Ishengoma DS1, Shayo A2,10, Mandara CI1, Baraka V1,3, Madebe RA1, Ngatunga D2,4, Kamugisha E5,Gesase S1, Ngadaya E6, Mghamba J7, Njau R8, Mandike R9, Mkude S9, Mohamed A9, Buzza J2 and Lemnge MM1
1National Institute for Medical Research, P.O Box 5004, Tanga, Tanzania
2The Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania
3Department of Epidemiology, University of Antwerp, International Health Unit, Antwerp, Belgium
4Tanzania Food and Drugs Authority, Dar es Salaam, Tanzania
5Catholic University of Health and Allied Sciences - Bugando, Mwanza, Tanzania
6National Institute for Medical Research, Muhimbili Research Centre, Dar es Salaam, Tanzania
7Epidemiology and Disease Control Section, Ministry of Health and Social Welfare, Dar es Salaam, Tanzania
8World Health Organisation Country Office, Dar es Salaam, Tanzania
9National Malaria Control Programme, Dar es Salaam, Tanzania
10Department of Biotechnology and Bioinformatics, The University of Dodoma, Dodoma, Tanzania
- *Corresponding Author:
- Deus S. Ishengoma
National Institute for Medical Research
Tanga Research Centre, P.O Box 5004, Tanga, Tanzania.
Tel: +255 754 528 891
Fax: +255 27 2642010
E-mail: deusishe@yahoo.com
Received date: March 16, 2016; Accepted date: May 28, 2016; Published date: May 31, 2016
Citation: Ishengoma DS,Shayo A, Mandara CI, et al. The Role of Malaria Rapid Diagnostic Tests in Screening of Patients to be Enrolled in Clinical Trials in Low Malaria Transmission Settings. Health Syst Policy Res. 2016, 3:2. doi:10.21767/2254-9137.100032
Keywords
Diagnostic tests; Malaria transmission; Screening
Background
Prompt diagnosis with parasitological confirmation and effective treatment with efficacious antimalarials have been advocated by the World Health Organisation (WHO), among the most effective strategies for malaria control which could help to reduce malaria related morbidity and mortality [1-3]. However, the recently reported artemisinin resistance in the Great Mekong sub-region (Cambodia, The Lao People’s Democratic Republic, Myanmar, Thailand and Vietnam) is a major concern for malaria control and therefore, urgent surveillance and/or containment measures need to be launched by all malaria endemic countries as recommended by WHO [4,5]. Continuous and sustained surveillances of antimalarial efficacy and parasite resistance are critical components of the WHO Global plan for artemisinin resistance containment (GPARC) [5].
Microscopic examination of blood smears to confirm the presence of malaria parasites remains the gold standard for malaria diagnosis and it is the most commonly used method in malaria clinical trials [6]. However, microscopy is limited by demands for equipment, laboratory infrastructures including electricity, skilled personnel and sustained delivery of laboratory supplies and consumables [6-8]. As a result of such limitations, WHO recommended use of malaria rapid diagnostic tests (mRDTs) for parasitological confirmation in routine practice as part of the strategies to improve case management and reduce over prescription of antimalarial (particularly artemisinin combination therapy-ACTs which are relatively expensive) [1]. The strategy is also aimed at improving the quality of care, reducing misuse of the drugs by targeting patients with parasites and preventing emergence of parasite resistance to ACTs [2,3,9]. Thus, most of malaria endemic countries have deployed mRDTs for parasitological confirmation of malaria in public health facilities particularly in remote areas with limited capacity for performing high quality microscopy [3,10].
The currently used mRDTs rely on detection of three different types of Plasmodium antigens namely, Plasmodium histidine rich protein 2 (pHRP-2), Plasmodium lactate dehydrogenase (pLDH) and Plasmodium aldolase (pAldo) [11,12]. The mRDTs based on pHRP-2 are only speciï¬ÂÂÂÂc to Plasmodium falciparum, while the tests which utilise pLDH and pAldo antigens are speciï¬ÂÂÂÂc to the four common malaria parasite species (P. falciparum, P. vivax, P. malariae and P. ovale) [11,12]. Plasmodium glutamate dehydrogenase (pGluDH) is another antigen which has also been considered for production of mRDTs for all malaria parasite species but such tests are not yet in the market [13]. By combining different antigens, mRDTs capable of detecting the four human malaria parasites have been developed and have received wide applicability in clinical settings [11,12]. Reports have shown that mRDTs have good operation accuracy despite wide variability which depends on other factors such as malaria endemicity, level of parasitaemia, type and thermo-stability of the tests, and user’s skills [14]. Overall, mRDTs are highly acceptable with high level of applicability for case management particularly in health facilities which lack equipment, resources and skilled technicians to perform good quality microscopy.
Tanzania introduced mRDTs for malaria diagnosis in all public health facilities and the phased introduction process was completed in 2012 (NMCP, unpublished data). To ensure all malaria cases receive prompt diagnosis, the National Malaria Control Program (NMCP) and its partners are developing strategies to introduce mRDTs in private outlets using an approach similar to the affordable medicine facilities for malaria medicines (AMFm) [15,16]. Five brands of mRDTs have been registered by the Tanzania Food and Drugs Authority (TFDA), namely Paracheck Pf® (Orchid Biomedical Systems - Mumbai, India), ParaHIT® (Span Diagnostics - Surat, India), ICT Malaria-Combo (ICT Diagnostics, South Africa), First® Response (Premier Medical Corporation Limited, India) and SD Bioline Malaria Ag Pf/Pan (Standard Diagnostics Inc., India) (S. Mkude, Personal Communication). The mRDTs are currently available in retail pharmacy shops and wholesale distributors for the private sector while the public sector depends on the medical stores department (MSD) which is a government owned procurement and distribution agent.
Although mRDTs are now widely used in public health facilities for malaria diagnosis in Tanzania and other malaria endemic countries, their applicability in clinical trials for screening and management of patients has not been well assessed. With declining malaria burden which has created a demand of screening many patients before confirming patients to be enrolled in clinical trials, there has been an interest in utilizing mRDTs to screen patients before recruitment [6,17]. Using mRDTs could help to quickly screen a large number of patients leading to saving time and resources which would otherwise be used for microscopy based screening process. However, the operational challenges and the benefits of using mRDTs for screening of patients before enrolment in clinical trials and other studies have not been rigorously assessed. The present study was therefore conducted as part of completed studies which were implemented in different parts of Tanzania to assess the performance of mRDTs when used for screening of patients to be enrolled in clinical trials and related studies.
Methods
Study sites
The data used in this study were obtained from studies which were conducted at five health facilities (HFs) of Mkuzi Health Centre and Muheza designated district hospital (DDH), Rubya DDH and Nachingwea district hospital, and Ujiji Health Centre in four districts of Muheza, Muleba, Nachingwea and Kigoma, respectively (Figure 1). The sites of Mkuzi/Muheza in Tanga region and Ujiji in Kigoma are among the eight sentinel sites of NMCP in Tanzania which are routinely used to monitor the efficacy of antimalarials [18,19].
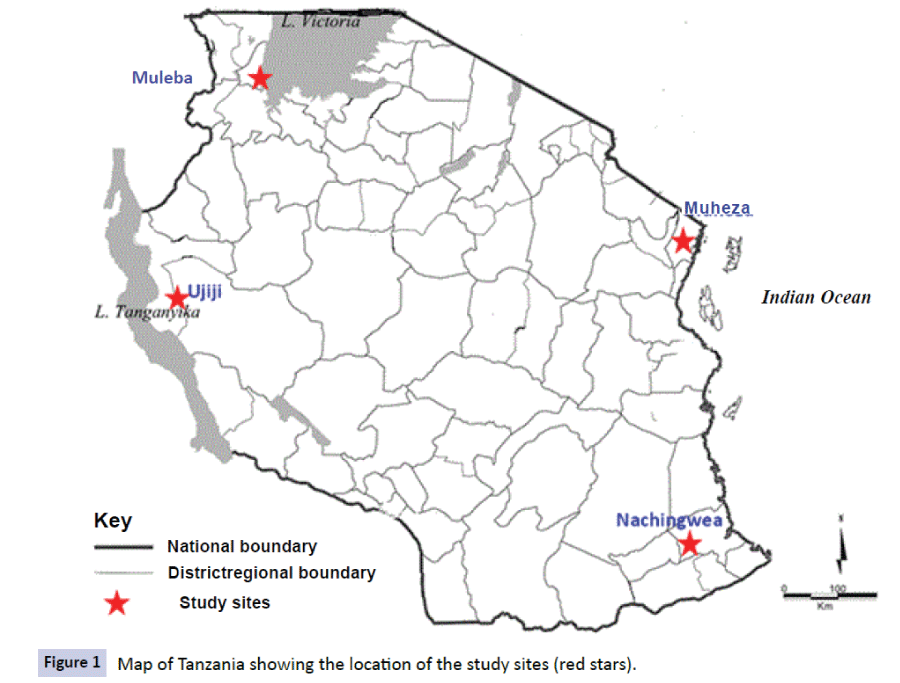
Figure 1: Map of Tanzania showing the location of the study sites (red stars).
Muheza is one of the eight districts of Tanga region which is located in north-eastern Tanzania. Malaria epidemiological profile of Muheza district has been well characterized and a detailed description has been given elsewhere [20,21]. However, recent studies conducted in Muheza have shown a significant decline of malaria burden [22] and shrinking as well as changes in the structure of mosquito populations [23,24]. Most studies have further shown that malaria transmission in Muheza and possibly other parts of the country is resilient to weather changes whereby a slight increase in rainfalls in 2013 [25] and 2014 (F. Francis et al., Manuscript in preparation) were significantly associated with resurgence of malaria.
Ujiji Health Centre is one of the 21 HFs in Kigoma district of Kigoma region. The region is located on the eastern shores of Lake Tanganyika in the north-western part of Tanzania between 3.5° - 6.5° south and 29.5° - 31.5° east. Kigoma district (Kigoma- Ujiji Municipality) with a population of 427,024 [26] is one of the six districts of Kigoma region and it is the region’s headquarters. The Municipality receives moderate rainfall ranging from 800mm to 1600 mm per year (between November and April) with relatively variable and unpredictable patterns. The 21 HFs in the district include two hospitals (one of them is the regional hospital, Maweni), three health centres and 16 dispensaries whereby five of these (three dispensaries, one health centre and a hospital) are private facilities. The district experiences perennial malaria transmission and malaria is the main cause of hospital attendances and admissions (Ujiji Municipal Council, Unpublished data). Kigoma region is among the areas of the country with high burden of malaria whereby parasite prevalence among underfives reported in 2012 was 26% [27].
Muleba is located on the western part of Lake Victoria in Kagera region in north-western Tanzania and is one of the districts which are prone to malaria epidemics in the country [28,29]. Recent studies conducted in Muleba have shown that malaria is still a major public health problem despite intensified control through deployment and wide coverage of different interventions including insecticide treated bed-nets/long lasting insecticidal nets (ITNs/LLINs) and indoor residual spraying (IRS) [30-32]. Muleba receives bimodal rainfall pattern and malaria transmission peaks during and after the rain seasons between August and January, and March and June. The main malaria vector in Muleba is An. gambiaess with no reports of changing vector population structure as reported in other areas with intensified vector control [33].
Lindi region located in south-eastern Tanzania has a population of 864,652 and Nachingwea (with a population of 178,464) [26] is one of the six districts of the region. The district has a total of 37 HFs including three hospitals, two Health Centres and 33 dispensaries. Of these, 36 are currently providing malaria diagnostic services using mRDTs while only 10 facilities are capable of conducting malaria diagnosis by microscopy (NMCP, unpublished DHIS data - 2014). The incidence of malaria in Nachingwea is estimated at 287/1000 cases and the parasite positivity rate is about 38% (NMCP 2014, unpublished DHIS and School survey data). Nachingwea is hyper-endemic to malaria and it is considered to be one of the areas with the highest burden of malaria in the country [27].
Study design and study populations
A cross sectional study (CSS) was conducted in three districts of Muheza, Muleba and Nachingwea in 2013 while two antimalarial efficacy trials were conducted at Mkuzi in 2013, and in Muheza and Ujiji in 2014 (in Muheza and Kigoma districts). The CSS enrolled patients aged ≥ 6 months with either uncomplicated or complicated/severe malaria from outpatient and inpatient departments. Convenient sampling was used to select patients with a history of fever in the past 24 hours or fever at presentation (defined as axillary temperature ≥ 37.5°C) who were screened for possible inclusion in the study. At Mkuzi site in 2013, Muheza DDH and Ujiji in 2014, the studies included patients enrolled in clinical trials to test the efficacy of antimalarial drugs (Shayo et al. [25] and Mandara et al., (manuscript in preparation). The efficacy studies enrolled patients aged 6 months to 10 years with malaria parasites confirmed by microscopy and meeting other inclusion criteria according to WHO guidelines [4]. At Mkuzi in 2013, patients who failed to meet inclusion criteria for enrolment in the efficacy studies (e.g. aged >10 or those with severe malaria) were assessed for possible inclusion in the CSS study.
All patients targeted for inclusion in the study were examined by the attending clinicians who recorded their clinical history and conducted a thorough clinical examination to assess eligibility for possible enrolment. Demographic, clinical and laboratory data obtained from study participants were recorded on case record forms (CRFs). Patients with positive mRDT results were treated with antimalarials and other drugs according to the presenting symptoms as per national guidelines for treatment of malaria [34] and guidelines for integrated management of childhood illnesses (IMCI) [35].
Inclusion and exclusion criteria
For the efficacy studies, samples were collected from enrolled patients according to the criteria specified in the respective protocols which were based on WHO guidelines for antimalarial efficacy testing [4]. However, for patients enrolled in the CSS, the criteria included age of patients ( ≥ 6 months), infection with P. falciparum detected by mRDTs (with or without other species), history of fever or other symptoms suggestive of malaria during the past 24 hours (with or without fever at presentation, axillary temperature ≥ 37.5°C) and informed consent from participants/ parents or guardians of children. For the CSS studies at all sites, patients with positive mRDT results and a history of taking antimalarials in the past 14 days before the study, which could lead to false positive mRDTs were excluded. Other exclusion criteria were presence of severe illness (including severe malaria) or severe anaemia (Hb<5 g/dl) and with general danger signs, and hospitalization with or without multiple blood sampling. Children with severe malnutrition (defined as growth standard below –3 z-score, symmetrical oedema involving at least the feet or midupper arm circumference <110 mm), and febrile conditions due to diseases other than malaria (e.g. acute lower respiratory tract infection, severe diarrhoea with dehydration) were not recruited. Patients with other known underlying chronic or severe diseases (e.g. cardiac, renal and hepatic diseases and HIV/AIDS) were also excluded.
Laboratory screening, sample collection and processing
Laboratory screening involved two steps whereby suspected patients were initially tested using malaria rapid diagnostic tests (mRDTs, with ParaHIT®, SD Bioline or First® Response) and blood smears were taken for confirmation of diagnosis. Thick and thin blood smears were used for detection and quantification of malaria parasites to confirm the eligibility criteria before enrolment in the respective studies.
The blood smears were dried at the sites and the thin films were fixed with methanol. The smears were stained on the same day using 3% Giemsa stain for 45 minutes and examined to detect parasite infection status and parasitaemia. Parasites were counted as asexual or sexual parasites per 200 or 500 White Blood Cells (WBCs), respectively. Parasite density was calculated by multiplying the number of asexual parasites by 40 and sexual parasites by 16 assuming that one microliter of blood contained 8000 WBCs. A blood smear was considered to be negative if no parasites were seen after examining 200 fields. All specimens were labelled anonymously using patients’ study number together with the code of each study site and the date of enrolment.
Sample size
This was an exploratory study and thus statistical based calculation was not used to determine the samples size for the data to be included in the analysis. For the CSS, the research team visited the selected health facilities for three weeks and targeted to recruit 100 patients of all age groups and both sexes at each of the three sites. In the efficacy studies, the sample size were calculated based on the study specific end points as described by Shayo et al. [25] and Mandara et al., (manuscript in preparation).
Ethical issues
The studies which provided data for this paper were approved by the Medical Research Coordination Committee of the National Institute for Medical Research (NIMR-MRCC). Permission to conduct the studies at the HFs was sought from the relevant regional and district authorities and the heads of the respective HFs. Before enrolment into the studies, written informed consent was sought from patients or parents/guardians in case of children.
Data management and statistical analysis
The data from both CSS and clinical trials were double entered into Microsoft Access database which incorporated consistency checks and validation while data of all screened patients but not enrolled were managed using Microsoft Excel. Data cleaning was performed followed by analysis using STATA version 11 (STATA Corp Inc., TX, USA). Categorical data were compared using chisquare test while continuous variables were tested using Students t-test or analysis of variance (ANOVA) for normally distributed data. Non-normal continuous variables such as parasite density were log transformed to normality. Linear regression models were used to test the relationship between the positivity rates as response variables against explanatory variables such as site, study type and fever, with adjustments for age of patients. Factors which determine the sensitivity of mRDTs (risk of obtaining false negative results) were assessed using a multivariate logistic regression model adjusting for age of study participants (under-fives vs. cases aged ≥ 5 years old), fever status (axillary temperature ≥ 37.5°C vs. temperature <37.5°C), parasite density, site and study type as a measure of malaria endemicity. For predictors of specificity of mRDTs (risk of false positive results), adjustment was done for age of study participants, site, study type and fever status. P-value ≤ 0.05 was considered significant.
Results
Baseline characteristics of study participants
A total of 1,910 participants with fever at presentation (axillary temperature ≥ 37.5°C) or a history of fever in the past 24 hours were screened for possible enrolment in the studies which were conducted in 2013 (n=819, 42.9%) and 2014 (n=1,091, 57.1%). Of these, 1,252 (65.6%) participants were screened in the clinical trials and 67.8% of all participants were under-fives. Most of the patients (n=963, 50.4%) were females and there was a significantly higher proportion of females in the CSS compared to the clinical trials due to a large number of female patients aged ≥ 10year in CSS (p<0.001) (Table 1). The mean age of study participants screened in the clinical trials was 3.4 years (range, 0.1 to 11.7 years) with a significantly lower mean age at Muheza in 2014 (p<0.001). In the CSS, the mean age was 14.0 years (range, 0.4 to 82.1 years) and there was no significant difference among the sites (p>0.55). Majority of the patients (56.6%) had fever at presentation (axillary temperature ≥ 37.5°C) with mean axillary temperature >37.5°C at all sites except Nachingwea. The mean axillary temperature was significantly different among the study sites and between the study types (p<0.001). However, the proportion of patients with fever (axillary temperature ≥ 37.5°C) was significantly different among the sites (p<0.001) and not between the study types (clinical trials vs. CSS, p=0.741) (Table 1).
| Study site |
N(%) |
Age, |
Sex-male(%) |
Axillary temp.
mean(range) |
Fever, |
| mean(range) |
N(%) |
| Efficacy studies |
| Muheza1 |
161(12.8) |
3.8(0.4-11.7) |
88(54.7) |
37.7(36.0-41.0) |
83(51.6) |
| Muheza3 |
468(37.3) |
2.6(0.0-10.3) |
270(58.2) |
38.1(35.1-40.9) |
307(65.6) |
| Ujiji |
626(49.9) |
4.7(0-10.4) |
312(49.8) |
37.7(35.1-40.9) |
325(51.9) |
| Total |
1255(100) |
3.8(0.2-11.7) |
670(53.6) |
37.9(35.1-41.0) |
615(57.0) |
| Cross-sectional study |
| Muheza2 |
172(26.1) |
16.2(0.5-75.0) |
74(43.0) |
37.7(36.0-41.0) |
119(69.2) |
| Muleba |
179(27.2) |
13.6(0.5-82.1) |
78(43.6) |
38.1(35.9-42.0) |
124(69.4) |
| Nachingwea |
307(46.7) |
13.0(0.4-78.0) |
123(40.1) |
37.3(35.5-41.0) |
126(41.0) |
| Total |
658(100) |
14.0(0.4-82.1) |
275(41.8) |
37.6(35.5-42.0) |
369(56.2) |
Note:Muheza1 and Muheza3=Efficacy studies involving children between 6 months and 10 years conducted in 2013 and 2014 respectively. Muheza2=cross-sectional study which was conducted in 2013 and involved patients of all age groups. N=number of patients, temp=temperature and %=percentage.
Table 1: Baseline characteristics of participants screened at the three districts of Muheza, Muleba and Nachingwea.
Parasite positivity rates by mRDTs and microscopy
Among the patients screened, 1,188 (62.1%) were positive by mRDTs and the positivity rates was significantly higher in the clinical trials compared to CSS. In the CSS, the mRDT positivity rate among the study sites was significantly different and the highest rate was seen at Muleba while the lowest was at Nachingwea (p<0.001). The positivity rate by mRDTs in the clinical trials was significantly higher at Ujiji compared to the two sites in Muheza (p<0.001). For microscopy and both types of studies, 1,019 (53.2%) participants had malaria parasites (P. falciparum). In the CSS, the positivity rates by microscopy were significantly higher at the two sites of Muleba and Muheza (>55.0%) compared to Nachingwea (35.8%, p<0.001). The highest parasite positivity rates in the clinical trials was reported at Ujiji and the difference among the sites was statistically significant (p=0.001). The overall parasite positivity rate detected by microscopy (for P. falciparum) was significantly higher in the clinical trials compared to the CSS studies (p=0.001). The geometric mean parasite density of P. falciparum asexual parasites/μl was significantly higher in the clinical trials compared to the CSS (p<0.001) and the differences among the sites by the type of study were also statistically significant (p<0.001 in the clinical trials and p=0.03 in the CSS). In cases where the mRDTs were based on pLDH antigens which could detect all species, 7/68(10.3%) patients with P. malariae were negative by mRDTs while 8/22 (36.4%) with P. ovale were not detected by mRDTs.
Accuracy of mRDTs when compared to microscopy
The sensitivity of mRDTs ranged from 97.3% to 99.3% at all sites with no significant differences between the sites (p>0.583) and the type of study (p>0.210) (Table 2). The sensitivity of mRDTs was higher even at low parasites density with a sensitivity of 96.4% at parasite density between 100 and <4000 asexual parasite/μl. The highest sensitivity of 99.3% was observed when the parasite density was ≥ 4000 asexual parasite/μl of blood, after adjusting for fever status, age, site and type of the study (OR=6.30, p=0.003) (Table 3). The specificity of mRDTs was generally lower, ranging from 64.9% to 87.7% (Table 2). Overall, mRDTs had higher negative but lower positive predictive values (Table 2). Although fever was a strong predictor of parasite positivity rate by microscopy (OR=2.32, p<0.001), the sensitivity of mRDTs was not affected by fever even after adjusting for age, parasite density, site and the study type (p>0.064). With the exception of low specificity at Muleba (OR=0.34, p=0.007) and Ujiji (OR=0.30, p<0.001), the specificity of mRDTs was similar in all patients even after adjusting for the effects of fever status, type of the study and age of participants (p ≥ 0.525) (Table 4).
| Study site |
mRDT +Ve |
BS +ve |
Sensitivity |
Specificity |
PPV |
NPV |
| Efficacy studies |
| Muheza1 |
97(60.3) |
90(55.9) |
98.9 |
87.7 |
91.8 |
98.4 |
| Muheza3 |
264(56.4) |
235(50.2) |
99.6 |
87.1 |
88.6 |
99.5 |
| Ujiji |
458(73.3) |
384(61.4) |
98.7 |
67.2 |
82.6 |
97.0 |
| Total |
819(65.3) |
709(56.4) |
99.0 |
78.5 |
85.7 |
98.4 |
| Cross-sectional study |
| Muheza2 |
106(61.6) |
96(55.8) |
97.9 |
84.2 |
88.7 |
97.0 |
| Muleba |
128(71.5) |
102(57.0) |
99.2 |
64.9 |
78.9 |
98.0 |
| Nachingwea |
135(44.0) |
110(35.8) |
97.3 |
85.8 |
79.3 |
98.3 |
| Total |
369(56.1) |
308(46.8) |
98.5 |
80.9 |
81.8 |
97.9 |
Note:mRDT=malaria rapid diagnostic test, BS=blood slide for detection of malaria parasites, +ve=positive test, N=number of patients, %=percentage, PPV=positive predictive value and NPV=negative predictive value.
Table 2: Sensitivity, specificity, negative and positive predictive values of mRDTs when used for screening of febrile patients compared to microscopy.
| |
Sensitivity(%) |
Unadjusted OR(p-value) |
Adjusted OR(p-value) |
| Age group |
|
|
|
| <5yrs |
646/652(99.1) |
reference |
reference |
| >5yr |
358/365(98.1) |
0.48(0.184) |
0.59(0.396) |
| Fever |
|
|
|
| No fever |
344/347(99.1) |
reference |
reference |
| With fever |
660/670(98.5) |
0.58(0.404) |
0.27(0.064) |
| Pf Density |
|
|
|
| <4000 |
188/195(96.4) |
reference |
reference |
| ≥4000 |
815/821(99.3) |
5.06(<0.004) |
6.30(0.003) |
| Study type |
|
|
|
| Clinical trials |
702/709(99.0) |
reference |
reference |
| CSS |
302/308(98.1) |
0.50(0.219) |
0.40(0.384) |
| Site |
|
|
|
| Muheza |
417/421(99.1) |
reference |
reference |
| Muleba |
101/102(99.0) |
0.97(0.978) |
3.00(0.382) |
| Nachingwea |
107/110(97.3) |
0.34(0.164) |
0.82(0.829) |
| Ujiji |
379/384(98.7) |
0.72(0.637) |
0.40(0.384) |
Note:mRDTs=malaria rapid diagnostic tests, OR=odds ratio, Pf=Plasmodium falciparum
Table 3: Factors which affect the sensitivity of mRDTs when used for screening of patients before enrolment in clinical trials and related studies.
| |
Specificity(%) |
Unadjusted OR(p-value) |
Adjusted OR(p-value) |
| Age group |
|
|
|
| <5yrs |
511/639(80.0) |
reference |
reference |
| >5yr |
200/256(78.1) |
0.89(0.538) |
0.88(0.540) |
| Fever |
|
|
|
| No fever |
381/481(79.2) |
reference |
reference |
| With fever |
330/414(79.7) |
1.03(0.854) |
1.05(0.776) |
| Study type |
|
|
|
| Clinical trials |
428/545(78.5) |
reference |
reference |
| CSS |
283/350(80.0) |
1.15(0.401) |
0.80(0.525) |
| Site |
|
|
|
| Muheza |
350/380(86.4) |
reference |
reference |
| Muleba |
50/77(64.9) |
0.28(<0.001) |
0.34(0.007) |
| Nachingwea |
169/197(85.8) |
0.91(0.725) |
1.15(0.713) |
| Ujiji |
162/241(67.2) |
0.30(<0.001) |
0.30(<0.001) |
Note:mRDTs=malaria rapid diagnostic tests, OR=odds ratio
Table 4 Factors which affect the specificity of mRDTs when used for screening of patients before enrolment in clinical trials and related studies.
Discussion
The findings of this study showed that mRDTs had very high sensitivity (>96%) which helped to exclude majority of the patients who had no malaria parasites. Unlike previous studies which showed that the sensitivity of mRDTs largely depended on the parasite density, fever status and the level of malaria transmission [36], the current study showed that parasite density was the only predictor of sensitivity of mRDTs. This could partly be due to the nature of the current studies and the fact that the largest proportion of patients (>80%) enrolled at the study sites had higher parasite density (with more than 4000 asexual parasites/μl of blood).
Fourteen patients (0.7%) had false negative mRDTs results, six of these had high fever at presentation (axillary temperature ≥ 37.5°C) and five had high parasitaemia (with 22,360; 79,489; 86,669; 189,333; 199,660 and 231,200 asexual parasites/μl). Two of these patients with higher parasitaemia had high fever at presentation (38.1°C and 40.2°C) suggesting that mRDTs can fail to pick up severely sick patients who might progress to severe malaria and other complications if left untreated. Previous studies conducted elsewhere have shown high sensitivity of mRDTs in severely sick patients [37-39]. The failure of mRDTs to detect such patients could be due to hrp-2 gene deletion, parasites expressing low level of target antigens, prozone effect or other factors which could not be established [40] and further assessment is urgently needed. Thus, febrile patients with occult symptoms suggestive of malaria and negative mRDTs results should whenever possible be confirmed using another test preferably microscopy to rule out malaria and support appropriate management of non-malaria febrile infections.
It was also shown that about 10.0% of patients with P. malariae and 36.0% with P. ovale could not be detected by mRDTs although the tests had the capacity to detect non-falciparum species. This could possibly be due to low parasitaemia among the patients whereby some of the patients missed could have had low parasite density (<200 asexual parasites/μl). However, only three patients had such low parasitaemia for both species suggesting that the failure could be due to other factors [40]. Furthermore, the limited performance of the mRDTs for detection of species other than P. falciparum could be attributed to limitations of pLDH antigen such as allelic variation and prozone effect as previously described [6]. Thus, further studies are needed to assess and determine the performance of mRDTs for detection of nonfalciparum species in areas of different epidemiological settings.
The results further showed that mRDTs had low specificity which might lead to recruitment of a significant number of patients without malaria parasites. In these studies, 184 patients (9.6%) had false positive results indicating that if mRDTs were used alone, such patients would have been wrongly enrolled in the studies. These patients might have recovered from malaria but still had circulating falciparum antigens or the results were due to cross-reactivity with non-specific antigens [6].
The specificity of mRDTs was not affected by any of the factors which have been shown to influence the risk of false positive results such as fever status and the level of transmission intensity [36]. Whereas previous studies showed that individuals with fever had high risk of false positive results possibly due to selftreatment, fever was not a risk factor of positive results in the current study. Similarly, it was shown that individuals from areas with low malaria transmission were less likely to have false positive results [36]. Surprisingly, false positivity rates in this study were independent of fever status and age although the risk was higher in Muleba and Kigoma districts. The high false positivity rates in Muleba could possibly be due to the outbreak of malaria which occurred a few months before the study (NMCP, unpublished reports). The outbreak could have caused prolonged exposure to malaria parasites, frequent illness leading to sustained levels of detectable antigens and also self-medication. The high false positivity rates at Kigoma, could possibly be due to high transmission intensity [27] which leads to high morbidity and increased chances of self treatment or recurrent infections.
This study covered areas with varying malaria transmission and some of the sites such as Muheza were previously reported to have relatively low malaria burden [22,27]. However, it was shown that Muheza had higher positivity rates and a large number of patients with fever. Together with Muleba and Ujiji, Muheza had a higher risk of malaria positivity rates. It was also shown that only at Nachingwea fever was not a strong predictor of the risk of malaria parasite infection. These variations could be attributed to the heavy rains of March to May 2013 and 2014 which resulted in increased malaria cases in many parts of the country and an outbreak of malaria in Muleba (NMCP, unpublished reports). Thus, more studies are needed to map the performance of mRDTs under different epidemiological settings due to the rapidly changing malaria transmission in Tanzania.
Due to declining malaria burden in Tanzania from 2008, it was increasingly becoming difficult to conduct studies to monitor the efficacy of ACT and other antimalarials as recommended by WHO. Studies conducted at Muheza and other parts of Tanzania from 2009 showed that it was difficult to find patients to recruit in clinical trials, even when enrolment duration and target age groups were extended (Kabanywanyi et al. Unpublished data). On the contrary, the study conducted in Muheza from May 2013 was able recruit sufficiently large number of patients in less than two months [25]. An increase in the number of cases in Muheza has been linked to high rainfall that was recorded from March 2013 [25] and 2014 (F. Francis, unpublished data). This further shows the significant impact of rainfall as a major determinant of malaria transmission and related morbidity and mortality [41-43]; suggesting that mapping of malaria case load will be required to identify areas suitable for drug resistance surveillance in Tanzania.
Conclusion
The findings showed high sensitivity of mRDTs in patients with different levels of parasitaemia suggesting that the tests can play a significant role in excluding majority of the patients without malaria and save time and other resources which would be used for microscopy. Few patients with high parasitaemia and some with non-falciparum infections could not be detected by mRDTs exposing such patients to the danger of developing severe malaria and related complications. Furthermore, the relatively low specificity of mRDTs could lead to enrolment of some patients without malaria parasites and compromise the quality of the trials. The different epidemiological picture and accuracy of mRDTs at the study sites suggest that more studies covering diverse sites are required to provide a detailed profile of malaria burden and performance of mRDTs in the country. In clinical trials, mRDTs should only be used for initial screening to exclude negative cases and all patients with positive results should be confirmed with microscopy or another test with high specificity such as PCR based assay.
Competing interest disclosure
The authors hereby declare to have no any competing interests.
Authors’ Contribution
DSI, AS, CIM, EN, JM, RNJ, RM, SM, AM, JB and MML conceived of the idea, DSI, AS, and CIM designed the study, and CIM, DSI and MML supervised the trials and overall implementation of the studies. AS, VB, RAM, CIM, DN and EK did the field and laboratory work. AS, CIM, VB and DSI wrote the manuscript and all authors read, and approved the final version of the manuscript.
Acknowledgements
The authors would like to thank the teams at the study sites for their cooperation and support during data collection. Special thanks to study participants and parents/guardians of the children who freely gave consent to participate in the different studies, and for adhering to the study protocols including the follow-up schedule. The technical support received from the staff of NIMR Tanga Centre (Filbert Francis, Geoffrey Makenga, Ludovick Rukeha, Mbaraka Muya Idd, Johari Sadi, August Nyaki, Ezekiel Malecela, Zacharia Savael, Juma Tupa, Zaina Maumba, Neema Barua, Benson Swai, Hatibu Athumani, Seth Nguhu, Salimu Tembo, Mary Lukindo, Fides Mumburi and Thomas Semdoe) is highly appreciated. The CSS study was conducted within the African Plasmodium diversity Network (PDNA, https:// www.cggh.org/collaborations/plasmodium-diversity-networkafrica) and the support from colleagues in the network is highly appreciated. Authors are grateful to Dr. Marian Warsame of the WHO Global Malaria Programme for reviewing the initial draft of the manuscript and providing critical comments. The studies which contributed data were funded by the Tanzania Commission for Science and Technology through the Nelson Mandela African Institution of Science and Technology, the UK Medical research Council (MRC) (G0600718 - Centennial award) and the World Bank through the East African public health laboratory network (EAPHLN) under the Tanzanian Ministry of Health and Social Welfare. Permission to publish the manuscript has been granted by the Director General of NIMR.
9690
References
- WHO (2010) Guidelines for the treatment of malaria, Second edition. Geneva, Switzerland.
- WHO (2011) Universal access to malaria diagnostic testing: An operational mannual. Geneva, Switzerland.
- WHO (2012) Scaling up diagnostic testing, treatment and surveillance for malaria. Geneva, Switzerland.
- WHO (2009) Methods for Surveillance of Antimalarial Drug Efficacy. Geneva, Switzerland.
- WHO (2011) Global plan for artemisinin resistance containment (GPARC). Geneva, Switzerland.
- Murphy SC, Shott JP, Parikh S, Etter P, Prescott WR, et al. (2013) Malaria diagnostics in clinical trials. Am J Trop Med Hyg 89:824-839.
- Obare P, Ogutu B, Adams M, Odera JS, Lilley K, et al. (2013) Misclassification of Plasmodium infections by conventional microscopy and the impact of remedial training on the proficiency of laboratory technicians in species identification. Malar J 12:113.
- Ohrt C, Obare P, Nanakorn A, Adhiambo C, Awuondo K, et al. (2007) Establishing a malaria diagnostics centre of excellence in Kisumu, Kenya. Malar J 6:79.
- Chuma J, Abuya T, Memusi D, Juma E, Akhwale W, et al. (2009) Reviewing the literature on access to prompt and effective malaria treatment in Kenya: implications for meeting the Abuja targets. Malar J 8:243.
- Bell D, Wongsrichanalai C, Barnwell JW (2006) Ensuring quality and access for malaria diagnosis: how can it be achieved? Nat Rev Microbiol 4:682-695.
- Murray CK, Gasser RA, Magill AJ, Miller RS (2008) Update on rapid diagnostic testing for malaria. Clin Microbiol Rev 21:97-110.
- Li Y, Ning YS, Li L, Peng DD, Dong WQ, et al. (2005) Preparation of a monoclonal antibodies against Plasmodium falciparum glutamate dehydrogenase and establishment of colloidal gold-immunochromatographic assay. 25:435-438.
- Moonasar D, Goga AE, Frean J, Kruger P, Chandramohan D (2007) An exploratory study of factors that affect the performance and usage of rapid diagnostic tests for malaria in the Limpopo Province, South Africa. Malar J 6:74.
- Thomson R, Festo C, Johanes B, Kalolella A, Bruxvoort K, et al. (2014) Has Tanzania embraced the green leaf? Results from outlet and household surveys before and after implementation of the Affordable Medicines Facility-malaria. PLoS ONE 9:e95607.
- Smith N, Obala A, Simiyu C, Menya D, Khwa-Otsyula B, et al. (2011) Accessibility, availability and affordability of anti-malarials in a rural district in Kenya after implementation of a national subsidy scheme. Malar J 10:316.
- Sykes A, Hendriksen I, Mtove G, Mandea V, Mrema H, et al. (2009) Azithromycin plus artesunate versus artemether-lumefantrine for treatment of uncomplicated malaria in Tanzanian children: a randomized, controlled trial. Clin Infect Dis 49:1195-1201.
- (2001) Monitoring antimalarial drug resistance within National Malaria Control Programmes: the EANMAT experience. Trop Med Int Health 6:891-898.
- (2003) The efficacy of antimalarial monotherapies, sulphadoxine-pyrimethamine and amodiaquine in East Africa: implications for sub-regional policy. Trop Med Int Health 8:860-867.
- Ellman R, Maxwell C, Finch R, Shayo D (1998) Malaria and anaemia at different altitudes in the Muheza district of Tanzania: childhood morbidity in relation to level of exposure to infection. Ann Trop Med Parasitol 92:741-753.
- Segeja MD, Mmbando BP, Kamugisha ML, Akida JA, Savaeli ZX, et al. (2008) Prevalence of glucose-6-phosphate dehydrogenase deficiency and haemoglobin S in high and moderate malaria transmission areas of Muheza, north-eastern Tanzania. Tanzan J Health Res 10:9-13.
- Ishengoma DS, Mmbando BP, Segeja MD, Alifrangis M, Lemnge MM, et al. (2013) Declining burden of malaria over two decades in a rural community of Muheza district, north-eastern Tanzania. Malar J 12:338.
- Derua YA, Alifrangis M, Hosea KM, Meyrowitsch DW, Magesa SM, et al. (2012) Change in composition of the Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania. Malar J 11: 188.
- Meyrowitsch DW, Pedersen EM, Alifrangis M, Scheike TH, Malecela MN, et al. (2011) Is the current decline in malaria burden in sub-Saharan Africa due to a decrease in vector population? Malar J 10:188.
- Shayo A, Mandara CI, Shahada F, Buza J, Lemnge MM, et al. (2014) Therapeutic efficacy and safety of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in North-Eastern Tanzania. Malar J 13:376.
- (2013) National Beareau of Statistics. Population distribution by age and sex. Dar es Salaam, Tanzania: United Republic of Tanzania.
- Salaam D (2014) Tanzania Commission for AIDS (TACAIDS) ZACZNBoSNOotCGSOaII. Tanzania HIV/AIDS and Malaria Indicator Survey 2011-2012.
- Kinung'hi SM, Mashauri F, Mwanga JR, Nnko SE, Kaatano GM, et al. (2010) Knowledge, attitudes and practices about malaria among communities: comparing epidemic and non-epidemic prone communities of Muleba district, North-western Tanzania. BMC Public Health 10:395.
- Mboera LE, Kitua AY (2001) Malaria epidemics in Tanzania: An overview. Afr J Health Sci 8:17-23.
- West PA, Protopopoff N, Rowland MW, Kirby MJ, Oxborough RM, et al. (2012) Evaluation of a national universal coverage campaign of long-lasting insecticidal nets in a rural district in north-west Tanzania. Malar J 11:273.
- West PA, Protopopoff N, Rowland M, Cumming E, Drakeley C, et al. (2013) Malaria risk factors in north west Tanzania: the effect of spraying, nets and wealth. PLoS ONE 8:e65787.
- West PA, Protopopoff N, Wright A, Kivaju Z, Tigererwa R, et al. (2014) Indoor residual spraying in combination with insecticide-treated nets compared to insecticide-treated nets alone for protection against malaria: a cluster randomised trial in Tanzania. PLoS Med 11:e1001630.
- Protopopoff N, Matowo J, Malima R, Kavishe R, Kaaya R, et al. (2013) High level of resistance in the mosquito Anopheles gambiae to pyrethroid insecticides and reduced susceptibility to bendiocarb in north-western Tanzania. Malar J 12:49.
- Ministry of Health (2006) National Guidelines for malaria diagnosis and treatment. United Republic of Tanzania, Ministry of Health and Social Welfare.
- WHO (1999) Improving child health: IMCI - The intergrated approach. Geneva, Switzerland.
- Ishengoma DS, Francis F, Mmbando BP, Lusingu JP, Magistrado P, et al. (2011) Accuracy of malaria rapid diagnostic tests in community studies and their impact on treatment of malaria in an area with declining malaria burden in north-eastern Tanzania. Malar J 10:176.
- Maltha J, Guiraud I, Lompo P, Kabore B, Gillet P, et al. (2014) Accuracy of PfHRP2 versus Pf-pLDH antigen detection by malaria rapid diagnostic tests in hospitalized children in a seasonal hyperendemic malaria transmission area in Burkina Faso. Malar J 13:20.
- Hendriksen IC, Mtove G, Pedro AJ, Gomes E, Silamut K, et al. (2011) Evaluation of a PfHRP2 and a pLDH-based rapid diagnostic test for the diagnosis of severe malaria in 2 populations of African children. Clin Infect Dis 52:1100-1107.
- Kyabayinze DJ, Tibenderana JK, Odong GW, Rwakimari JB, Counihan H (2008) Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for Plasmodium falciparum malaria in a hyperendemic region of Uganda. Malar J 7:221.
- Cheng Q, Gatton ML, Barnwell J, Chiodini P, McCarthy J, et al. (2014) Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J 13:283.
- Briet OJ, Vounatsou P, Amerasinghe PH (2008) Malaria seasonality and rainfall seasonality in Sri Lanka are correlated in space. Geospat Health 2: 183-190.
- Briet OJ, Vounatsou P, Gunawardena DM, Galappaththy GN, Amerasinghe PH (2008) Temporal correlation between malaria and rainfall in Sri Lanka. Malar J 7:77.
- Odongo-Aginya E, Ssegwanyi G, Kategere P, Vuzi PC (2005) Relationship between malaria infection intensity and rainfall pattern in Entebbe peninsula, Uganda. Afr Health Sci 5:238-245.