Introduction
Arenaviruses are rodent-borne, enveloped ambi-sense RNA negative-strand RNA viruses that are divided into two groups: the Lassa-Lymphocytic choriomeningitis serocomplex and the Tacaribe serocomplex. The Lassa-Lymphocytic choriomeningitis serocomplex (Old World arenaviruses) consists of Lassa virus (LASV) and Lujo virus; both of which have the capacity to cause hemorrhagic fever. LASV is the most prevalent hemorrhagic fever arenavirus, infecting a quarter of a million individuals in endemic regions of West Africa annually [1]. The Tacaribe complex arenaviruses (New World arenaviruses) are endemic in the Americas and are classified into three or four clades. The New World arenaviruses which cause hemorrhagic fever in humans include Junín virus (JUNV), Machupo virus (MACV), Guanarito virus (GTOV), Chapare virus and Sabiá virus. All of these Clade B New World arenaviruses are category A priority pathogens and classified as HHS (Health and Human Services) Select Agents [2] and require BioSafety Level (BSL-4) laboratories for study in the United States [3]. The Clade A arenaviruses consist of Pirital virus (PIRV), Pichindé virus (PICV), Flexal virus (FLEV), Parana virus (PARV), and Allpahuayo virus (ALLV). Arenaviruses are maintained in nature in a specific rodent reservoir and some of these viruses have the ability to cause a wide spectrum of human disease including hemorrhagic fever manifestations [4]. Infected rodents tend to have chronic persistent infection with virus shedding through urine, feces, and/or saliva [5, 6]. Rodents and nonhuman primates have been historically utilized as animal models for arenavirus research. Guinea pigs and hamsters have been the most common rodent used for arenavirus pathogenesis studies. The guinea pig has previously been used to study LASV and GTOV pathogenesis because infection of the guinea pig results in viremia, morbidity, and mortality. Hamsters have been commonly used to study PIRV pathology. Infection of the hamster with PIRV results in viremia, pathologic abnormalities in specific tissues, coagulopathy, morbidity, and mortality. Because hamsters and guinea pigs have been historically used as small animal models for other arenaviruses, it is logical to use these animals when discerning a new animal model for another arenavirus.
FLEV is pathogenic Clade A New World arenavirus that was isolated in Brazil from the Oryzomys spp. (Rice rat) and is known to cause overt disease [7]. Clinical manifestations include fever, chills, headache, general myalgia, nausea, vomiting, dizziness, and diarrhea. FLEV is considered a HHS Select Agent and requires BSL-3 containment. Though this virus was isolated over 30 years ago, very little is known about the pathogenesis associated with human disease. Additionally, no animal model has been described. Therefore, this study aimed to delineate a suitable animal model to study the pathogenesis associated with FLEV infection.
Materials and Methods
Cell lines, virus, and reagents
FLEV (BeAn 293022) and PIRV (VAV-488) was obtained from Dr. Robert B. Tesh at the University of Texas Medical Branch (Galveston, TX). The virus was passed up to five times in Vero E6 cells (ATCC) using RPMI 1640 media (Gibco) plus antibiotics (diluted 1:100) and diluted in Minimal Essential Media prior to challenge. The animals were challenged with 9.96 x 106 pfu of FLEV or 5.26 x 106 pfu of PIRV administered by intraperitoneal injection in 1 mL.
Plaque assay
Plaque assays were performed as previously described [8]. Briefly, Vero E6 cells (ATCC) were seeded on 12-well plates (seeding density of 3x105 cells/well) 24 hours prior to inoculation. After 10 fold dilutions of viral samples, 100 uL was added to each well in triplicate. The plates were incubated at 37.0°C (5% CO2) for 60 minutes with rocking every 15 minutes. A 0.7% methyl-cellulose overlay (containing EMEM, antibiotics, non-essential amino acids, and 10% FBS) was added to the inoculum prior to incubating at 37.0°C (5% CO2) for 96 hours. The overlay was aspirated and replaced with an identical overlay supplemented with 4% Neutral Red (Sigma) and incubated an additional 24 hours (37.0°C, 5% CO2). The wells were then fixed with a 2% paraformaldehyde solution prior to visual analyses.
Animal Studies
Twenty (24) female Syrian golden hamsters (Mesocricetus auratus) (twelve at 13-15 weeks of age, and twelve at 5-6 weeks of age at the beginning of the study), and twelve Hartley guinea pigs were received from Charles River Kingston (Kingston, NY) and randomly associated into six groups. Groups 1-3 consisted of ten animals per group and Groups 4-6 each consisted of two animals per group. The animals were individually housed in isolator cages and allowed food and water ad libitum. All study procedures were approved in accordance to the guidelines by the Institutional Animal Care and Use Committee. All work involving infected animals or virus was performed in the BSL-3 laboratory.
Blood Sampling
Terminal blood samples were obtained from animals that were euthanized due to moribundity via cardiac puncture. Sera was collected for viremia analysis.
Assessment of vascular leakage
Vascular permeability was determined by injection of Evans Blue Dye (EBD) (Sigma-Aldrich, St. Louis, MO) into the vena cava and quantifying its diffusion into various tissues. Animals found moribund from groups 1, 2, and 3 and uninfected animals from groups 4, 5, and 6 were anesthetized, and injected with 0.4 mL 0.5% EBD in PBS by vena cava. Animals were euthanized after 30 minutes, and the vasculature was extensively perfused transcardially with 30 mL of PBS to remove residual blood. Section of the liver, heart, spleen, lung, pancreas, bronchial lymph nodes, kidney, brain, adrenal glands, small intestine, and large intestine were harvested, weighed, and immersed in 0.5 mL of 10% formamide. EBD extraction was then performed by incubating tissue samples at 37°C overnight, heat inactivating for 2 hours at 56°C, homogenizing, and centrifuging to pellet debris. A 0.1 mL volume from each sample was placed into a 96-well microtiter plate, and the absorbance at 630 nm was measured. Data is calculated as a ratio of absorbance to grams of tissue.
Statistical analyses
Animal survival data was analyzed using the SAS® MULTTEST procedure to adjust for multiple comparisons at the 0.05 level of significance using the Bonferroni-Holm Method. A time-to-death analysis was performed based on the length of survival model and the Kaplan-Meier estimator and logrank tests were used to determine any significant differences between groups.
Results
Morbidity and mortality associated with FLEV infection in the Syrian golden hamster and the guinea pig.
The Syrian golden hamsters and guinea pigs were infected with FLEV or PIRV by intraperitoneal (i.p.) inoculation. FLEV infection resulted in 60% mortality (6/10) in 5-6 week old hamsters and 80% mortality (8/10) in 13-15 week old hamsters, while only 20% mortality occurred in FLEV-infected guinea pigs (Figure 1). Of the infected hamsters (13-15 weeks of age), 8 of 10 were lethargic and exhibited huddled posture and ruffled fur. The 2 surviving animals never exhibited any signs of disease and 6 of the 8 that died demonstrated petechial rashes, hemorrhaging from the nose and/or mouth, and labored breathing. The petechial rashes and hemorrhaging was observed in these 6 animals from days 6 through 10. Once hemorrhaging was observed, animal deaths occurred within 24 to 48 hrs. These 6 animals all died within 10 days after challenge. Two of the 13-15 week old hamsters were euthanized on 14 and 18 days post-challenge. These experienced significant weight loss (> 20%), labored respiration, and lethargy. Neither of these animals hemorrhaged externally. In all, the mean day of death for the animals that died was calculated to be 10 days post-challenge.
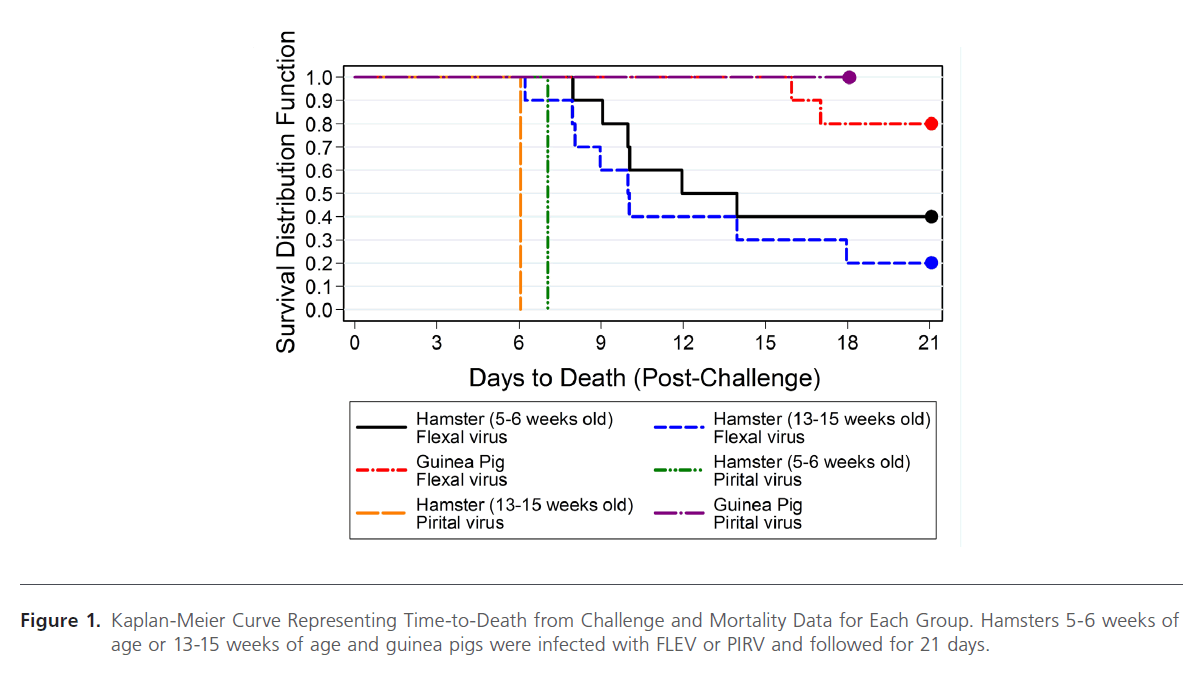
Figure 1: Kaplan-Meier Curve Representing Time-to-Death from Challenge and Mortality Data for Each Group. Hamsters 5-6 weeks of age or 13-15 weeks of age and guinea pigs were infected with FLEV or PIRV and followed for 21 days.
Similar results were observed in the 5-6 week old hamsters; 5 of the 6 animals that died demonstrated petechial rashes and hemorrhaging 24 to 48 hrs prior to death. These 5 animals died within 12 days post-challenge. The remaining animal did not demonstrate petechial rash or any hemorrhaging, but was euthanized 14 days post-challenge due to decreased grooming, lethargy, and significant weight loss (>20%). The mean day of death for the animals that died was calculated to be 9 days post-challenge.
Only 2 of the 10 FLEV-challenged guinea pigs succumbed to disease. Both of these animals demonstrated huddled posture, ruffled fur, and labored respiration and were euthanized 16 and 17 days post-challenge. The remaining 8 animals never demonstrated any signs of overt disease.
As controls, a total of 2 hamsters (5-6 weeks of age and 13- 15 weeks of age) and 1 guinea pig were inoculated with PIRV, since PIRV infection in hamsters results in 100% mortality, while PIRV infection in guinea pigs does not result in morbidity or mortality. This was performed to guarantee the validity of the animal model system. As expected, both hamsters infected with PIRV succumbed to disease, while the guinea pig remained healthy and survived to the end of the study.
The unadjusted pairwise log-rank tests suggest a significant difference in the distribution of time to death among each of the hamster groups and the guinea pig group (Table 1). After adjusting for multiplicity, the 5 – 6 weeks old hamster group was no longer significantly different from the guinea pig group. No significant difference could be inferred between the two hamster groups. The Kaplan-Meier estimator of the survival functions for these three groups indicate that the hamsters were more susceptible than the guinea pigs to the FLEV. The two FLEV-challenged hamster groups had a shorter time to death and greater lethality rates when compared to the guinea pigs challenged with FLEV.
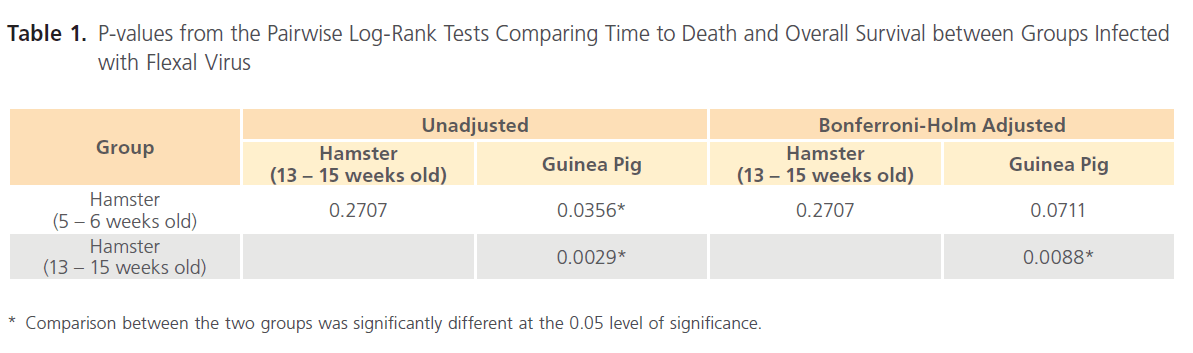
Table 1: P-values from the Pairwise Log-Rank Tests Comparing Time to Death and Overall Survival between Groups Infected with Flexal Virus
Effects of FLEV infection on animal weights
Weight loss associated with FLEV-infection in the hamsters and guinea pigs appeared to correlate with disease. FLEV infection in hamsters (5-6 weeks of age) resulted in 20% weight loss in 40% of the animals (Figure 2A). All of these animals were euthanized due to FLEV-associated disease. Two of the four surviving hamsters lost weight before recovering, and the other two survivors gained weight throughout the study. As a whole, FLEV-infected hamsters (13-15 weeks of age) lost weight. Sixty percent of these hamsters lost at least 20% of their baseline body weight; all of these animals were euthanized due to FLEV-associated disease. The two surviving hamsters lost weight throughout the course of the study; however, the weight loss never reached 20% and these hamsters recovered from disease signs that were present after challenge. Only one guinea pig lost 20% of its baseline body weight. This animal was euthanized 16 days post-challenge due to a combination of weight loss and FLEV-associated disease. The other guinea pig that was euthanized due to the disease lost 10% of its body weight. Weight loss was not associated with the surviving guinea pigs. In all, weight loss was associated with disease in the hamsters (5-6 weeks of age and 15-16 weeks of age).
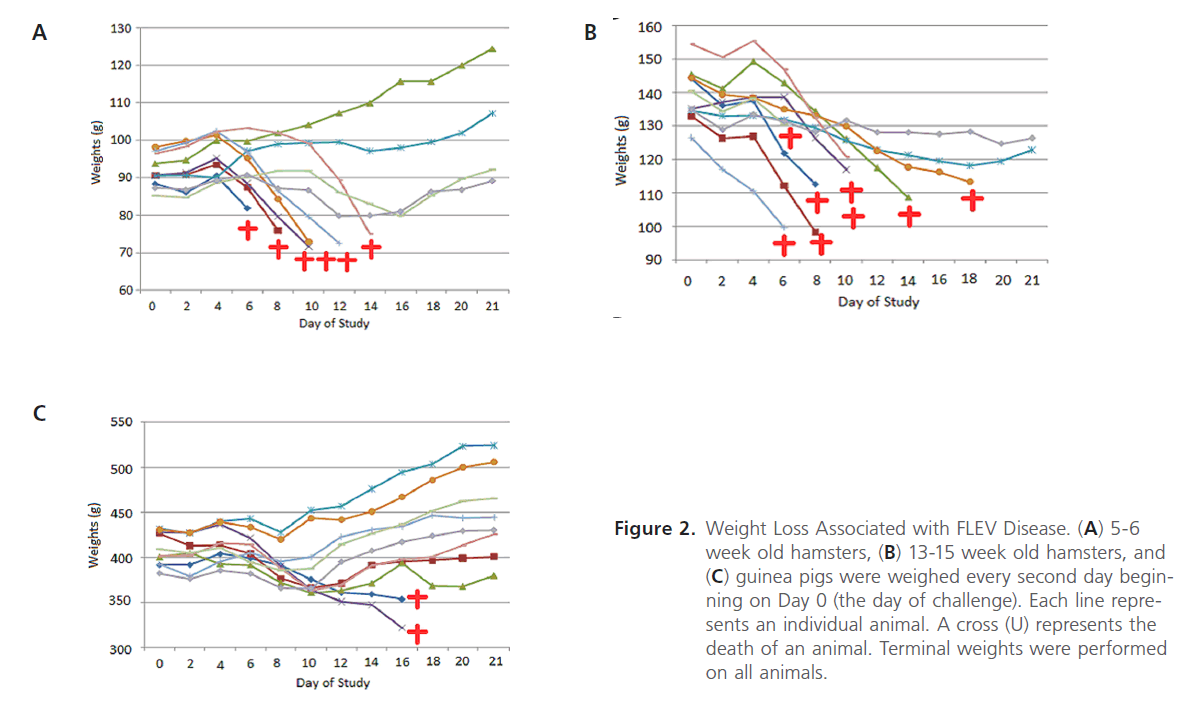
Figure 2: Weight Loss Associated with FLEV Disease. (A) 5-6 week old hamsters, (B) 13-15 week old hamsters, and (C) guinea pigs were weighed every second day beginning on Day 0 (the day of challenge). Each line represents an individual animal. A cross (U) represents the death of an animal. Terminal weights were performed on all animals.
FLEV distribution in infected animals
FLEV was associated with the pancreas, kidney, adrenal gland, heart, lung, bronchial lymph node, brain, small intestine, liver, and spleen in terminal hamsters and guinea pigs as determined by plaque assay analysis (Figure 3A). Viral titers were also observed in the large intestine of terminal FLEVinfected hamsters; however, no viral titers were detected in the large intestines of the terminal guinea pigs, which may be due to the sensitivity of the assay. A significant difference in viral titers was observed when comparing the titers associated in the brain of the hamsters to the guinea pigs. Viremia was also detected in all terminal animals. Interestingly, FLEV titers were detected in the kidneys of surviving hamsters and guinea pigs; in the adrenal gland of hamsters (5-6 weeks of age); in the bronchial lymph nodes of surviving guinea pigs; in the large intestines in surviving hamsters (13-15 weeks of age); and in the spleen of surviving hamsters (5-6 weeks of age) (Figure 3B). No viremia was measured in the terminal blood samples from the survivors. In all, higher levels of viral titers were observed in the non-surviving hamsters when compared to the non-surviving guinea pigs.
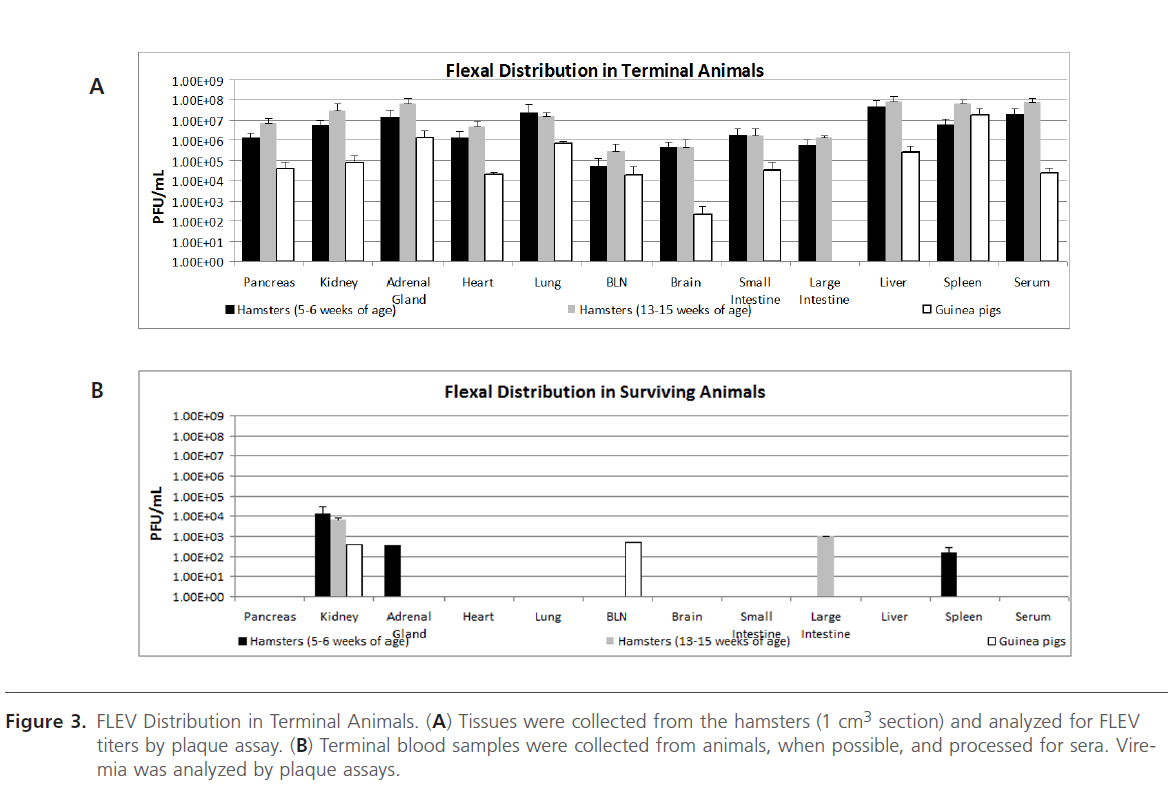
Figure 3: FLEV Distribution in Terminal Animals. (A) Tissues were collected from the hamsters (1 cm3 section) and analyzed for FLEV titers by plaque assay. (B) Terminal blood samples were collected from animals, when possible, and processed for sera. Viremia was analyzed by plaque assays.
Vascular permeability associated with FLEV infection
To assess vascular permeability, EBD was infused into animals prior to euthanasia via the vena cava. This procedure was performed to demonstrate whether mortality was associated with vascular permeability. Animals were anesthetized prior to EBD infusion and then euthanized after 30 min. Tissue samples from infected and uninfected animals were collected and assayed for EBD concentration. The amounts of EBD, as assayed by OD 630/g, were compared amongst the hamsters that died as a result of challenge and the hamsters that survived. Vascular leakage was associated with the large intestines, spleen, adrenal glands, lung, and brain in 5-6 week old hamsters and in the spleen, lung, and brain in the 13-15 week old hamsters (Figure 4). Additionally, the EBD numerical data is supported by visual observations of the tissues (Figure 5). The liver, large intestine, and lung of a FLEV-infected hamster and mock-infected hamster is representative of the visual observations from other animals. Typically, a visual difference could be observed in FLEV-infected and mock-infected hamsters, with specific tissues from FLEV-infected hamster showing more intense blue color when compared to mock-infected hamsters. These data demonstrate vascular permeability in specific tissues in hamsters infected with FLEV.
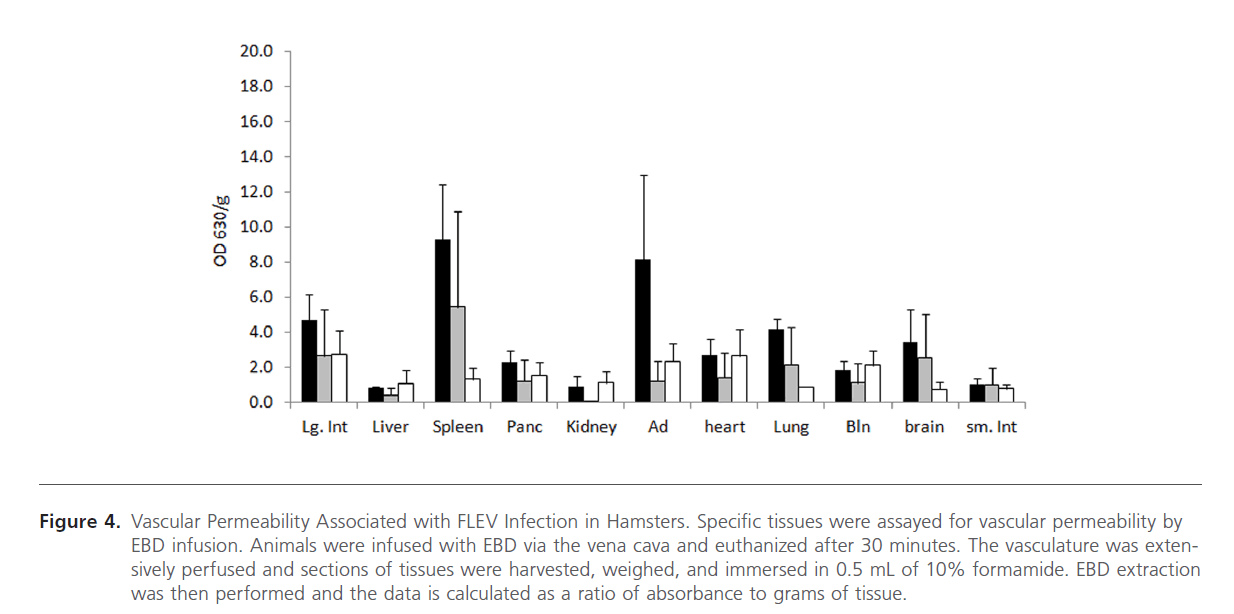
Figure 4: Vascular Permeability Associated with FLEV Infection in Hamsters. Specific tissues were assayed for vascular permeability by EBD infusion. Animals were infused with EBD via the vena cava and euthanized after 30 minutes. The vasculature was extensively perfused and sections of tissues were harvested, weighed, and immersed in 0.5 mL of 10% formamide. EBD extraction was then performed and the data is calculated as a ratio of absorbance to grams of tissue.
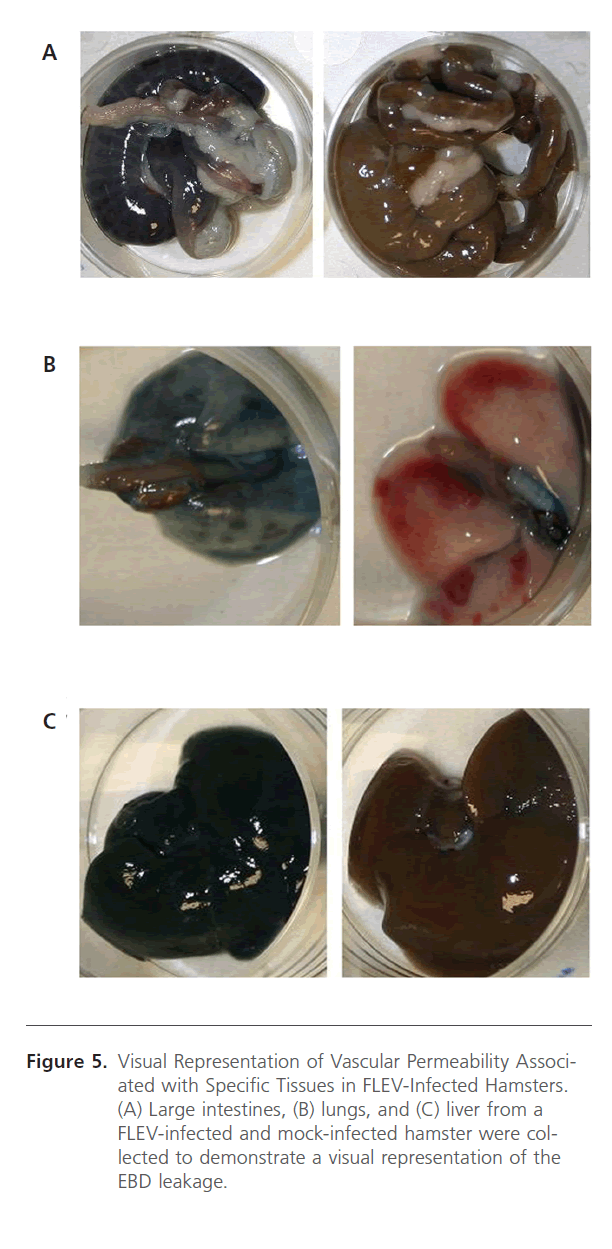
Figure 5: Visual Representation of Vascular Permeability Associated with Specific Tissues in FLEV-Infected Hamsters. (A) Large intestines, (B) lungs, and (C) liver from a FLEV-infected and mock-infected hamster were collected to demonstrate a visual representation of the EBD leakage.
Discussion
Arenaviral disease is often associated with fever, malaise, myalgia, and fatigue during the early stages of disease prior to progressing to petechial rashes, hemorrhage, delirium, tremors, and hyporeflexia [9, 10]. Advanced stages of disease is often associated with respiratory distress, vascular permeability, seizures, shock, and death. Several arenavirus models have been described. Rodents have been mainly utilized as small animal models for arenavirus pathogenesis research. Of the rodents, guinea pigs and hamsters have been the most common small animal used to describe arenavirus hemorrhagic fever. The guinea pig has previously been used to study LASV and GTOV pathogenesis, while hamsters have been commonly used to study PIRV pathology. LASV infection of guinea pigs results in viremia, tissue viral titers, histological lesions, and morbidity and mortality. Infection of the guinea pig with GTOV results in viremia, viral antigen in endothelial cells, thrombocytopenia, petechia, epistaxis, adrenal hemorrhage, bone marrow depletion, and morbidity and mortality. To avoid performing research in a BSL-4 environment, some investigators have used the PICV-guinea pig model to study hemorrhagic fever pathogenesis [11-15], while others may use the PIRV-hamster model [16-19]. These different models do provide some pathogenic similarities to hemorrhagic fever caused by LASV, JUNV, MACV, or GTOV, but there are also distinct differences. PIRV infection in the hamster results in viremia, tissue viral titers, pathologic abnormalities in the liver, spleen, and lungs, anorexia, lethargy, pulmonary hemorrhage, splenomegaly, intestinal hemorrhage, coagulopathy, and morbidity and mortality. Infection of the guinea pig with the PICV P18 variant results in viremia, tissue viral titers, terminal shock, and morbidity and mortality. Additionally, the PICV and PIRV models provide a safer environment to study hemorrhagic fever pathogenesis since these viruses are not known to cause overt disease in humans. Lastly, studies utilizing PICV requires BSL-2 containment, while studies using PIRV requires BSL-3 containment. Utilizing these models negates the requirement for BSL-4 containment, which allows for studies to be performed at a more cost-efficient and rapid manner.
Flexal virus is a pathogenic arenavirus (identified as a HHS and USDA Select Agent) that was isolated in Brazil from the Oryzomys spp. (Rice rat) and is known to cause overt disease [7]. At least 2 symptomatic laboratory infections have been observed; however, the disease has not been completely defined [20]. Thus, this pathogen may act as a bridge to study various arenavirus hemorrhagic fever treatments prior to studying such products in the BSL-4. To clarify, products aimed to treat LASV (or the other BSL-4 arenaviruses) infection, could be first screened in the BSL-2 PICV or BSL-3 PIRV model because the work is cost-efficient, rapid, and safer. Products that demonstrate efficacy could then be tested in the FLEV BSL-3 model prior to the testing in a BSL-4 model since FLEV is a known human pathogen. Additionally, infections of all hamsters with comparable titers of PIRV (when compared to FLEV) result in mortality, while some of the hamsters infected with FLEV recover and survive. This scenario is more realistic when compared to arenavirus infections that result in hemorrhagic fever since these infections do not result in a 100% mortality rate. Thus, this study aimed to define the disease progression associated with FLEV infection in the Syrian golden hamster model. Characterization of this model is important to future vaccine, prophylactic, and therapeutic work.
Syrian golden hamsters serve as an adequate animal model to study FLEV infection and pathogenesis and can be used to test the efficacy of prophylactics, therapeutics, and vaccines aimed at arenaviral hemorrhagic fever and disease. Some differences exist when comparing the results of challenge in the 5-6 week old hamsters and the 13-15 week old hamsters in regards to survival and the disease progression. However, differences have also been reported when hamsters, of the approximate ages, were infected with PIRV [16-19]. Thus, these results were not surprising. More studies involving higher animal numbers may be necessary to delineate whether the age of the animals influence mortality and overt disease.
Specific tissues were assayed in animals that survived and died as a result of FLEV challenged. The highest titers of virus were observed in the adrenal gland, lung, and liver in the 5-6 week old hamsters and in the adrenal gland, liver, and pancreas in the 13-15 week old hamsters. Less viral titers were associated with the tissues in the guinea pigs that died when compared to the hamsters. No virus was observed in the large intestines of the guinea pigs; however, this may be because the amount of virus present was below the limit of detection. Viremia was also measured in all hamsters and guinea pigs that died as a result of FLEV challenge. This demonstrates that viremia existed throughout the course of the disease. No viremia, above the limit of detection, was exhibited in the surviving animals.
Additionally, the data suggest that mortality may be correlated to vascular permeability, since vascular permeability was associated with tissues from the hamsters that died. These results are in agreement with previous published reports demonstrating vascular permeability in PICV-infected hamsters [21]. Vascular leakage was observed in the large intestines, spleen, adrenal glands, lung, and brain in the 5-6 week old hamsters and in the spleen, lung, and brain in the 13-15 week old hamsters. However, no correlation could be discerned when comparing the amount of virus in a specific tissue with EBD leakage. Thus, viral titers in specific tissues do not seem to correlate with vascular permeability under the correct conditions.
In all, the data suggest that FLEV in the hamster can be (1) used as a surrogate model to study arenaviral hemorrhagic fever pathogenesis, (2) used to screen antivirals and vaccines for arenavirus infection, and (3) used to study FLEV-associated pathogenesis. Unlike some of the other arenavirus animal models, FLEV infection of the hamster does not result in 100% mortality. Thus, mortality resembles what is observed in humans infected with an arenavirus hemorrhagic fever. The observed viremia, tissue viral titers, petechiae, loss of weight, and morbidity and mortality resembles what is observed in humans suffering from arenavirus-induced hemorrhagic fever.
Conclusion
In conclusion, these data suggest that either 5-6 week old hamsters or 13-15 week old hamsters can be used when evaluating the efficacy of a vaccine, therapeutic, or prophylactic against arenaviral hemorrhagic fever. In all, it can be concluded that FLEV infection in hamsters leads to overt disease, viremia, weight loss, viral titers in specific tissues, and vascular permeability.
Acknowledgment
The authors would like to acknowledge Drs. Jim Blank, Carol Sabourin, and Lena Furci for providing technical review. This work was funded through the Battelle National Security Global Business Independent Research and Development Program.
157
References
- McCormick JB, Webb P, Krebs J, Johnson K, Smith E (1987) A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis 155: 437-444.
- Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, et al. (2002) Hemorrhagic Fever Viruses as Biological Weapons: Medical and Public Health Management. JAMA: The Journal of the American Medical Association 287: 2391-2405.
- CDC/NIH (2007) Biosafety in Microbiological and Biomedical Laboratories, Fifth edn, Washington DC.
- Peters CJ (2002) Arenaviruses. In: Richman DD, Whitley RJ, Hayden FG (eds) Clinical Virology. Washington DC: ASM Press. 949-969
- Walker D, Wulff H, Lange JV, Murphy F (1975) Comparative pathology of Lassa virus infection in monkeys, guniea-pigs, and Mastomysnatalensis. Bull World Hearh Org 52: 523-534.
- Peters C (1997) Viral hemorrhagic fevers. In: Nathanson N, Ahmed R, Gonzalez-Scarano F, Griffin D, Holmes K, Murphy F, Robinson H (eds) Viral Pathogenesis. Philadelphia: Lippincott-Raven. 779-799.
- Archer AM, Rico-Hesse R (2002) High Genetic Divergence and Recombination in Arenaviruses from the Americas. Virol 304: 274-281.
- Vela E, Zhang L, Colpitts TM, Davey RA, Aronson JF (2007) Arenavirus entry occurs through a cholesterol-dependent, non-caveolar, clathrin-mediated endocytic mechanism. Virol 369: 1-11.
- de Manzione N, Salas RA, Paredes H, Godoy O, Rojas L, et al. (1998) Venezuelan hemorrhagic fever: clinical and epidemiological studies of 165 cases. Clin Infect Dis 26: 308-313.
- McCormick JB, Fisher-Hoch SP (2002) Lassa fever. Curr Top Micro Immunol 262: 75-109.
- Aronson JF, Herzog NK, Jerrels TR (1994) Pathological and virological features of arenavirus disease in guinea pigs. Am J Pathol 145: 228- 235.
- Bowick GC, Fennewald SM, Scott EP, Zhang L, Elsom BL, et al. (2007) Identification of Differentially Activated Cell-Signaling Networks Associated with Pichinde Virus Pathogenesis by Using Systems Kinomics. J Virol 81: 1923-1933.
- Scott EP, Aronson JF (2008) Cytokine patterns in a comparative model of arenavirushaemorrhagic fever in guinea pigs. J Gen Virol 89: 2569- 2579.
- Zhang L, Marriott K, Harnish D, Aronson J (2001) Reassortant analysis of guinea pig virulence of Pichindeviurs variants. Virol 290: 30-38.
- Zhang L, Marriott K, Aronson JF (1999) Sequence analysis of the small RNA segment of guinea pig-passaged Pichinde virus variants. Am J Trop Med Hyg 61: 220-225.
- Sbrana E, Mateo RI, Xiao S-Y, Popov VL, Newman PC, et al. (2006) Clinical laboratory, virologic, and pathologic changes in hamsters experimentally infected with Pirital virus (Arenaviridae): a rodent model of Lassa fever. Am J Trop Med Hyg 74: 1096-1102.
- Vela EM, Knostman KA, Warren RL, Garver JN, and Stammen R (2010) The disease progression associated with Pirital virus infection in the Syrian golden hamster. J Infect Dis Imm 2: 15-23.
- Vela EM, Knostman KA, Mott JM, Warren RL, Garver JN, Vela LJ, and Stammen RL (2010) Genistein, a general kinase inhibitor, as a potential antiviral for arenaviralhemorrhagic fever as described in the Pirital virus-Syrian golden hamster model. Antiviral Res 87: 318-328.
- Xiao S-Y, Zhang H, Yang Y, Tesh RB (2001) Pirital virus (Arenaviridae) infection in the Syrian golden hamster, Mesocricetusauratus: A new animal model for arenaviral hemorrhagic fever. Am J Trop Med Hyg 64: 111-118.
- Enria D, Bowen M, Mills J, Shieh W, Bausch D, et al. (1999) Arenavirus Infections. In: Guerrant R, Walker D, Weller P (eds) Tropical Infectious Diseases. Philadelphia: Churchill Livingstone, 1191-1212.
- Gowen BB, Julander JG, London NR, Wowng M-H, Larson D, et al. (2011) Assessing changes in vascular permeability in a hamster model of viral hemorrhagic fever. Virol J 12: 240.











