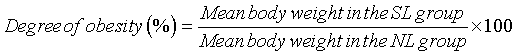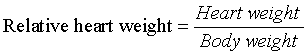Keywords
Adiponectin, Cytokines, Obesity, Early, Heart
Introduction
As obesity is a global problem in each country, treatment and prophylaxis of the obesity have been important issue. Control of obesity during childhood can be important role in prophylaxis of obesity because the obesity in childhood easily induces adult obesity. Stettler et al. have focused on this problem and interestingly, it appears that there are critical periods during development that strongly increase the risk of long-term obesity. Weight gain in the first week of life can increase the risk of permanent overweight in adulthood [1] and being overweight in childhood can increase the risk of coronary heart disease in adulthood [2].
Cardiac tissue mainly consists of myoctyes which are surrounded by extracellular matrix and the major component of the extracellular matrix is collagen [3]. In other words, cardiac tissue is composed of cellular and non-cellular components. The cellular components are made up of major myocytes and minor endothelial cells. The non-cellular components are made up of the extracellular matrix and the interstitial fluid. The major component of the extracellular matrix is collagen. The interstitial fluid is associates with cytokines released by cells that have a specific effect on the interactions between cells. The cytokines includes cell signal molecules, such as tumor necrosis factor and the interferons, which trigger inflammation [4].
Therefore, the first, we investigated PCNA cells and apoptotic cells to observe turnover (proliferation and necrosis) of myocytes. The up-regulation of cellular turnover in myocytes, especially PCNA cells and apoptotic cells in early obesity was confirmed in our previous study [5]: early obesity showed different patterns of cellular changes [6]. The second, we surveyed the deposition of the collagen in the interstitial space because an increase in collagen deposition (fibrosis) in almost all cardiac diseases is observed [3] and obesity induces cardiac fibrosis due to increases of collagen during early development in rats [7]. The last, we investigated the cytokines in cardiac obesity associated with turnover of the extracellular matrix and inflammation. Adipose cytokines (adipokines; adioponectin, PAI-1), inflammatory cytokines (TNF-α, ED-1, MCP-1) and extracellular matrix cytokines (MMP, TIMP) are associated with child obesity and metabolic syndrome and are investigated to treat early obesity [8-10].
Materials and Methods
Twenty virgin Sprague–Dawley female rats at the age of 3 months mated with the same species of male rats at the almost same time to obtain time-matched siblings. The pups were delivered from pregnant mother rats after 21 days and the pups which have extremely small or large body weights with more than two standard errors were excluded. The male pups among the pups at the first day of life were selected and female pups were discarded. The individual litters were randomly distributed to the real dams or surrogate dams. We defined 10 male pups per dam as the control group [the normal litter (NL) group)] and 3 male pups per dam as the obesity group [the small litter (SL) group].
The definition of 10 male pups in the normal litter (NL) as the control group means that a rat dam has 10 nipples and 10 male pups compete with each other to gain much milk because of spatial and quantitative limitations. Also this condition is similar to the natural rearing status. The definition of 3 male pups in the small litter (SL) means that 3 male pups eat sufficient milk without competing with siblings. This condition is artificial rearing status but there was no other food intervention except enough maternal breast feeding. The body weights of pups in each group were measured every 3 days from the 1st to the 28th day after birth. All laboratory procedures were performed with the approval of the Animal Care Committee of Korea University Guro Hospital and in accordance with the recommendations of the Guidelines for the Care and Use of Laboratory Animals.
Animal preparation
The pups in each group were sacrificed on the 28th day after birth and their heart organs were extracted from the body to prepare the Masson trichrome (MT) staining and Western blotting. The blood was collected to measure serum glucose with use of the Glucostick®.
Masson trichrome (MT) stain
Obesity usually causes systemic hypertension and it can burden the left ventricle as a systemic ventricle rather than both the atria and right ventricle [11]. Therefore, we chose to perform MT staining and Western blotting to investigate pathologic change of the left ventricles by effects of obesity. MT staining is a threecolored staining system to detect collagen and other components, and the collagen is differentially stained blue. The amount of collagen is proportional to the increase in fibrotic changes. Point detection was used to identify the amount of interstitial collagen. Latticed squares buried in the microscopic lens were used for the point detection method. A larger square that was 25 × 25 μm2 had 100 smaller squares that were 2.5 × 2.5 μm2. The 100 smaller squares comprised a total of 121 vertexes in a larger square. If the stained collagen fiber crossed at the individual vertexes of the smaller square, then that was defined as a positive detection. The final average of the positive detection of collagen was counted and compared between two groups. The myocytes were measured via microscopy at 400 × in 20 fields as non-overlapped areas. We then compared the amount of collagen fibers between two groups.
Western Blotting
Western blotting was performed with tissue preparation, gel electrophoresis, transfer, blocking, and detection with primary (Santa Cruz, California, USA) and secondary antibodies (anti-rabbit IgG, Millipore, Temecula, USA; anti-mouse IgG, KPL, Gaithersburg, USA; anti-goat IgG, R & D systems, Minneapolis, USA). We used primary antibodies against obesity-induced inflammatory cytokines such as matrix metalloproteinase 9 (MMP-9), tissue inhibitor of MMPs type 1 (TIMP-1), plasminogen activator inhibitor 1 (PAI-1), tissue necrosis factor-α (TNF-α), anti-CD68 antibody of tissue macrophages (ED-1), monocyte chemotactic protein 1 (MCP-1) and adiponectin. The protein density for each antibody was measured by computerized densitometry using the Image PC alpha 9® program (NIH, Bethesda, MD, USA), and those values were divided by the density of β-actin, which is regularly expressed in all cells. We compared each value of protein density/ β-actin density in the SL group with that in the NL group.
Statistical analysis
The means with standard errors for body weight and Western blotting in the SL group were compared with those of the NL group using t-tests. Statistical significance was adopted at P values < 0.05 and the statistics program, Sigma Stat® (Version 2.03, Systat Software, Point Richmond, CA, USA) was used for all statistical analyses.
Results
Body weights were not significantly different between the two groups on the first day after birth, but a significant difference developed from the 4th day to the 28th day (all P < 0.001; Figure 1). On the 28th day, body weights and heart weights increased significantly in the SL group compared to those in the NL group (P < 0.001 and P = 0.003, respectively) and the mean relative heart weight decreased significantly in the SL group compared to those in the NL group (P = 0.025; Table 1). Serum glucose level and collagen were increased in the SL group compared to those in the NL group on MT staining, but these changes were not significant (Table 1 and Figure 2).
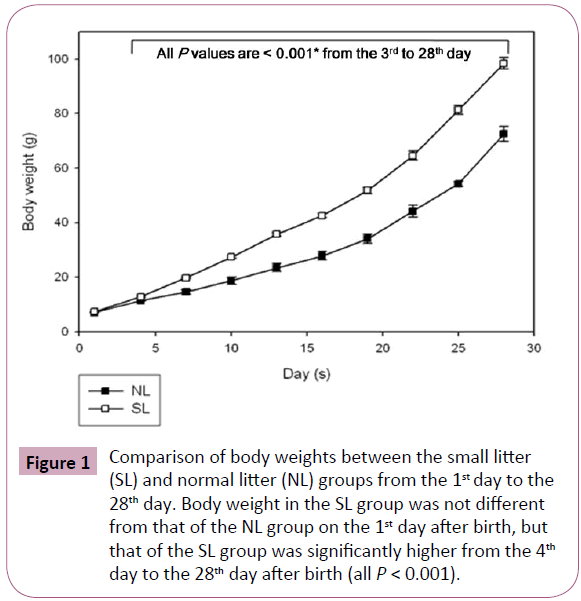
Figure 1: Comparison of body weights between the small litter (SL) and normal litter (NL) groups from the 1st day to the 28th day. Body weight in the SL group was not different from that of the NL group on the 1st day after birth, but that of the SL group was significantly higher from the 4th day to the 28th day after birth (all P < 0.001).
| |
NL group (n = 50) |
SL group (n = 15) |
P value* |
| Body weight (g) at 1 day |
7.45 ± 0.04 |
7.57 ± 0.11 |
0.453 |
| Body weight (g) at 28 days |
70.23 ± 1.75 |
98.00 ± 3.50 |
< 0.001* |
| Heart weight (g) at 28 days |
0.45 ± 0.02 |
0.57 ± 0.02 |
0.003* |
| Relative heart weight at 28 days |
(61.2 ± 1.8) ? 10-4 |
(55.6 ± 2.1) ? 10-4 |
0.025* |
| Serum glucose (mg/dL) |
147 ± 7.1 |
153 ± 5.8 |
0.148 |
*P < 0.05 in the SL compared with the NL by use of the t-test. NL: normal litter; SL: small litter; g: gram
Table 1: Body weight, heart weight, relative heart weight (heart weight divided by body weight), and serum glucose
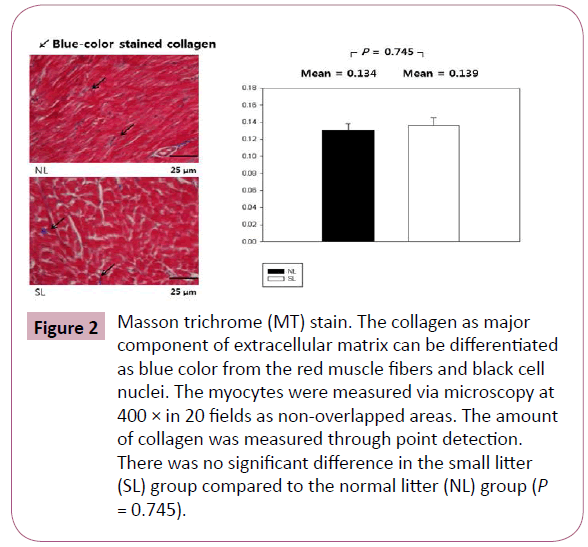
Figure 2: Masson trichrome (MT) stain. The collagen as major component of extracellular matrix can be differentiated as blue color from the red muscle fibers and black cell nuclei. The myocytes were measured via microscopy at 400 × in 20 fields as non-overlapped areas. The amount of collagen was measured through point detection. There was no significant difference in the small litter (SL) group compared to the normal litter (NL) group (P = 0.745). Figure
The inflammatory cytokines associated with obesity were measured by Western blotting. The inflammatory cytokines associated with turnover in the extracellular matrix are MMP-9 and TIMP-1. Protein expression of MMP-9 in the SL group was increased significantly compared to those in the NL group (P < 0.001; Figure 3), but the increase of TIMP-1 in the SL group was not significant compared to those in the NL group (P = 0.340; Figure 4). The inflammatory cytokines associated with proinflammatory reactions (adipokines) are PAI-1 and TNF-α; protein expression of PAI-1 in the SL group was significantly increased compared to those in the NL group (P < 0.001; Figure 5), but the increase in TNF-α was not significant (P = 0.221; Figure 6). Adiponectin is an inflammatory cytokine associated with antiinflammatory activity; the protein expression of adiponectin in the SL group was significantly increased compared to those in the NL group (P = 0.009; Figure 7). The inflammatory cytokines associated with macrophage recruitment are ED-1 and MCP-1; the protein expression of ED-1 and MCP-1 in the SL group was not increased compared to those in the NL group (P = 0.254 and P = 0. 657, respectively; Figures 8 and 9).
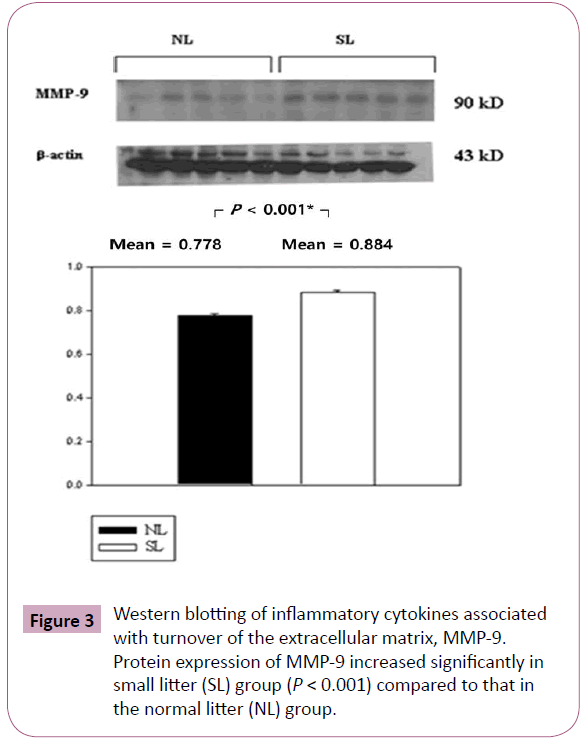
Figure 3: Western blotting of inflammatory cytokines associated with turnover of the extracellular matrix, MMP-9. Protein expression of MMP-9 increased significantly in small litter (SL) group (P < 0.001) compared to that in the normal litter (NL) group.
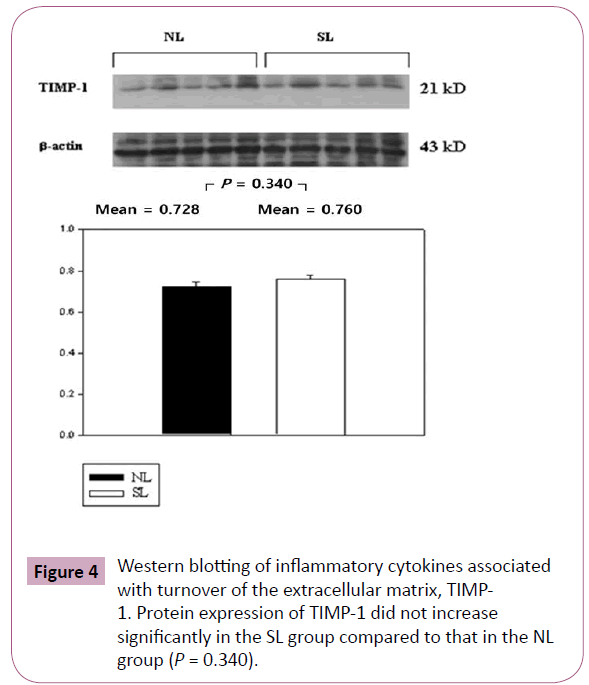
Figure 4: Western blotting of inflammatory cytokines associated with turnover of the extracellular matrix, TIMP- 1. Protein expression of TIMP-1 did not increase significantly in the SL group compared to that in the NL group (P = 0.340).
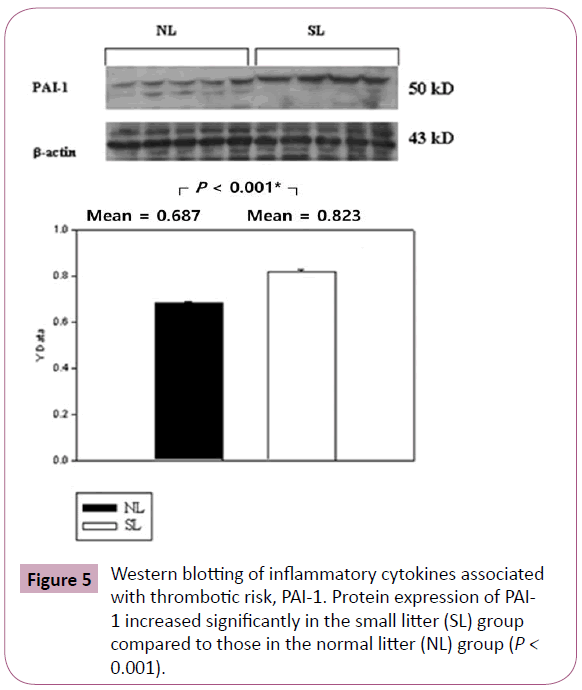
Figure 5: Western blotting of inflammatory cytokines associated with thrombotic risk, PAI-1. Protein expression of PAI- 1 increased significantly in the small litter (SL) group compared to those in the normal litter (NL) group (P < 0.001).
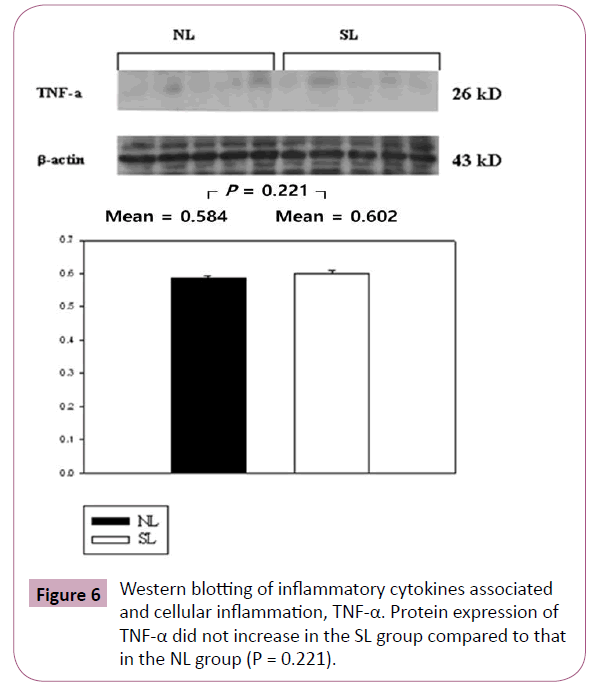
Figure 6: Western blotting of inflammatory cytokines associated and cellular inflammation, TNF-α. Protein expression of TNF-α did not increase in the SL group compared to that in the NL group (P = 0.221).
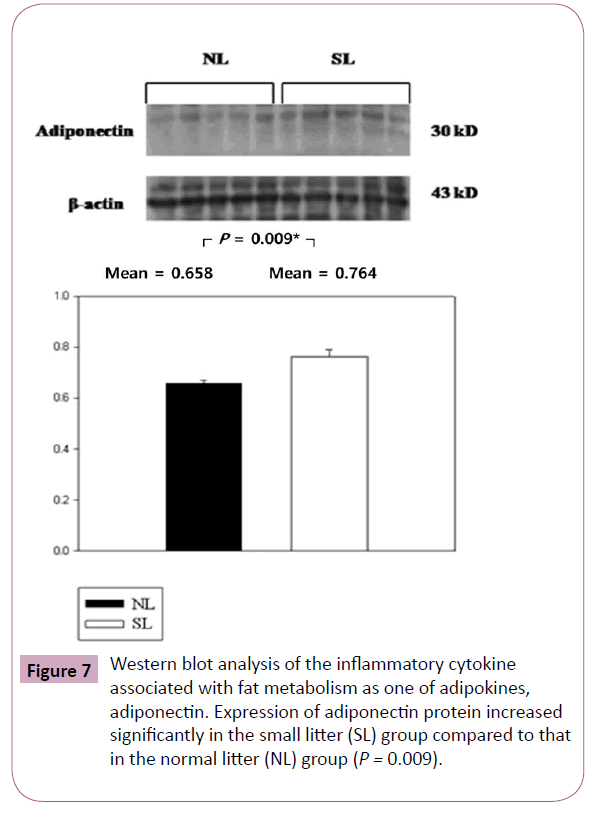
Figure 7: Western blot analysis of the inflammatory cytokine associated with fat metabolism as one of adipokines, adiponectin. Expression of adiponectin protein increased significantly in the small litter (SL) group compared to that in the normal litter (NL) group (P = 0.009).
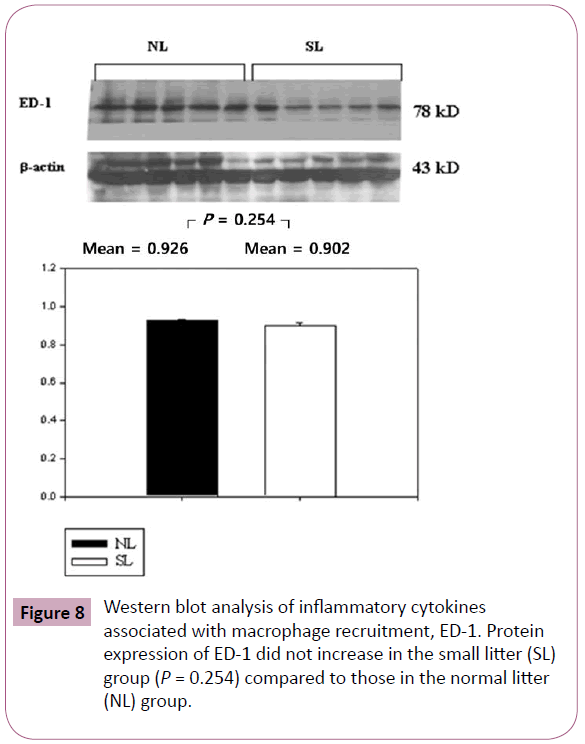
Figure 8: Western blot analysis of inflammatory cytokines associated with macrophage recruitment, ED-1. Protein expression of ED-1 did not increase in the small litter (SL) group (P = 0.254) compared to those in the normal litter (NL) group.
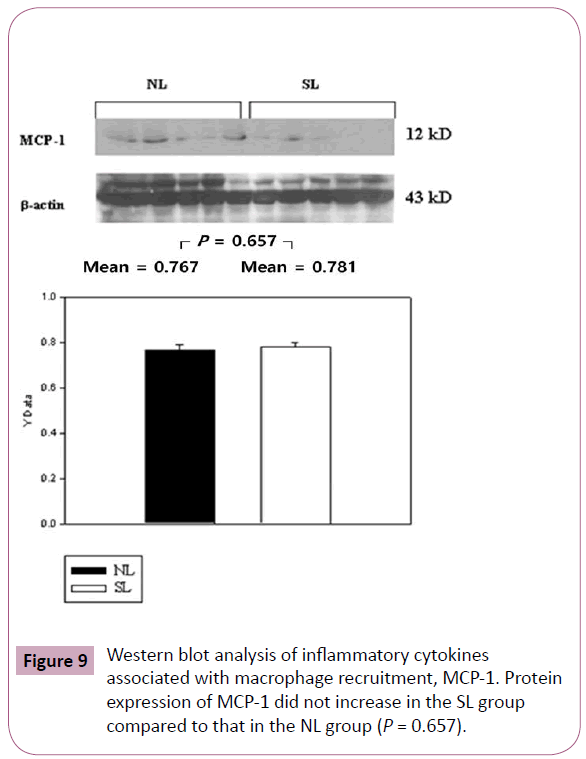
Figure 9: Western blot analysis of inflammatory cytokines associated with macrophage recruitment, MCP-1. Protein expression of MCP-1 did not increase in the SL group compared to that in the NL group (P = 0.657).
Discussion
The critical periods for developing obesity are the prenatal period, early infancy, the period between 5 and 7 years of age, and adolescence [12]. Infancy in human is one of these critical periods because of weaning from maternal milk at about 1 year old, similar to weaning at the 28th day in rats. Therefore, we selected this period because it is important to understand the pathophysiology of early obesity.
We compared the degree of obesity in overfed rats (the obesity, SL group) with that of the control, NL group. The degree of obesity in the SL group was expressed as follows:
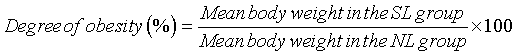

The degree of obesity at the 28th day was 139.54% (98 / 70.23 × 100 (Figure 1 and Table 1, P < 0.001) and the value of the SL group at the 28th day can be defined as obesity although the definition is from human research [13].
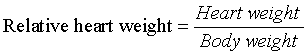
Mean relative heart weight in the SL group decreased more than that in the NL group, indicating that the growth rate of the total body was much faster than that of the heart. This may cause relative cardiac burden for maintaining the body. However it is unclear that this imbalance is associated with cardiac morbidity and mortality or not, because an increase in body weight should not correspond in a proportional increase in heart weight and there are coexisting substantial risk factors.
Myocytes are the major cellular component of cardiac cells and their proliferation is suppressed under normal physiologic conditions within 30 days after birth at rat [14]. However, our previous research showed that early-onset obesity induced reactivation of cellular components while they were physiologically suppressed [5]. Therefore, we were interested in looking at the changes in extracellular components as well as cellular components. Collagen is the main cardiac component of the extracellular matrix; early obesity induces increases in fibrotic deposition of collagen in sheep [15]. In order to investigate the changes in collagen in the obesity group, we performed Masson trichrome staining of the heart. Collagen was increased in the obesity group, but this change was not statistically significant. Early obesity causes changes in extracellular components through the increase in collagen as well as cellular components.
The most important molecules for molecular turnover in the extracellular matrix are MMP and TIMP. As clinical data support an important role MMP-9 and TIMP-1 in cardiovascular risk, we chose to analyze MMP-9 and TIMP-1 [16,17]. MMP-9 has a specific interaction with type IV collagen and is more involved in turnover of the extracellular matrix compared to other MMPs [18]. MMP- 9 is found at elevated concentrations in obese subjects [19,20] and increased MMP-9 is a risk factor for cardiovascular morbidity and mortality. 16 Increased TIMP-1 levels are also associated with obesity [21] Therefore, early obesity induced an imbalance in MMP and TIMP and eventually increased MMP-9 significantly and TIMP-1 nonsignificantly. Increases in MMP-9 and TIMP-1 may influence turnover of extracellular matrix and affect cardiac morbidity in early obesity.
PAI-1 is a principle inhibitor of tissue plasminogen and the inflammatory cytokine produced in adipose tissue [22]. Upregulation of PAI-1 in obesity induces endogenous thrombosis and cardiac fibrosis, and an increase in PAI-1 enhances the risk of cardiovascular disease [23]. Our result showed that early obesity increased protein expression of PAI-1. This reaction may be related to increases in thrombotic risk during early development of the rat heart.
The level of adiponectin varies with different inflammatory diseases and it is related to pro- and anti-inflammatory cytokines in obesity [24]. Adiponectin is inversely correlated with body fat mass in adults and children (adiponectin levels are decreased in obese subjects) [25]. Adipokines (such as leptin, TNF-α, PAI-1, IL-1 beta, IL-6 and IL-8) are proinflammatory cytokines and they are increased in obesity.8 Adiponectin inhibits the expression of TNF-α in adipocytes, and, reciprocally, TNF-α inhibits the production of adiponectin [26,27]. Our results showed that adiponectin was increased and TNF-α was increased (nonsignificantly) in the obesity group. As adiponectin levels are decreased in obese subjects [27]. The role of adiponectin in the inflammatory processes during early obesity changes; in other words, up-regulation of adiponectin occurs in early-onset obesity. This fact can be supported by Inami et al. who revealed that the level of adiponectin during the first few days of life correlates positively with neonatal adiposity and increased adiponectin and has a good effect on postnatal growth and development [28]. Also, Shibata et al. insisted that adiponectin mediates cardiovascular protection through attenuation of inflammatory responses and apoptotic activities in the heart [29]. Therefore, an increase in adiponectin in early obesity can be associated with cardiac growth and protection against inflammatory progression, in contrast to the decrease in adiponectin seen in late obesity. Also, adiponectin in early obesity may influence the level of TNF-α or other pro-inflammatory cytokines, and this needs to be further investigated.
Increases in adiponectin during youth are a strong and independent correlate of insulin sensitivity; the anti-diabetogenic property of adiponectin is also evident early in life [30]. Our results showed that the serum level of glucose increased, but the change was non-significant in the SL group (Table 1). Adiponectin may have an influence on insulin sensitivity in obesity and the relationship between the two should be investigated.
ED-1 (CD68) and MCP-1 mediate recruitment of macrophages and they are involved in inflammatory reactions of cardiovascular disease [31]. We looked for increases in ED-1 and MCP-1, but there were no significant changes in the obesity group. Other pathways involved in changes from recruiting macrophages should be researched.
Early obesity causes the over-expression of inflammatory cytokines such as MMP-9, TIMP-1, PAI-1, and TNF-α. These cytokines have an influence on the extracellular matrix and thrombotic morbidity associated with inflammatory reaction. The level of adiponectin is increased in other ways because adiponectin in early obesity is associated with cardiac growth and protective mechanisms against inflammatory progression. The anti-inflammatory reaction of the adiponectin is most prominent in the early obesity period around the 28th day after birth. We should investigate how long anti-inflammatory and cardiac protective effects of the adiponectin reaction continue to provide protection against obesity. If we can determine when the antiinflammatory effect of the adiponectin ends, we will learn more about the pathophysiology of obesity. This study provides new findings regarding early-onset obesity.
Our study had a few limitations. It is not clear whether the early findings in rats can be generalized to humans because experimental data obtained from animal experiments do not always correlate to human biology.
Acknowledgements
This work was supported by the Korea Research Foundation (K. H. Yoo) funded by the Korean Government (KRF-2008-313-E00308).
8855
References
- Stettler N, Stallings VA, Troxel AB, Zhao J, Schinnar R, et al. (2005) Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation 111: 1897-1903.
- Baker JL, Olsen LW,Sørensen TI (2007) Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 357: 2329-2337.
- de Jong S, van Veen TA, de Bakker JM, van Rijen HV (2012) Monitoring cardiac fibrosis: a technical challenge. Neth Heart J 20: 44-48.
- vanKimmenade RR, Januzzi JL Jr (2012) Emerging biomarkers in heart failure. ClinChem 58: 127-138.
- Ha KS, Yoo KH, Yim HE, Jang GY, Bae IS, et al. (2011) Cellular and RAS changes in the hearts of young obese rats. PediatrCardiol 32: 659-666.
- Cayli S, Ustünel I, Celik-Ozenci C, Korgun ET, Demir R (2002) Distribution patterns of PCNA and ANP in perinatal stages of the developing rat heart. ActaHistochem 104: 271-277.
- Velkoska E, Cole TJ, Dean RG, Burrell LM, Morris MJ (2008) Early undernutrition leads to long-lasting reductions in body weight and adiposity whereas increased intake increases cardiac fibrosis in male rats. J Nutr 138: 1622-1627.
- Guzzardi MA, Iozzo P (2011) Fatty heart, cardiac damage, and inflammation. Rev Diabet Stud 8: 403-417.
- Alikasifoglu A, Gönç N, Özön ZA, Sen Y, Kandemir N (2009) The relationship between serum adiponectin, tumor necrosis factor-alpha, leptin levels and insulin sensitivity in childhood and adolescent obesity: adiponectin is a marker of metabolic syndrome. J Clin Res PediatrEndocrinol 1: 233-239.
- Chavey C, Mari B, Monthouel MN, Bonnafous S, Anglard P, et al. (2003) Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J BiolChem 278: 11888-11896.
- Devereux RB, Roman MJ (1999) Left ventricular hypertrophy in hypertension: stimuli, patterns, and consequences. Hypertens Res 22: 1-9.
- Dietz WH1 (1994) Critical periods in childhood for the development of obesity. Am J ClinNutr 59: 955-959.
- Shim TS, Ko KW (1986) The growth standard of Korean children in 1985. Korean Journal of Pediatrics 29: 1-21.
- Marino TA, Cao W, Lee J, Courtney R (1996) Localization of proliferating cell nuclear antigen in the developing and mature rat heart cell. Anat Rec 245: 677-684.
- Huang Y, Yan X, Zhao JX, Zhu MJ, McCormick RJ, et al. (2010) Maternal obesity induces fibrosis in fetal myocardium of sheep. Am J PhysiolEndocrinolMetab 299: E968-975.
- Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, et al. (2003) Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 107: 1579-1585.
- Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, et al. (2006) Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart J 151: 1101.
- Vu TH, Werb Z (2000) Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev 14: 2123-2133.
- Derosa G, Ferrari I, D'Angelo A, Tinelli C, Salvadeo SA, et al. (2008) Matrix metalloproteinase-2 and -9 levels in obese patients. Endothelium 15: 219-224.
- Andrade VL, Fernandes KS, Bosco AA, Tanus-Santos JE, Sandrim VC (2012) Functional polymorphism located in MMP-9 gene promoter is strongly associated with obesity. DNA Cell Biol 31: 1054-1057.
- Głowińska-Olszewska B, Urban M (2007) Elevated matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 in obese children and adolescents. Metabolism 56: 799-805.
- De Taeye B, Smith LH, Vaughan DE (2005) Plasminogen activator inhibitor-1: a common denominator in obesity, diabetes and cardiovascular disease. CurrOpinPharmacol 5: 149-154.
- Gils A, Declerck PJ (2004) The structural basis for the pathophysiological relevance of PAI-I in cardiovascular diseases and the development of potential PAI-I inhibitors. ThrombHaemost 91: 425-437.
- Aprahamian TR, Sam F (2011) Adiponectin in cardiovascular inflammation and obesity. Int J Inflam 2011: 376909.
- Garg MK, Dutta MK, Mahalle N (2012) Adipokines (adiponectin and plasminogen activator inhhibitor-1) in metabolic syndrome. Indian J EndocrinolMetab 16: 116-123.
- Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 2001;50:2094-9.
- Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, et al. (2003) Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. BiochemBiophys Res Commun 301: 1045-1050.
- Inami I, Okada T, Fujita H, Makimoto M, Hosono S, et al. (2007) Impact of serum adiponectin concentration on birth size and early postnatal growth. Pediatr Res 61: 604-606.
- Shibata R, Murohara T, Ouchi N (2012) Protective role of adiponectin in cardiovascular disease. Curr Med Chem 19: 5459-5466.
- Bacha F, Saad R, Gungor N, Arslanian SA (2004) Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care 27: 547-552.
- Niu J, Kolattukudy PE (2009) Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. ClinSci (Lond) 117: 95-109.