Introduction
|
| The sodium/glucose co-transporter (SGLT) is a new class of antidiabetic drugs. These drugs act on a unique target in kidneys causing loss of glucose in urine. The glucosuric effect is produced by inhibition of the renal Na/glucose co-transporter that will lead to a unique hypoglycemic, hypotensive and favourable weight loss. For this novel mechanism of action, SGLT inhibitors can be used for both type 1 and type 2 diabetic cases. Drug discovery and introduction of new SGLT inhibitors is still growing and more drugs are expected to be introduced. This is enforced by the high selectivity and efficacy of the novel inhibitors. |
Diabetes
|
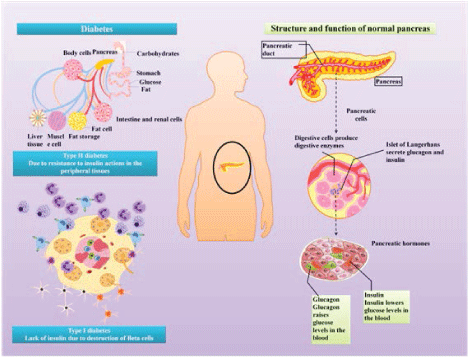
| Diabetes mellitus (DM) comprises chronic sugar toxicity due to loss of control on the blood sugar level. The human pancreas secretes two important hormones that control the blood glucose level. The first is insulin which lowers the blood glucose level. In contrast, glucagon increases the level of blood sugar. Disturbance in the pancreatic function or peripheral response to pancreatic hormones will lead to diabetic conditions (Figure 1). There are two main types of DM. Type 1 DM (insulin dependent DM) in which there is progressive loss of pancreatic beta cells leading to loss of endogenous production of insulin. Therefore, patients of this type will depend on external source of insulin during the rest of their lives. In type 2 DM, there is normal or subnormal levels of endogenous insulin. However, the peripheral tissues are devoid of sufficient response to insulin action (insulin resistance). The complications of diabetes include microvascular and neurological complications that interfere with major body functions and causing disorders as retinopathy, renopathy, cardiopathy, neuropathy and other complications [1-3]. |
Treatment of DM
|
|
Type 1 DM
|
| Insulin is the choice for type 1 DM. The required dose of insulin, frequency and type of insulin are determined on individual bases. |
|
Type II diabetes
|
| Oral hypoglycemics, diet restrictions, exercise and/or insulin are required for type 2 DM (Table 1). |
Role of Kidney in Glucose Homeostasis
|
| Kidney is a major organ in glucose homeostasis. The normal kidney reabsorbs the filtered glucose into the circulation. It is estimated that about 180-220 gram of glucose are reabsorbed into the circulation again after being filtered from the blood [4]. This function is managed by an efficient glucose transport system in the renal tubules. This system includes two important glucose transporters called sodium/glucose co-transporters (SGLTs). There are two main types of SGLTs involved in glucose transport, SGLT1 and SGLT2. The differences between these two transporters are summarized in Table 2. |
|
SGLT inhibitors
|
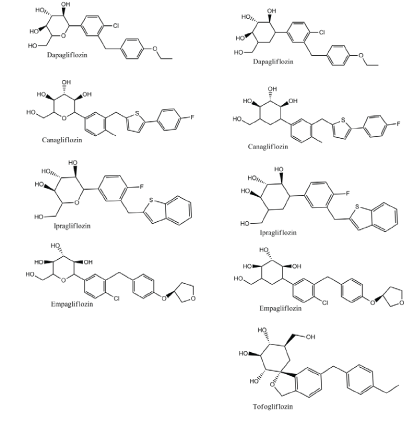
| SGLT inhibitors act by inhibiting SGLT leading to loss of glucose in urine. The action of SGLTs is depending on the amount of filtered glucose. Therefore higher amount of glucose loss is expected during glucosuria. The concept of SGLT inhibition and its related antidiabetic effect was introduced in 2001 [5]. The first compound known to inhibit glucose reabsorption was phlorizin (isolated from the bark of apple tree), which was used as antipyretic and induced glucosuria. Several phlorizin derivatives were tested for their antidiabetic efficiency [5]. This concept was first introcduced and followed by extensive drug discovery studies to find new SGLT inhibitors (Fujimori et al., 2008; Gao et al., 2010; Katsuno et al., 2007; Meng et al., 2008; Nomura et al., 2010; Ohsumi et al., 2003; Tsujihara et al., 1996; Tsujihara et al., 1999; Zhou et al., 2010). Recently, three SGLT inhibitors are approved for clinical use in treatment of DM, dapagliflozin, canagliflozin and empagliflozin (Figure 2) [6,7]. Other SGLT inhibitors under preclinical studies include ipragliflozin, sergliflozin remoglilozin, , and tofogliflozin [8]. |
Development of New Inhibitors
|
| Drug discovery is the only reliable way to combat the infectious and noninfectious disease by using various in silico and in vivo techniques. In this context, mechanisms that are unique to the foreign organism or affected in the host at very low concentrations can be targeted to combat diseases [9-12]. SGLT inhibitors showed extensive drug discovery studies that lead to discovery of hundreds of new SGLT inhibitors and many other are still under development. The wide variety of chemical modifications and its favourable inhibitory properties accounts for the growing repository of SGLT inhibitors. in the development of new SGLT inhibitors, two critical measurements are to be considered. The first is the potency of SGLT2 inhibition and the second is the selectivity of SGLT2 over SGLT1. The desired drugs could have higher selectivity for SGLT2 to avoid the side effects produced after inhibition of SGLT1. Table 3 summarizes the inhibitory properties and selectivity of some SGLT inhibitors (the best compound in each group). |
Tables at a glance
|
|
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |







