Keywords
|
| Chronic obstructive pulmonary disease; Lung cancer; Survival; Metaanalysis |
Abbreviations
|
| OPD: Chronic Obstructive Pulmonary Disease; NSCLC: Non-small Cell Lung Cancer; SCLC: Small Cell Lung Cancer |
Highlights
|
| • COPD preceding lung cancer diagnosis has a deleterious impact on survival of lung cancer |
| • This negative impact of COPD is more pronounced in patients with non-small cell lung cancer, at an early-stage, and who received surgical treatment. |
Introduction
|
| Chronic obstructive pulmonary disease (COPD) and lung cancer are two leading causes of death in the world, which are projected to rank fourth and sixth by 2030 [1], respectively. Both are caused by cigarette smoking and there is an increasing evidence linking the two diseases through epidemiologic and genetic studies [2,3]. In clinical practice, the prevalence of COPD is estimated at 50% to 70% among patients diagnosed with lung cancer [4]. Recently, Durham and Adcock [5] performed a review on the relationship between COPD and lung cancer, aiming to expand the understanding on mechanistic possibility of these two linked diseases. Despite many studies from large national databases reporting the survival for primary lung cancer or COPD [6- 9], the outcome of lung cancer coexisting with COPD, namely the prognostic significance of COPD in lung cancer is poorly understood. Lee et al., [10] reported that COPD did not worsen the prognosis for lung cancer after adjustment for baseline characteristics while Tammemagi et al., [11] found that the presence of COPD was an independent factor of a poor prognosis regardless of cancer stages. A recent meta-analysis by Gao et al., [12] discussed the impact of COPD and emphysema on lung cancer and indicated these as predictors of poor survival, but the study population included patients with emphysema without evidence of airway obstruction [13] and congestive heart failure [14], resulting in a high heterogeneity, thus some conclusions may be biased. Since COPD is very prevalent in lung cancer patients and the conclusions from previous studies remain conflicting regarding the prognostic significance of COPD preceding lung cancer diagnosis, we aimed to systematically review the current available literature to verify and quantify the impact of COPD on survival of lung cancer patients. |
Methods
|
|
Literature search strategy
|
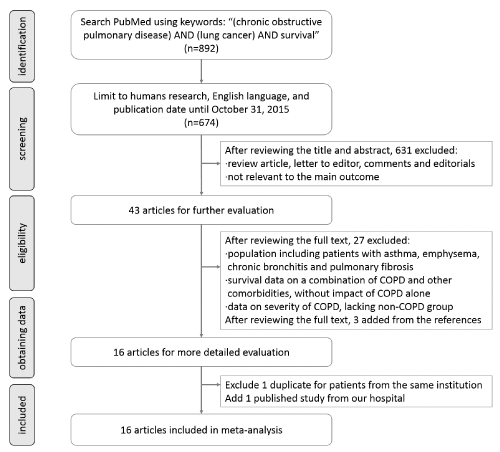
| A systematic search was performed using the PubMed database to identify articles mentioning the impact of COPD on overall survival (OS) in patients with lung cancer. Inclusion criteria were: (1) peer-reviewed and published original articles, (2) study populations involving 20 or more cases in each group (COPD group and non-COPD group), and (3) a hazard ratio (HR) and 95% confidence interval (CI) were stated, or could be calculated in the article. Publications were excluded if the study (1) was published in a book or in non-English, (2) lacked accessibility of HR and 95%CI, or (3) only described the severity of COPD and its relationship to lung cancer prognosis. If the enrolled patients came from the same institution and in the same period, the most proper study would be chosen according to the needs of stratified analysis. |
| A search strategy using the keywords “(chronic obstructive pulmonary disease) AND (lung cancer) AND survival” with the limitation of English language, human research and publication date through October 31, 2015 identified 672 articles (Figure 1). Of these, 629 were excluded on the basis of title or abstract and the full-text of 43 articles was reviewed. Three articles [15-17] were published from a single institution with the same study periods, so one study [15] was excluded and the other two articles [16,17] were divided into different analyses (i.e., stratified analyses by different cancer stages and treatment modalities) to avoid double counting the patients cohorts. Three additional studies [11,18,19] were identified from the reference in obtained articles. One published study [6] from our hospital was also added. Finally, 16 studies were included in the meta-analysis. |
|
Data extraction
|
| The relevant variables from the selected studies were collected by two researchers independently (J.D. and M.L.). The extracted data included (a) year of publication, (b) study design, (c) ethnic population, (d) number of patients, (e) diagnostic method of COPD, (f) histopathology and stage of lung cancer, (g) treatment for lung cancer, (h) OS in each group, and (i) HR and 95%CI in each study. |
|
Statistical analysis
|
| For each study, the log (HR) and its standard error were used as the outcome variables for data combination [20]. For the studies in which HR could not be achieved directly, the Kaplan-Meier survival curves from original papers would be read by Engauge Digitize version 4.1 to extract data and to calculate the HR according to the methods introduced by Tierney et al., [20]. As shown in Table 1, the HRs in 3 studies [16,21,22] were calculated by data reading from Kaplan-Meier survival curves. It is noted that there are obvious typographical errors in two papers [23,24] which give the HR and 95%CI as 1.15[0.04-2.23] and 0.74[0.83- 2.23], respectively, and therefore, these HRs were regenerated as 1.15[0.93, 1.42] and 1.36[0.83, 2.23] by RevMan Calculator. Meta-analysis was performed with RevMan version 5.1 using a random-effects model or a fixed-effect model according to the results of heterogeneity test. The heterogeneity among studies was assessed with the Cochrane Q test and I2 statistics. The publication bias was detected by funnel plot visually, and analyzed by Egger’s test quantitatively through Stata 12.0 |
Results
|
| The characteristics of the included studies are summarized in Table 1. Most studies were based on the retrospective analysis except for two (by Lopez-Encuentra [22] and Xie [6]), in which patients were enrolled prospectively. The data in four studies [18,21,22,25] came from population-based studies or multicenter trials. The size of the cohorts varied from 114 to 19,337, with a total number of 38,966 patients. COPD was mainly diagnosed by spirometry. |
|
Impact of COPD on survival of lung cancer in all patients
|
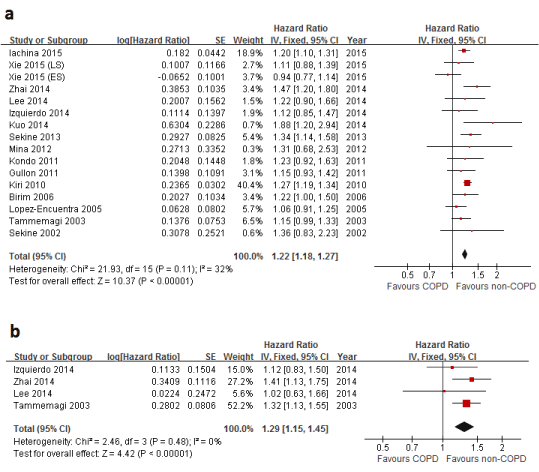
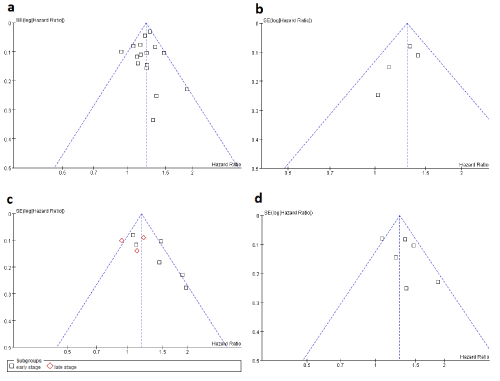
| A meta-analysis for all 15 publications reporting the impact of COPD on OS of lung cancer was shown in Figure 2A and 3A. The statistical heterogeneity was non-significant (p=0.11, I2=32%), and thus a fixed-effect model was used. The result suggested that COPD was an adverse prognostic factor in lung cancer (HR, 1.22; 95%CI, 1.18-1.27). The funnel plot displays a symmetric distribution (Figure 4A), and no sign of publication bias was proved by Egger’s test (p=0.760). |
| After adjustment for important covariates such as age, gender, smoking status, performance status and stage of lung cancer. The result (Figure 2B), on the basis of adjusted HRs in 4 available studies [10,11,26,27], suggested that COPD was an independent deleterious factor (HR, 1.29; 95%CI, 1.15-1.45). No significant heterogeneity was found (p=0.48, I2=0%). The funnel plot (Figure 4B) shows symmetry indicating no obvious publication bias, as confirmed by Egger’s test (p=0.062). |
|
Impact of COPD on survival of lung cancer in different ethnic populations
|
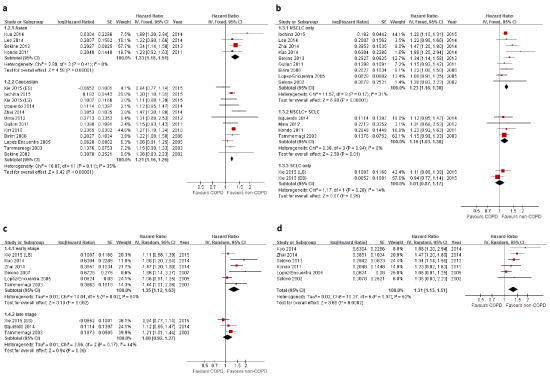
| When stratified by different ethnicities, the association remained significant in both Asians (HR, 1.33; 95%CI, 1.18-1.51) and Caucasians (HR, 1.21; 95%CI, 1.16-1.26), respectively (Figure 3A). There was no significant heterogeneity detected in any subgroups; with respective Cochran test and I2 statistics for Asian and Caucasian population being p=0.41, I2=0% and p=0.11, I2=35%. |
|
Impact of COPD on survival of lung cancer in different cancer types
|
| Histopathologic types of lung cancer were non-small cell lung cancer (NSCLC) in nine studies, small cell lung cancer (SCLC) in one study, and mixed types (NSCLC+SCLC) in four studies (Figure 3B). A stratified analysis showed that the difference in OS between patients with and without COPD was significant in NSCLC (HR, 1.23; 95%CI, 1.16-1.30), less significant in mixed types (HR, 1.16; 95%CI, 1.03-1.30), but not significant in SCLC (HR, 1.01; 95%CI, 0.87-1.17). No obvious heterogeneity in each group (p=0.17, I2=31%; p=0.94, I2=0%; p=0.28, I2=14%) or publication bias (p=0.462) was detected. |
|
Impact of COPD on survival of lung cancer in different cancer stages
|
| Seven studies reported the impact of COPD in stage-specific lung cancer including six publications studying early stage (stage I-II or limited stage) and three publications studying late stage (stage III-IV or extensive stage) (Figure 3C). A stratified analysis showed that COPD had a significantly negative impact on early stage lung cancer (HR, 1.35; 95%CI, 1.12-1.63) but not on late stage lung cancer (HR, 1.08; 95%CI, 0.92-1.27). Because the statistical heterogeneity was moderate in the early stage subgroup (p=0.03, I2=62%), a random-effects model was used. No significant publication bias was found by either funnel plot (Figure 4C) or Egger’s test (p=0.058). |
|
Impact of COPD on survival of lung cancer in different treatment modalities
|
| Only one study [27] focused on patients receiving non-surgical treatment (chemotherapy and/or tyrosine kinase inhibitors) and the result suggested that COPD has no impact on the mortality in this population (HR, 1.12; 95%CI, 0.85-1.47). In patients receiving surgical treatment (Figure 3D), six studies were included and a pooled analysis showed a significant association between the presence of COPD and worse OS (HR, 1.31; 95%CI, 1.13-1.51). The statistical heterogeneity was mild (p=0.07, I2=52%), so that a random-effects model was used. The funnel plot shows symmetry (Figure 4D) and no publication bias was detected (p=0.316). |
Discussion
|
| This meta-analysis based on 16 studies which examined the association between the presence of COPD and the prognosis of lung cancer has verified that COPD had a significant deleterious impact on lung cancer survival regardless of the ethnic groups studied. In addition, the impact of COPD appeared to be more pronounced in NSCLC, early stage, and surgically-treated patients. |
| Recently, many studies were keen on the relationship between COPD and the risk of lung cancer [3,4,28]; however, the prognostic impact of COPD on lung cancer is not clearly defined. To our knowledge, this is the first meta-analysis to quantify its impact based on a strict screening for patients with COPD. In previous studies, COPD was shown to correlate with higher rates of tumor recurrence and metastasis [17,29], with underlying mechanism by which COPD affects the prognosis of lung cancer remaining elusive. Some studies indicated that a host environment with chronic inflammation could contribute to the poor prognosis [30,31]. Besides, genetic and epigenetic pathway in, for example, SPARC, p16 and smoking-related CXCL14 gene may also modulate the prognosis [32,33]. |
| COPD is characterized by two components: airflow obstruction (bronchitis) and peripheral airspace disease (emphysema), and chronic inflammation is involved in both of their pathogenesis ,34]. One common feature of this chronic inflammation is the influx of neutrophils [35] while the neutrophilic inflammation in the context of lung cancer may act as a dual-edged sword [36]. On one hand, it can suppress tumor progression by means of direct tumor cytotoxicity [37]. On the other hand, it may possess a tumorpromoting effect not only to survive cancer cells by suppressing the action of cytotoxic lymphocytes [35], but also to promote tumor metastasis by facilitating tumor cell transendothelial migration [38]. As a result, many studies indicated that the neutrophil content or neutrophil to lymphocyte ratio in the tumor and/or in the peripheral blood inversely correlated with the outcome in various types of solid cancers, including SCLC [6] and NSCLC [35,39]. |
| Another mechanistic explanation proposed by Dvorak [40] is that chronic inflammation can activate angiogenesis and increase vascular permeability, which provides support for the malignant cells, by which tumors are likely to behave as “wounds that do not heal” [41]. Besides, in some cases, inflammation could diminish the beneficial effects of therapy [42]. Clinically, Berry et al., [43] has disclosed an apparent decrement in survival for patients with lower pulmonary function. Therefore, considering the results documented previously and presented in our study, COPD did negatively impact the long-term outcome in lung cancer, and this disadvantage was also observed after the adjustment for important prognostic factors (e.g., smoking status, performance status, and stage of disease). Further studies including genomic and epigenetic analysis to explore and verify this prognostic link are still warranted, which could serve as a novel biologic target for both prevention and treatment of lung cancer with COPD.In this meta-analysis, examinations for pooled HR were performed among all ethnic, pathological, and staging subgroups. The presence of COPD had a negative influence in both examined ethnicities (Asian and Caucasian). Histopathologically, the association of COPD with NSCLC survival seemed more pronounced than that with SCLC, because the pooled HR was significant in NSCLC but not significant in SCLC. We hypothesize that this subgroup-specific association can be partly explained by a highly aggressive nature and a rapid disease progression of SCLC, which could minimize or overwhelm any potential influence of patient’s comorbidities like COPD on survival [44]. |
| Although there likely be an interaction between stage of disease and choice of treatment for lung cancer, we are unable to conduct a multivariate model for the meta-analysis because of the inconsistent data provided in the published studies. When analyzing stage-specific subgroup separately, the pooled HR was significant in early stage but not significant in late stage lung cancer. Although only three studies were eligible for analysis in the late stage subgroup, the HR in each study was merely marginally significant or even not significant. With regard to treatment modalities, one study by Izquierdo et al., [27] demonstrated no influence of COPD on OS of lung cancer in patients receiving non-surgical treatment whereas our meta-analysis revealed a worse survival in surgically-treated patients with COPD. Owing to the fact that surgical treatment is commonly associated with diseases in the early stage, it is reasonable to infer that the influence of COPD is more evident in patients at an early stage and/or receiving surgical treatment. However, this result did not mean that patients with COPD were unfit for surgery, because lung resection for tumor sometimes had volume reduction benefit, contributing to improved quality of life [45]. The findings of this investigation highlight the association of COPD and lung cancer prognosis, disclosing the inhomogeneous magnitude of COPD in different phases of lung cancer, thus aiding the decision-making process when clinicians are selecting best possible strategies for the management and long-term monitoring in this particular population, especially in the field of multidisciplinary care for inflammation control and pulmonary rehabilitation because both may be beneficial to lung cancer outcome [46]. |
| There were several limitations in the current study. First, the majority of the enrolled studies were retrospective and the numbers of studies in the subgroups were relatively small, which might be subject to some biases, such as selection bias. Second, due to the lack of accessible data, we were unable to perform a stratified analysis by severity of COPD since the result by Sekine et al., [16] suggested that the severity of COPD was related to lung cancer prognosis. Third, two HRs in this meta-analysis were obtained from the data in 2-year and 3-year follow-ups [19,21], which may be not as accurate as the HRs calculated from 5-year follow-up information. Future studies should supplement the impact of COPD on survival of SCLC and in patients with other nonsurgical treatment, and investigate the mechanisms underlying the association of COPD with lung cancer prognosis. |
Conclusion
|
| In conclusion, our meta-analysis not only has confirmed that COPD is an independent prognostic factor of lung cancer survival, but also demonstrated that the deleterious impact tends to be more pronounced in patients with non-small cell lung cancer, at an early-stage, and on those who received surgical treatment. Further mechanistic investigations for this relationship and potential clinical interventions are warranted. |
Contributors
|
| J.D. and M.L. searched the literatures and collected the data. J.D. undertook the analysis and G.J. supervised analysis. G.J. interpreted the results and J.D. drafted the paper. P.Y. revised the paper. All authors contributed to the approved the final draft for publication. |
Acknowledgement
|
| The authors appreciate Ms Connie E. Edwards, for her professional editing and technical assistance with the manuscript. |
Tables at a glance
|
 |
| Table 1 |
|
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|









