibiotics. Comprehensive Medicinal Chemistry II. Oxford: Elsevier.
- Čulić O, Eraković V, Parnham MJ (2001) Anti-inflammatory effects of macrolide antibiotics. Eur J Pharmacol 429: 209-229.
- Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H (2010) Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract Res Clin Anaesthesiol 24: 573-594.
- Villa P, Bigini P, Mennini T, Agnello D, Laragione T, et al. (2003) Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Clin Exp Med 198: 971-975.
- Dunn LK, Durieux ME, Nemergut EC (2016) Non-opioid analgesics: novel approaches to perioperative analgesia for major spine surgery. Best Pract Res Clin Anaesthesiol. 30: 79-89.
- Kang SH, Kim YS, Hong TH, Chae MS, Cho ML, et al. (2013) Effects of dexmedetomidine on inflammatory responses in patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiol Scand 57: 480-487.
- Lee CY, Jan WC, Tsai PS, Huang CJ (2011) Magnesium sulfate mitigates acute lung injury in endotoxemia rats. J Trauma Acute Care Surg 70: 1177-1185.
- Liu X, Fan XL, Zhao Y, Luo GR, Li XP, et al. (2005) Estrogen provides neuroprotection against activated microglia‐induced dopaminergic neuronal injury through both estrogen receptor‐α and estrogen receptor‐β in microglia. Journal of neuroscience research. 81: 653-665.
- Jakobsson U (2010) The epidemiology of chronic pain in a general population: results of a survey in southern Sweden. Scand J Rheumatol 39: 421-429.
- Gómez-Hernández J, Orozco-Alatorre AL, Domínguez-Contreras M, Oceguera-Villanueva A, Gómez-Romo S, et al. (2010) Preoperative dexamethasone reduces postoperative pain, nausea and vomiting following mastectomy for breast cancer. BMC cancer 10: 692.
- Holte K, Kehlet H (2002) Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg 195: 694-712.
- Papich MG (2007) Saunders handbook of veterinary drugs. St. Louis, MO: Saunders Elsevier.
- Tan DX, Manchester LC, Hardeland R, Lopez‐Burillo S, Mayo JC, et al. (2003) Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. Journal of pineal research 34: 75-78.
- Cuzzocrea S, Reiter RJ (2002) Pharmacological actions of melatonin in acute and chronic inflammation. Curr Top Med Chem 2: 153-165.
- Rasmussen LS, Larsen K, Houx P, Skoygaard LT, Hanning CD et al. (2001) The assessment of postoperative cognitive function. Acta Anaesthesiol Scand 45: 275-289.
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, et al. (1998) Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet 351: 857-861.
- Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, et al. (2001) Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 344: 395-402.
- Bryson GL, Wyand A (2006) Evidence-based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction. Can J Anaesth 53: 669.
- Krenk L, Rasmussen LS (2011) Postoperative delirium and postoperative cognitive dysfunction in the elderly-what are the differences?. Minerva Anestesiol 77: 742-749.
- Grape S, Ravussin P, Rossi A, Kern C, Steiner LA (2012) Postoperative cognitive dysfunction. Trends Anaesth Crit Care 2: 98-103.
- Ballard C, Jones E, Gauge N, Aarsland D, Nilsen OB, et al. (2012) Optimised anaesthesia to reduce post-operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PloS One 7: 1-9.
- Perouansky M (2008) General anesthetics and long-term neurotoxicity. Handb Exp Pharmacol 182: 147-157.











