Keywords
Breast cancer; Somatostatin; Retinoids; Prolactin’ inhibitors; Melatonin
Introduction
Breast cancer is usually treated with surgery, which may be associated singly or in various forms with chemotherapy, estrogen block, radiotherapy and selective monoclonal antibodies for epidermal growth factor receptors (EGFR). Breast cancer is the most frequent form of cancer in women and the leading cause of death in women.
Numerous in vitro studies carried out on various cell lines have demonstrated the marked anticancer effects of DBM components’, clarifying the mechanisms of action and paving the way to the achievement of encouraging results in clinical practice [1-16]. We report a retrospective observational study with 5-year follow-up, carried out on 297 patients affected by breast cancer and treated with the Di Bella Method biological therapy.
Materials and Methods
Enrolment criteria of 297 cases of breast cancer
Only patients with a histological diagnosis of breast cancer and disease characteristics that could be measured according to the Response Evaluation Criteria in Solid Tumors (RECIST) were enrolled [17].
The study included 297 patients suffering from breast cancer and each one of these were subject to blood chemistry, hystological and immunoistocheminal tests expected for this pathology.
The patients were divided into three groups according to the stage of their breast cancer at the start of the treatment.
• The first group, Regional Breast Cancer, consisted of patients whose tumor was limited to a single anatomical district.
• The second group, Local Breast Cancer, consisted of patients whose tumor had spread to the lymph nodes.
• The third group, Advanced Breast Cancer, consisted of patients with metastases.
The DBM was administered in one of the following forms:
1) First-line treatment, for patients who had previously undergone only surgery
2) Second-line treatment, for patients who had previously followed traditional adjuvant oncological protocols for a maximum of 6 months.
3) Last-resort treatment, for patients with stage IV cancer who had undergone repeated multi-chemo/radiotherapy treatments for more than a year.
The objective clinical responses were statistically classified in three groups: REGRESSION, STABILITY, PROGRESSION. Among the effects of the DBM, toxicity and quality of life were also evaluated. RELATIVE survival was also assessed, i.e. the percentage of patients still alive 5 years after the diagnosis of breast cancer, compared to the average survival of a group of women of the same age, from the same geographical area, and in good health. This last index was then compared with the results published by the National Cancer Institute (Figure 1 statistical analysis of cases by stage), the leading American agency for the collection and management of data relative to cancer patients (Figure 1).
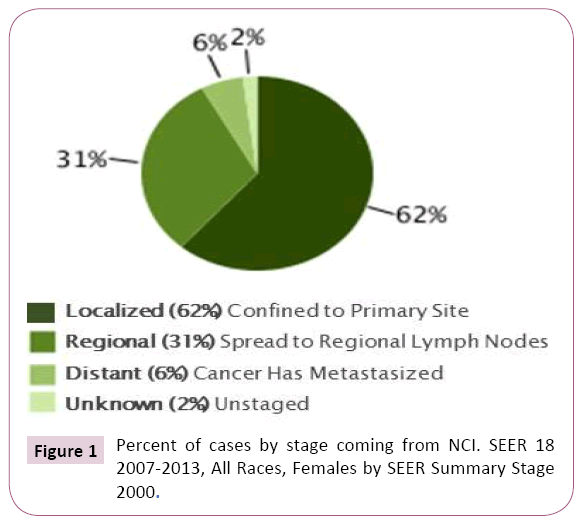
Figure 1: Percent of cases by stage coming from NCI. SEER 18 2007-2013, All Races, Females by SEER Summary Stage 2000.
Enrolment criteria 40 cases of breast cancer- Sub-analysis
A sub-analysis is setup with only 40 cases, treated with DBM in Exclusive Therapy. In the present study only patients with an Eastern Cooperative Oncology Group (ECOG) status 2 were diagnosed with histologic diagnosis of breast cancer and measurable disease characteristics in accordance with the Solid Tumors Response Evaluation Criteria (RECIST) [17]. Another requirement for enrollment was the absence of standard therapeutic regimens (surgical interventions, polycotherotherapy, radionuclide therapy, monoclonal antibodies), and to accept, with prior informed consent, the administration of biological therapy as a first-line treatment. The 40 cases are classified according to the type of response received during the observation period (according to the RECIST criteria):
• OVERALL RESPONSE=CR(Complete) - PR(Partial) – SD (Stable Disease)-PD (Progressive Disease)
• DFS=Disease-Free Survival
• PFS=Progression-Free Survival
Therapeutic treatment
All the patients received a daily combination of Somatostatin/ octreotide, Melatonin, Retinoids solubilized in Alpha Tocopherol Acetate, dopaminergic agonists, estrogen inhibitors and minimal doses of cyclophosphamide.
These components were administered as follows:
Gradual oral dosages of:
• Retinoic acid, [ATRA-All Trans Retinoc Acid] 0.25 gr (488.372 IU), + axerophthol palmitate 0.25 gr (909000 IU) + Betacarotene 1 gr (3 334 000 IU) solubilized in alpha tocopherol acetate 500 gr, stoichiometric ratio 1:1:4:2)(1×106 IU); fasting, once a day for seven days, then twice a day for 7 days; from week 3 for the rest of the treatment period, 3 times a day, orally.
• Dihydrotachysterol 10 drops with each administration of retinoids (15 200 IU), 30 drops a day.
• Somatostatin at gradually increasing doses (1 mg for the first 7 days, increasing 1 mg for week, to 3 mg at day 21).
• Tetracosactide acetate (0.25 mg) added every other day to the syringe with somatostatin, compatibly with pressure and glycemia.
• Octreotide long-acting release (LAR) (20 mg) every 3 weeks, intramuscular.
• Triptoreline analogues LH- FSH (Follicle-Stimulating Hormone) 3.75 mg every 4 weeks, intramuscular.
• Melatonin hydrosolubilized in hydrogen bond with Adenosinae, stabilized with Glycine in 5 mg tablets, orally: 3 tabs (15 mg) at midday and in the evening at meals plus 10 tabs (50 mg) before going to bed (average total daily dose=80 mg).
• Cabergoline orally with the main meal, 1 mg (equal to 12 tablet) twice a week.
• Bromocriptine (2.5 mg) orally, half a tablet morning and evening.
• Anastrozole 1 mg tablet.
• Cyclophosphamide (50-100 mg) orally, variable dosage: start with 1 tablet with the main meal for 1 week, then 1 tablet in the morning and the evening one day and the next day just in the evening.
• Hydroxyurea 500-1000 mg/day; replace with Endoxan if not tolerated or if cerebral metastases are present due to the ability of hydroxyurea to pass through the blood-brain barrier.
• Ascorbic Acid (Vit C) orally: 1/2 teaspoon (4 gr) in a glass of water during the midday and evening meals together with:
• Calcium lactate gluconate +calcium carbonate equal to 1000 mg of calcium, half a sachet in the same glass.
• Chondroitin sulfate (500 mg) one tablet in the morning, at midday and in the evening, with meals.
• Ferrous Sulphate (329,7 mg) one tablet 2 – 3 times a week, depending on sideremia and hemochrome values.
• Calcium levofolinate one 22 mg tablet a day.
• Ursodeoxycholic acid (UDCA) 300 mg to contrast the choleretic and cholagog inhibition of the SST (Somatostatin) and/or octreotide.
• Taurine 1500-2000 mg/day when necessary, to improve cardiac and hepatic function.
Therapeutic treatment has been administered continuously, even after complete remission, and its effects are appropriately and consistently monitored over time, through chemotherapy and with the aid of instrumental diagnostics.
As long as the tumor is in progress, the therapy should be given without any variation, or with the possible changes that may be made on the course, and only after obtaining complete remission over time can be appropriately reduced in a very gradual manner until reaching a "maintenance" therapeutic threshold, also calibrated on the basis of individual individual staging and histological features. Somatostatin was administered subcutaneously by means of a timed 10-hour syringe, taking into account the short half-life (3 minutes) and concurrent GH nighttime peak.
Evaluation of safety and toxicity
For the toxicity evaluation, only the side effects that could have been due to this specific treatment were considered (degrees of correlation: possible, probable or certain, expressed as absolute frequency (n), relative frequency (%), and 95% confidence interval (CI), as described by the National Cancer Institute (NCICTC) criteria (https://www.eortc.be/services/doc/ctc/).1
All patients therefore gave their informed consent to participate in the study.
The most frequent toxicity phenomena found in the study, grade I and II, were the following:
• Hematological toxicity: Leucopenia 33%,
• Gastrointestinal: nausea 25%,
• Drowsiness (36%).
These phenomena were the only ones observed, and generally there was a subsequent, progressive and gradual adaptation and improvement within a few weeks. A reduction, delay or temporary suspension of treatment due to toxicity has been necessary in patients with leucopenia (cyclophosphamide suspension until recovery of leukocyte counts) and in cases of severe gastrointestinal effects (Somatostatin).
Such circumstances, though limited, have occurred much more frequently and more clearly, especially in patients with critical stage, advanced especially if chemotherapy/radiotherapy/ monoclonal antibodies are pre-treated.
There have been no deaths associated with the treatment.
Results and Discussion
Table 1 shows the clinical result in terms of REGRESSION, STABILITY and PROGRESSION of the disease. The best clinical outcomes were achieved, as expected, in the first group of patients, those with Regional Breast Cancer, with a clinical response of regression always greater than 80%. In the second group of patients with locally advanced cancer, the most promising results were seen with the DBM administered in the second-line form, where 87.5% of patients achieved regression. As regards the patients with advanced stage breast cancer, both the first and second-line forms of treatment achieved regression in 20% of cases, while the success rate was only 10% in patients who had repeatedly undergone multiple sessions of chemo- and radiotherapy, were no longer responsive and were generally in critical conditions before starting the DBM (Table 1).
| Staging at the date of Enrolment |
Treatment Modality |
Patients n. |
Result |
% |
| Early Stage Breast Cancer (Stage I - II - III) |
Exclusive Therapy |
27 |
Remission=23 |
81.50% |
| Stability=1 |
3.70% |
| Progression=3 |
14.80% |
| Adjuvant Therapy |
87 |
Remission=74 |
81.50% |
| Stability=2 |
2.30% |
| Progression=11 |
12.60% |
| 2nd/ 3rdLine Therapy or Supportive |
34 |
Remission=28 |
82.40% |
| Stability=5 |
14.70% |
| Progression=1 |
2.90% |
| Locally advanced |
Exclusive Therapy |
4 |
Remission=1 |
25% |
| Stability=1 |
25% |
| Progression=2 |
50% |
| Adjuvant Therapy |
8 |
Remission=7 |
87.50% |
| Progression=1 |
12.50% |
| 2nd/ 3rdLine Therapy or Supportive |
3 |
Remission=1 |
33.30% |
| Progression=2 |
66.70% |
| Metastatic Breast Cancer |
Exclusive Therapy |
9 |
Remission=2 |
22.20% |
| Stability=2 |
22.20% |
| Progression=5 |
55.60% |
| Adjuvant Therapy |
18 |
Remission=4 |
22.20% |
| Stability=1 |
5.60% |
| Progression=13 |
72.20% |
| 2nd/3rd Line Therapy or Supportive |
104 |
Remission=11 |
10.60% |
| Stability=20 |
19.20% |
| Progression=73 |
70.20% |
| Stage not defined |
3 |
- |
- |
| Total |
297 |
Table 1: Percentage of patients with remission, stability and progression, after DBM with different treatment modality (exclusive, adjuvant or 2nd/ 3rd line therapy), for different staging at the date of enrolment.
In order to compare our results with NCI data, a preliminary Relative Survival is obtained for every stage and reported in Table 2. In Figure 2 is shown an evaluation of the percentage of women still alive 5 years after diagnosis, treated with the DBM, versus data on official oncological treatment reported by the National Cancer Institute. DMB method appears more efficient for the treatment of distant tumor in particular (Table 2) (Figure 2).
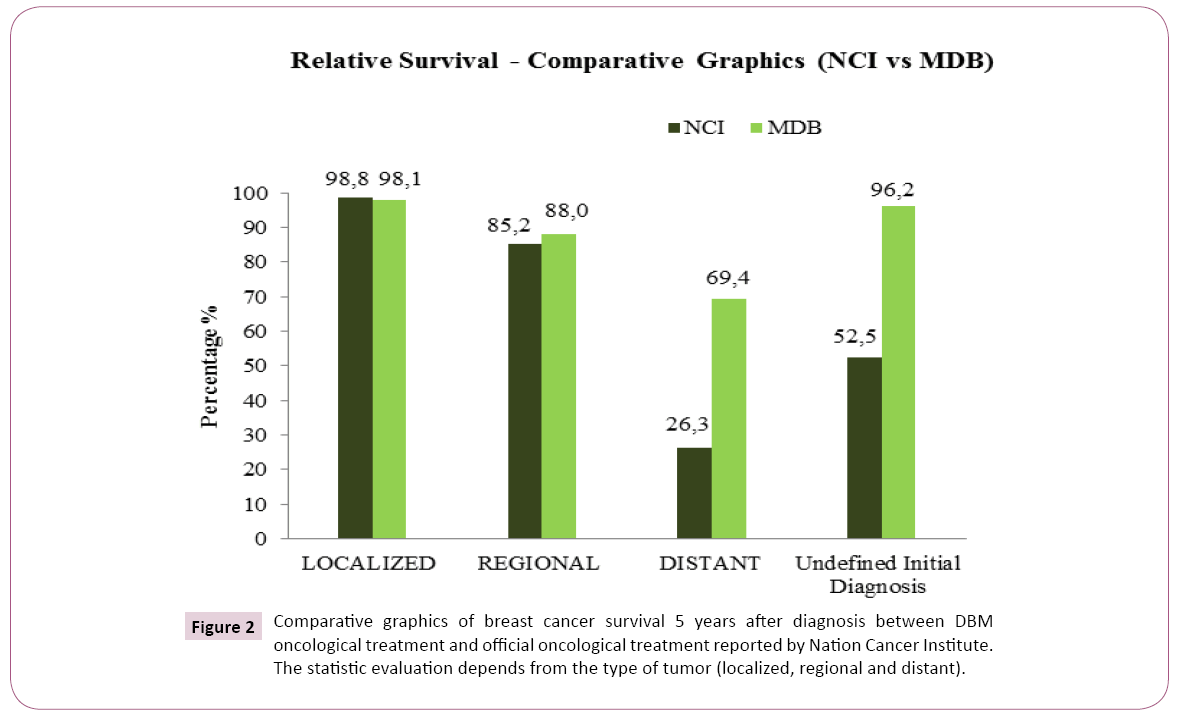
Figure 2: Comparative graphics of breast cancer survival 5 years after diagnosis between DBM oncological treatment and official oncological treatment reported by Nation Cancer Institute. The statistic evaluation depends from the type of tumor (localized, regional and distant).
| MDB- Observed and Relative Survival (5 years) |
| Initial diagnosis |
Observed patients N. |
Survived patients n. |
Observed Survival (%) |
Relative Survival (%) |
| Localized |
52 |
49 |
94.2% |
98.1% |
| Regional |
91 |
77 |
84.60% |
88% |
| Distant |
15 |
10 |
66.70% |
69.40% |
| Undefined initial diagnosis |
29 |
27 |
93.1% |
96.2% |
| SubTotal |
187 |
163 |
87.2% |
90.4% |
| Others (data/diagnosis <5years) |
107 |
- |
- |
- |
| No classiable |
3 |
- |
- |
- |
| Total |
297 |
- |
- |
- |
Table 2: Statistical Observation on Survival (Observed and Relative) at 5 years after diagnosis and initial stage: (Localized=confined to primary site - Regional=extended to regional lymph nodes - Distant=metastatic tumor).
Sub-analysis
This sub-analysis highlights:
• Clinical Target Benefit (CR - PR - SD) in 85% of patients with initial stage disease (I - II - IIIA)
• a percentage of 44% positive results (2 PR-2 SD) in the 9 metastatic tumor patients and an average survival time of approximately 30 months (75-42-42-41-24-21-17-6 -6).
Based on the classification criteria used by N.C.I (Project SEER 18 - 2007-2013), the 40 cases are also classifiable:
• 19 localized tumors (11 CR - 6 PR - 1 SD - 1 PD).
• 12 locoregional tumors (5 CR - 2 PR - 1 SD - 4 PD).
• 9 metastatic tumors (2 PR - 2 SD - 5 PD).
By identifying Progressive Survival Survival (PFS) - Free Disease Survival (DFS) and Global Response (OR) as the primary endpoint, the following summary was elaborated (Table 3).
| Identity and Staging |
Overall Response (Global Response) |
Maintenance of clinical remission |
PFS |
| Patient ID |
Age at Diagnosis |
Age at Enrolment |
Grade |
Stage |
CR |
PR |
SD |
PD |
Start therapy |
Stable response over time |
Temporary answer |
No answer |
DFS (month) |
Conclusion of the observation |
Observation period (months) |
PFS (month) |
PFS / observation period |
| 138 |
51 |
51 |
n.d. |
IIA |
X |
- |
- |
- |
Dec-04 |
X |
- |
- |
105 |
Nov-14 |
119 |
119 |
100% |
| 586 |
62 |
68 |
n.d. |
IV |
- |
X |
- |
- |
Dec-06 |
X |
- |
- |
_ |
Oct-10 |
42 |
42 |
100% |
| 601 |
52 |
52 |
G2 |
I |
X |
|
- |
- |
Mar-06 |
X |
- |
- |
104 |
Still under obser |
118 |
118 |
100% |
| 614 |
56 |
56 |
n.d. |
IIA |
- |
X |
- |
- |
Jul-06 |
X |
- |
- |
_ |
Jul-11 |
60 |
60 |
100% |
| 657 |
57 |
57 |
n.d. |
IIA |
- |
X |
- |
- |
Jun-06 |
X |
- |
- |
_ |
Oct-07 |
17 |
17 |
100% |
| 799 |
42 |
43 |
G3 |
IIIC |
- |
- |
- |
X |
Jan-07 |
- |
X |
- |
_ |
May-08 |
18 |
2 |
11% |
| 895 |
72 |
72 |
n.d. |
IIIB |
- |
- |
- |
X |
Mar-07 |
- |
- |
X |
_ |
Feb-08 |
5 |
0 |
0% |
| 970 |
69 |
69 |
G3 |
IV |
- |
- |
- |
X |
Apr-08 |
- |
X |
- |
_ |
Sep-09 |
17 |
2 |
12% |
| 994 |
54 |
55 |
G3 |
IV |
- |
- |
- |
X |
May-09 |
- |
X |
- |
13 |
Jan-15 |
75 |
41 |
55% |
| 1101 |
50 |
51 |
nd |
IIB |
X |
- |
- |
- |
Mar-07 |
X |
- |
- |
106 |
Still under obser. |
117 |
117 |
100% |
| 1941 |
47 |
47 |
n.d. |
IIA |
X |
- |
- |
- |
Apr-09 |
X |
- |
- |
45 |
Sep-13 |
65 |
65 |
100% |
| 2178 |
68 |
68 |
n.d. |
IIA |
- |
X |
- |
- |
Jun-09 |
X |
- |
- |
_ |
Still under obser. |
81 |
81 |
100% |
| 2224 |
52 |
65 |
G1 |
IV |
- |
- |
- |
X |
Aug-09 |
- |
X |
- |
_ |
Aug-11 |
24 |
10 |
42% |
| 2580 |
55 |
55 |
G2 |
IIIA |
- |
- |
- |
X |
Mar-10 |
- |
X |
- |
_ |
Jul-13 |
40 |
0 |
0% |
| 2708 |
37 |
37 |
G2 |
IIB |
- |
X |
- |
- |
May-10 |
X |
- |
- |
_ |
Sep-12 |
27 |
27 |
100% |
| 2898 |
55 |
55 |
G2 |
I |
X |
- |
- |
- |
Sep-10 |
X |
- |
- |
5 |
Nov-13 |
22 |
22 |
100% |
| 3029 |
45 |
45 |
G1 |
IIA |
X |
- |
- |
- |
Dec-10 |
X |
- |
- |
48 |
Still under obser. |
72 |
72 |
100% |
| 3048 |
49 |
49 |
G3 |
IIB |
X |
- |
- |
- |
Dec-10 |
X |
- |
- |
34 |
May-14 |
42 |
42 |
100% |
| 3056 |
62 |
62 |
G2 |
IIB |
- |
X |
- |
- |
Dec-10 |
X |
- |
- |
_ |
Still under obser. |
92 |
92 |
100% |
| 3361 |
34 |
35 |
G2 |
IIIB |
X |
- |
- |
- |
Feb-11 |
X |
- |
- |
59 |
Still under obser. |
74 |
74 |
100% |
| 3617 |
41 |
41 |
G1 |
IIA |
X |
- |
- |
- |
Jul-11 |
X |
- |
- |
48 |
Still under obser. |
60 |
60 |
100% |
| 3696 |
53 |
53 |
G2 |
IIA |
X |
- |
- |
- |
Sep-11 |
X |
- |
- |
9 |
Nov-12 |
14 |
14 |
100% |
| 3734 |
37 |
39 |
n.d. |
I |
- |
X |
- |
- |
Oct-99 |
X |
- |
- |
_ |
Still under obser. |
171 |
171 |
100% |
| 3950 |
64 |
64 |
n.d. |
IIA |
- |
- |
- |
X |
Dec-11 |
- |
X |
|
_ |
Jan-15 |
36 |
25 |
69% |
| 4253 |
49 |
56 |
G1 |
I |
- |
X |
- |
- |
Mar-12 |
X |
- |
- |
_ |
Still under obser. |
57 |
57 |
100% |
| 4358 |
43 |
43 |
G2 |
IIIA |
X |
- |
- |
- |
Apr-12 |
X |
- |
- |
41 |
Still under obser. |
45 |
45 |
100% |
| 4548 |
45 |
45 |
n.d. |
IIB |
- |
- |
- |
X |
Jul-12 |
- |
X |
|
_ |
Still under obser. |
57 |
25 |
44% |
| 4614 |
48 |
48 |
n.d. |
I |
X |
- |
- |
- |
Sep-12 |
X |
- |
- |
42 |
Still under obser. |
54 |
54 |
100% |
| 4688 |
67 |
67 |
G3 |
IIIB |
- |
- |
X |
- |
Sep-12 |
X |
- |
- |
_ |
Nov-15 |
36 |
36 |
100% |
| 4851 |
31 |
31 |
n.d. |
IIB |
X |
- |
- |
- |
Aug-12 |
X |
- |
- |
22 |
Oct-14 |
26 |
26 |
100% |
| 4967 |
64 |
65 |
n.d. |
IIA |
X |
- |
- |
- |
Mar-12 |
X |
- |
- |
19 |
Still under obser. |
51 |
51 |
100% |
| 4971 |
46 |
46 |
G3 |
IV |
- |
- |
- |
X |
Feb-13 |
- |
X |
- |
_ |
Aug-16 |
42 |
16 |
38% |
| 5502 |
59 |
59 |
G2 |
I |
- |
- |
X |
- |
Sep-13 |
X |
- |
- |
_ |
Still under obser. |
38 |
38 |
100% |
| 5779 |
60 |
60 |
G3 |
IV |
- |
- |
X |
- |
Sep-13 |
X |
- |
- |
_ |
Mar-14 |
6 |
6 |
100% |
| 5826 |
64 |
64 |
G2 |
IV |
- |
- |
X |
- |
Jun-13 |
X |
- |
- |
_ |
Still under obser. |
41 |
41 |
100% |
| 6167 |
48 |
48 |
n.d. |
IIA |
X |
- |
- |
- |
Oct-13 |
X |
- |
- |
24 |
Still under obser. |
41 |
41 |
100% |
| 6287 |
68 |
68 |
G2 |
IIA |
X |
- |
- |
- |
Jul-12 |
X |
- |
- |
19 |
Still under obser. |
56 |
56 |
100% |
| 6435 |
41 |
42 |
n.d. |
IV |
|
X |
- |
- |
May-15 |
X |
- |
- |
- |
Still under obser. |
21 |
21 |
100% |
| 6514 |
54 |
54 |
n.d. |
I |
|
X |
- |
- |
Mar-13 |
X |
- |
- |
- |
Still under obs. |
50 |
50 |
100% |
| 6624 |
47 |
48 |
G2 |
IV |
- |
- |
- |
X |
May-14 |
- |
- |
X |
_ |
Nov-15 |
6 |
0 |
0% |
| CR=Complete Response; PR=Partial Response; SD=Stable Response; PD=Progressive Response; DFS=Disease Free Survival; PFS=Free from Progressive Survival. |
Table 3: Observation of therapeutic response of 40 cases of breast cancer treated with only DBM.
Conclusion
The DBM therapy proposes to counter the progression of the neoplastic phenotype by:
a) Inhibiting the neoplastic proliferation by cell processes of apoptosis/necrosis and depletion of hormones and cell growth factors;
b) Countering the marked mutagenic tendency by direct activation of the DNA repair systems, and by epigenetic cell programming.
c) Blocking neoplastic progression by reducing the formation of new blood vessels (Neoangiogenesis- Lymphoangiogenesis) and of cell motility phenomena (migration), essential for neoplastic dissemination
d) Reinforcing natural defense mechanisms against neoplastic aggression (natural and acquired immunity and improvement of vital functions).
In cases of breast cancer monitored at 5 years, the DBM biological multitherapy significantly improved quality of life, objective response and survival with respect to the same stages of breast cancer treated with conventional oncological protocols. The negative regulation of the main interactive mitogen systems - GH–GF–Prolactin-Estrogen – by means of somatostatin and analogues, D2R agonists, aromatase inhibitors, analogues of LHFSH, together with the differentiating, cytostatic, homeostatic action of Retinoids, Vitamins E, D3, and C, Folates, Ca, MLT (Melatonin), Proteoglycans with factorial interaction, without significant toxicity, made this result possible [18-22]. We report these therapeutic results to improve the prognosis of tumors in general and of breast cancer in particular. Breast cancer remains the leading cause of death worldwide in women, demonstrating the deficiencies of the current measures of cancer prevention and treatment and the need to overcome these serious limits of oncological therapy by taking advantage of still underestimated scientific evidence.
Acknowledgements
Thanks to Alessandro Ricchi from University of Bologna for his support in the collection of data and statistical analysis.
Conflict of Interest
No conflicts of interest to disclose.
1Note: This is a study on the combined use of drugs that have already passed all the reliability and anticancer tests. Thus, since all the drugs have been extensively tested and their use has been approved by the international health organizations, and here they are merely used in a new combination, it was decided not to submit the study protocol to an ethics committee. The study was carried out in accordance with The Good Clinical Practices directives and the Declaration of Helsinki.
22684
References
- Seitz S (2013) Targeting triple-negative breast cancer through the somatostatin receptor with the new cytotoxic somatostatin analogue AN-162 [AEZS-124], Anticancer. Drugs 24: 150-157.
- Sanchez-Barcelo EJ, Mediavilla MD, Alonso-Gonzalez C, Reiter RJ (2012) Melatonin uses in oncology: breast cancer prevention and reduction of the side effects of chemotherapy and radiation. Expert OpinInvestig Drugs 21: 819-831.
- Tang XH, GudasLJ (2011) Retinoids, retinoic acid receptors, and cancer.Annu Rev PatholMech Dis 6: 345-364.
- Mehta RG, Peng X, Alimirah F, Murillo G, Mehta R (2013) Vitamin D and breast cancer: Emerging concepts. Cancer Lett334: 95-100.
- Frati A, Antoine M, Rodenas A, Gligorov J, Rouzier R, et al. (2011) “La somatostatinedans le cancer du sein Somatostatin in breast cancer. Ann BiolClin69: 385-391.
- FulanH (2011) Retinol, vitamins A, C, and E and breast cancer risk: A meta-analysis and meta-regression. Cancer Causes Control 22: 1383-1396.
- Proietti S, Cucina A, Reiter RJ, Bizzarri M (2013) Molecular mechanisms of melatonin’s inhibitory actions on breast cancers,” Cell Mol Life Sci 70: 2139-2157.
- MargheriM (2012) Combined effects of melatonin and all-trans retinoic acid and somatostatin on breast cancer cell proliferation and death: molecular basis for the anticancer effect of these molecules. Eur J Pharmacol681: 34-43.
- Cescon DW, Ganz PA, Beddows S, Ennis M, Mills BK, et al. (2012) Feasibility of a randomized controlled trial of vitamin D vs. placebo in women with recently diagnosed breast cancer. Breast Cancer Res Treat 134: 759-767.
- Ostendorf GM (2012) High dosage vitamin C in breast cancer? Versicherungsmedizin64: 85.
- Suhail N (2012) Effect of vitamins C and E on antioxidant status of breast-cancer patients undergoing chemotherapy. J Clin Pharm Ther 37: 22-26.
- Zhang Y, Zhang H, Wang X, Wang J, Zhang X, et al. (2012) The eradication of breast cancer and cancer stem cells using octreotide modified paclitaxel active targeting micelles and salinomycin passive targeting micelles. Biomaterials33: 679-691.
- Proietti(2010) Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFβ-1-dependent growth inhibition of breast cancer cells. J Pineal Res
- He Y (2009) The antiproliferative effects of somatostatin receptor subtype 2 in breast cancer cells. Acta Pharmacol Sin 30: 1053-1059.
- Watt HL, Kharmate GD, Kumar U (2009) “Somatostatin receptors 1 and 5 heterodimerize with epidermal growth factor receptor: Agonist-dependent modulation of the downstream MAPK signalling pathway in breast cancer cells,” Cell Signal21: 428-439.
- Lung-Ta Lee CL, Schally AV,Ping-Ping MTL (2008) “Dephosphorylation of cancer protein by tyrosine phosphatases in response to analogs of luteinizing hormone-releasing hormone and somatostatin,” Anticancer Res., vol. 28, pp. 2599–2606.
- Therasse P(2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205-216.
- Di Bella G, Mascia F, Ricchi A, Colori B (2013) Evaluation of the safety and efficacy of the first-line treatment with somatostatin combined with melatonin, retinoids, vitamin D3, and low doses of cyclophosphamide in 20 cases of breast cancer: a preliminary report. Neuro Endocrinol Lett 34: 660-668.
- Di Bella G (2008) Complete objective response to biological therapy of plurifocal breast carcinoma. Neuro Endocrinol Lett 29: 857-866.
- Di Bella G (2011) The Di Bella Method (DBM) improved survival, objective response and performance status in a retrospective observational clinical study on 122 cases of breast cancer. Neuro Endocrinol Lett32: 751–762.
- Di Bella G (2010) The Di Bella Method (DBM). Neuro Endocrinol Lett 31: 1-42.
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS (1996) Evidence based medicine: what it is and what it isn’t. BMJ312: 71-72.







