Lida Karimi Behbahany1, Fatemeh Keshavarzi2* and Gholamreza Homayounpour3
1Department of Biology, Kurdistan Science and Research Branch, Islamic Azad University, Sanandaj, Iran
2Department of Biology, Sanandaj Branch, Islamic Azad University, Sanandaj, Iran
3Central Office of Legal Medicine, Kurdistan Province, Sanandaj, Iran
*Corresponding Author:
Fatemeh Keshavarzi
Department of Biology, Sanandaj Branch
Islamic Azad University, Sanandaj, Iran
Tel: +98 91 8370 4918
E-mail: gol.keshavarzi@gmail.com
Received Date: September 3, 2018; Accepted Date: September 12, 2018; Published Date: September 15, 2018
Citation: Behbahany LK, Keshavarzi F, Homayounpour G (2018) The Frequency of Chromosomal Abnormality in Individuals with Susceptibility of Abortion or Infertility by Cryptogenic Method. Ann Clin Lab Res Vol.6 No.4:258. DOI: 10.21767/2386-5180.100258
Background and Aim: Abnormality karyotype of couples can produce susceptibility to infertility or abortion. Therefore, the first step to study these couples should be determining karyotype by cytogenetic method. In this study assay the prevalence of abnormalities chromosomal these couples from western provinces of Iran.
Method: A cytogenetic study was performed on 200 individuals (124 women (28.1 ± 5) and 76 males (30.7 ± 5) with infertility and/or abortion with age average 30.81 ± 5 using lymphocyte culture and high-resolution G-banding method.
Results: Normal karyotype and various types of chromosomal abnormalities were observed in 138 (69%) and 62 (31%) individuals, respectively. Also, the frequency of translocations, insertions, deletions, inversions and duplications were 40.32% (25), 22.6% (14), 17.74% (11), 9.7% (6) and 9.7% (6) respectively, that the highest frequency was related to changes in transposition or chromosomal displacement with 25 cases (40.3%) In addition, the types of chromosomal abnormalities involving translocations, insertions, deletions, inversions and duplications were detected in 15, 9, 6, 3 and 2 persons, respectively, from 35 patients with RLP and 10, 5, 5, 3 and 4 persons, respectively, of 27 patients with infertility.
Conclusion: This issue can also play a role in the planning of pregnant mother’s health.
Keywords
Abortion; Infertility; Chromosomal abnormalities; Karyotype
Introduction
Clinically, abortion is defined as the termination of pregnancy before the 18th to 20th week of pregnancy, with an embryo weighing less than 500 grams [1]. Also, Recurrent abortion or recurrent pregnancy loss (RLP) is defined as; is three or more consecutive pregnancy losses prior to 20 weeks from the last menstrual period. Studies strongly suggest that in fertile couples (infertility is: the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse), pregnancy occurs in at least 60% of normal cycles. Studies also suggest that 50% of these fertility end in the uterus before implantation. The ending date for pregnancy is about 30% shortly after implantation (before the clinical diagnosis of pregnancy), even after clinical diagnosis of pregnancy, 25% of pregnancies usually end in abortion during the first 14 weeks. The most dangerous time is between 6 and 8 weeks after the last menstrual period, with more than 50% of aborted foetuses at this time having chromosomal problems [2-5]. In fact, more than 80% of abortions occur in the first 12 weeks of pregnancy, and chromosomal abnormalities are at least responsible for 50% of them. After the first trimester, abortion rates and incidence of chromosomal abnormalities decrease [3]. Chromosomal abnormalities may occur due to structural disturbances and the displacement of chromosomal fragments in the parent chromosomes or disruptions in the number of embryo chromosomes. Parental chromosomal complexation in 2-3% is abnormal abortion, usually due to chromosomal displacement, chromosome reversal, ringing, and chromosomal microscopy [4-8]. The rate of abortion is 30% immediately after implantation (before the pregnancy is clinically diagnosed). Even after having a pregnancy diagnosed clinically, about a quarter of pregnancies are aborted, which usually occurs within the first 14 weeks. The most dangerous time between weeks 6 and 8 is from the last menstruation. Half of the babies aborting at this time have a chromosomal abnormality. Birth defects have been observed in 3% of the live births, with a significant proportion of these defects (20%) related to chromosomal abnormalities or gene mutations. Some of the aneuploidy, including anioplose, 13, 18, 21, X, and Y chromosomes can lead to the birth of the baby alive and abnormal [9-14]. These disorders do not pose a problem for the parents themselves, but make it difficult to divide and create male and female sex cells in the ganglion. These disorders are detectable by performing a karyotype test. Therefore, abnormal karyotype in any of the couples can be a source of infertility or frequent abortion. In the present study, the prevalence of chromosomal abnormalities in these couples in the western province of is to examine the importance of testing the pre-pregnancy and even marriage karyotype awareness.
Materials and Methods
This study was a descriptive cross-sectional research and the samples consisted of 200 patients with recurrent abortions and/or infertility with mean age of 30.83 ± 5 from western provinces. 124 women (28.1 ± 5) and 76 males (30.7 ± 5) were identified and selected through genetic laboratories, genetic counselling centres, forensics and infertility centres, as well as obtaining written consent from them. Blood samples were collected and metaphase chromosome spreads were prepared from phytohemagglutinin (PHA)-stimulated cultures of peripheral blood lymphocytes at the 800-1000 band level using standard cytogenetic methods. Briefly At first 10 ml syringes were washed with heparin solution. The specimens were then transferred to sterile tubes and stored at 4°C until the test (maximum 7 hours). Before using the specimens, tubes containing blood were shaken several times. Then, the blood sample was homogenized in heparinous tubes of 3 to 5 cc, which causes the plasma to be mixed with the blood sample. For each patient, 2 Falcon 15 cc tubes were selected. One tube was added to the new borne cell serum, 4 cc hamf 10 and 0.1 cc PHA (culture stimulant). In another tube, 5 cc pbmax was added, which is a completely nutritious medium including a culture stimulant. At that time, 500 lindas were sampled in each tube (because of the use of two separate tubes, one tube should be used if one does not grow). After sampling, the tubes containing the sample were kept in a 37°C incubator for 72 hours. After 72 hours and reaching the harvests stage, 120 landas were added to each tube and stored at 37°C for 12 minutes. After 5 minutes of 12 minutes, both tubes were identical (because at this stage, each tube grown and reached the time of the harvested so as to have enough of the two precipitates). After the completion of 12 minutes, the tubes (which were joined at this time) were centrifuged at 1500 and 10 minutes. After 10 minutes, the tubes were centrifuged and the supernatant was poured. A hypotonic solution (0.75 g of powdered KCl in 100 ml of pb) was added to the tubes for 14 minutes and continued for 25 minutes at Ben Mari 37°. After 25 minutes, 1 cc of fixation solution (to fix the wind cells that occurred during the hypotonic phase) was poured into 1500 centrifuge for 10 minutes. After 10 minutes, samples were pipetted with glass pipettes. Firstly, 10 drops of slow-air tweeter were added to the tubes. By adding the fixator, the fixed-winded cells shrink and the metaphases out of the cells. After completion of pipetting, the samples were centrifuged for 1500 rpm for 10 minutes. After this stage the precipitate was applied to the tapered tube of the Falcon tube and the supernatant was discarded and a 5 cc fixator was added and the tubes were shaken vigorously (This step is done to wash the sample so that the remaining RBCs were removed). After the addition, the 5 cc sample fixators were centrifuged for 10 minutes with 1500 rpm. After the end of the centrifugation, the samples were ready to stick. At this stage, a humid environment is required to completely open metaphases. For this purpose, an incinerator is used. Ensure that the ambient temperature reaches the desired level for 30 minutes before starting the machine. The slides were cleaned with sterilized gas and then washed away. After wetting the lambs from the patient's sample, 2 to 3 drops were poured onto the lamella (to the extent that it covers the lamina and drops do not come onto each other). After dropping the drops, they drowned 2 drops of the fixator. The fixator proves the cells and metaphases on the lam. After drying the lam is seen under the microscope. At the end stage, first, 0.5 g of trypsin powder was dissolved by 50 cc PBS, considered as stock, then 10 cc of the stock was loaded with 90 cc PBS, and slides that were previously incubated at 70°C between 5 and 7 hours, they were placed within this solution for 30 seconds. After 30 seconds, the slides were quickly inserted into PBS to eliminate the corrosivity of the trypsin, after which the slides were removed from PBS. Then they were painted with Gemsa color, diluted with PBS, for 3 minutes. In order to examine the karyotype under the microscope, a series of chromosomes was first identified. Then homologous chromosomes were identified. The chromosomes and their pairs were numbered then the chromosome abnormalities were identified. Typically, 20 metaphases were studied for each patient. In the presence of mosaic, 50 metaphases were examined and the results recorded. Infect, the chromosomes were treated with trypsin and then stained with Giemsa stain after ageing. GTG high resolution–banded chromosomes were analysed 20 random metaphases spreads from two independent cultures photographing four metaphase spreads for karyotyping (karyotyping system Ikaros, MetaSystem, USA) according to the International System for Human Cytogenetic Nomenclature criteria.
Results
Of the 200 individuals who became karyotypes, 138 (69%) healthy karyotypes and 62 (31%) patients had one of the chromosomal defects of Inversion, Deletion, Insertion, Duplication and Trans the location was translocation (Figure 1). Of the total 200, 138 were healthy, with 81 of them female and 57 males. Of the 62 (31%) patients with chromosomal abnormalities, 41 were females and 21 were male (Table 1).
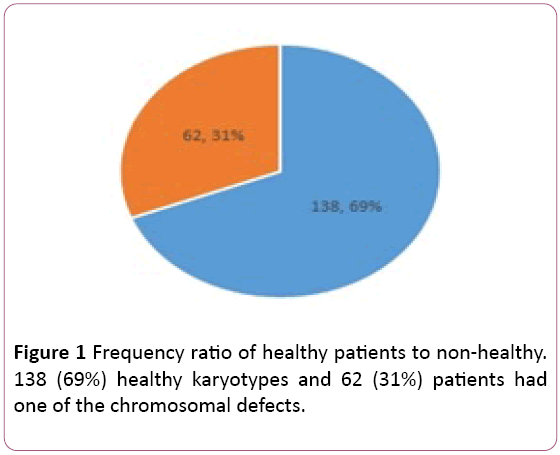
Figure 1: Frequency ratio of healthy patients to non-healthy. 138 (69%) healthy karyotypes and 62 (31%) patients had one of the chromosomal defects.
In addition, out of 62 chromosomal changes, the frequency of translocations, insertions, deletions, inversions and duplications were 40.32% (25), (22.6%) (14), (17.74%) (11), (9.7%) (6) and (9.7%) (6) respectively. The highest frequency was related to changes in transposition or chromosomal displacement with 25 cases (40.3%). The list of identified disorders is presented in Table 2. Other results were that chromosome number 3 had the most changes, and the chromosomes 16, 17, 18 and Y chromosomes were unchanged.
Discussion
Clinically, abortion is defined as the termination of pregnancy before the 20th or 24th week of pregnancy, with an embryo weighing less than 500 grams. In this study, 200 individuals with a mean age of 30.83 ± 5 years with a history of repeated abortion and infertility were examined by cytogenetic method. According results 138 (69%), 62 (31%) of patients had been normal and abnormal karyotype, respectively. The frequency of translocations, insertions, deletions, inversions and duplications were 40.32% (25), (22.6%) (14), (17.74%) (11), (9.7%) (6) and (9.7%) (6) respectively. Also, the results showed that 35 and 27 persons, respectively, complaining of RLP and infertility had chromosomal abnormalities from 62 individuals with abnormality. The frequency of translocations, insertions, deletions, inversions and duplications were 40.32% (25), 22.6% (14), 17.74% (11), 9.7% (6) and 9.7% (6), respectively. In addition, the types of chromosomal abnormalities involving translocations, insertions, deletions, inversions and duplications were detected in 15, 9, 6, 3 and 2 persons, respectively, from 35 patients with RLP and 10, 5, 5, 3 and 4 persons, respectively, of 27 patients with infertility. The highest frequency was related to changes in transposition or chromosomal displacement with 25 cases (40.3%) that was more common than others in both samples. Frequent abortions (two or more unsuccessful pregnancies) affect 2-5% of couples (15). More than 50% of couples who have a history of recurrent abortions are referred to as unspecified or idiopathic causes [8,9]. Studies show that chromosomal analysis can determine the cause of 80% of cases of repeat unexplained abortions in women over 35 years of age [15-18]. The cause of 50% of early pregnancy is the most common cause of abnormal abortion during the first trimester of pregnancy, chromosomal abnormalities [19]. More than 80% of abortions occur in the first 12 weeks of pregnancy, and chromosomal abnormalities are at least responsible for 50% of them. After the first trimester, the rate of abortion and the incidence of chromosomal aberrations decrease [20]. Since 1962, Smith has shown that cytogenetic abnormalities of parents are associated with frequent abortions in the family. Several studies have been carried out in this area around the world [21]. In a study conducted in Saudi Arabia, al-Hussein et al. Showed 193 couples with a history of repeated abortions that among the mothers with an average age of 30.31 years, genetic abnormalities were found in 11 (2.85%) cases [22].
In a study conducted by Hugo et al. over a four-year period on abortion products in the United States, it was shown that of 273 abortions, 17(6.3%) were caused by chromosomal abnormalities such as displacement and drug abuse [21]. In a study by Rozana Oliveira Gonçalves and colleagues on 240 abortion patients in Brazil, the prevalence of chromosomal abnormalities was 7.3% in women, the most abnormality associated with X chromosome mosaic and the frequency of chromosomal abnormalities in males was 2.1% [23]. A similar study was conducted in the north-eastern Iran on 728 couples by Ghazaey et al., out of 11.7%, they had non-healthy karyotype [24]. Most of the changes identified were related to a balanced transposition with a frequency of 37.85% among other changes. In a study in India, the 1484 aborted patients by Dubey et al. Found that the frequency of chromosomal abnormalities in this population was 2%. Among the identified abnormalities in this population, the most change was related to transcription with the frequency of 68.2% [25]. However, in a study by Rajasekhar and colleagues on 420 Indian patients, the frequency of chromosomal abnormalities was reported to be 8.57% [26].
Conclusion
The high prevalence of chromosomal anomalies in the studied population (31%) makes it necessary to carry out genetic counselling and subsequent genetic tests for couples with abortion and infertility before going to pregnancy to prevent the birth of more affected families. This can play a significant role in the planning of pregnant women's health.
Author’s Contributions
Fatemeh keshavarzi designed the study and wrote the paper; Lida Karimi Behbahany conceived the experiments, prepared the figures; collected the samples. Gholamreza Homayounpour conducted research. All authors gave final approval for the manuscript to be submitted for publication.
Ethics Statement
The study was approved by the regional Ethics Committee of Sanandaj branch, Islamic Azad University, Sanandaj, Iran. Research is based on Miss. Lida Karimi Behbahani's master's thesis.
Acknowledgments
The authors appreciate the entire individual who participated in the survey.
Conflict of Interest
The authors declare no conflict of interests.
23494
References
- Vendrell X, Carrero R, Alberola T, Bautista-Llácer R, García-Mengual E, et al. (2009) Quality management system in PGD/PGS: Now is the time. J Assist Reprod Genet 26(4): 197-204.
- Estandarte AKC (2002) A review of the different staining techniques for human metaphase chromosomes. Department of Chemistry, University College, London.
- Rius M, Daina G, Obradors A, Ramos L, Velilla E (2011) Comprehensive embryo analysis of advanced maternal age–related aneuploidies and mosaicism by short comparative genomic hybridization. Fertil Steril 95(1): 413-416.
- Alberman E, Beard RW (1988) Early pregnancy loss: Mechanisms and trearment. New York: Springer-Verlag, USA. pp. 9-17.
- Practice Committee of the American Society for Reproductive Medicine (2013) Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil Steril 99(1): 63.
- Stephenson M, Kutteh W (2007) Evaluation and management of recurrent early pregnancy loss. Clin Obstet Gynecol 50(1): 132-145.
- Jauniaux E, Farquharson RG, Christiansen OB, Exalto N (2006) Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Human Reprod 21(9): 2216-2222.
- Hellani A, Abu-Amero K, Azouri J, El-Akoum S (2008) Successful pregnancies after application of array-comparative genomic hybridization in PGS-aneuploidy screening. Reprod Biomed Online 17(6): 841-847.
- Marquard K, Westphal LM, Milki AA, Lathi RB (2010) Etiology of recurrent pregnancy loss in women over the age of 35 years. Fertil Steril 94(4): 1473-1477.
- Capalbo A, Treff NR, Cimadomo D, Tao X, Upham K, et al. (2015) Comparison of array comparative genomic hybridization and quantitative real-time PCR-based aneuploidy screening of blastocyst biopsies. Eur J Human Genet 23(7): 901.
- Gutiérrez-Mateo C, Colls P, Sánchez-García J, Escudero T, Prates R, et al. (2011) Validation of microarray comparative genomic hybridization for comprehensive chromosome analysis of embryos. Fertil Steril 95(3): 953-958.
- Wells D, Kaur K, Grifo J, Glassner M, Taylor JC, et al. (2014) Clinical utilisation of a rapid low-pass whole genome sequencing technique for the diagnosis of aneuploidy in human embryos prior to implantation. J Med Genet 51(8): 553-562.
- Schoolcraft WB, Fragouli E, Stevens J, Munne S, Katz-Jaffe MG, et al. (2010) Clinical application of comprehensive chromosomal screening at the blastocyst stage. Fertil Steril 94(5): 1700-1706.
- Fragouli E, Alfarawati S, Goodall NN, Sánchez-García JF, Colls P, et al. (2011) The cytogenetics of polar bodies: insights into female meiosis and the diagnosis of aneuploidy. MHR: Basic Sci Reprod Med 17(5): 286-295.
- Stephenson MD, Sierra S (2006) Reproductive outcomes in recurrent pregnancy loss associated with a parental carrier of a structural chromosome rearrangement. Human Reprod 21(4): 1076-1082.
- Lledó B, Ortiz JA, Morales R, Ten J, De la Fuente PE, et al. (2010) The paternal effect of chromosome translocation carriers observed from meiotic segregation in embryos. Human reproduction 25(7): 1843-1848.
- Feichtinger M, Stopp T, Göbl C, Feichtinger E, Vaccari E, et al. (2015) Increasing live birth rate by pre implantation genetic screening of pooled polar bodies using array comparative genomic hybridization. PLoS One 10(5): e0128317.
- Handyside AH, Harton G, Mariani B, Thornhill AR, Affara NA, et al. (2010) Karyomapping: A universal method for genome crossovers between parental haplotypes wide analysis of genetic disease based on mapping. J Med Genet 47(10): 651-658.
- Lebedev IN, Ostroverkhova NV, Nikitina TV, Sukhanova NN, Nazarenko SA (2003) Molecular cytogenetic characteristics of chromosome imbalance in cells of spontaneous human abortion fetuses with low proliferative activity in vitro Russian Journal of Genetics 39(8): 1111-1122.
- Ferro, V Serra (2003) Improved accuracy of hysteroembryoscopic biopsies for karyotyping early missed abortions. Fertil Stril 80(5): 1260-1264.
- Hogge WA, Byrnes AL, Lanasa MC, Surti U (2003) The clinical use of karyotyping spontaneous abortions. Am J Obstet Gynecol 189(2): 397-400.
- Talib ZA, Zaki OK (2000) Cytogenetic study in cases with recurrent abortion in Saudi Arabia. Ann Saudi Med 20(3-4): 233-236.
- Gonçalves RO, Santos WV, Sarno M, Cerqueira BA, Gonçalves MS, et al. (2014) Chromosomal abnormalities in couples with recurrent first trimester abortions. Revista Brasileira de Ginecologia e Obstetrícia 36(3): 113-117.
- Ghazaey S, Keify F, Mirzaei F, Maleki M, Tootian S, et al. (2015) Chromosomal analysis of couples with repeated spontaneous abortions in North-eastern Iran. Royan Institute of Iran. Int J Fertil Steril 9(1): 47-54.
- Dubey S, Chowdhury MR, Prahlad B, Kumar V, Mathur R, et al. (2005) Cytogenetic causes for recurrent spontaneous abortions–an experience of 742 couples (1484 cases). Ind J Hum Gene 11(2): 95-98.
- Rajasekhar M, Gopinath PM, Sreelakshmi K, Satyamoorthy K (2013) A cytogenetic study of couples with miscarriages: An experience from Manipal Referral Centre. Int J Hum Genet 13(2): 93-97.







