Keywords
Organoids; IPSC (Induced Pluripotent Stem Cells); Ethics; Diseases
Introduction
Elucidating the cellular and molecular basis of human neocortex development and evolution has profound importance for understanding our species-specific cognitive abilities as well as our susceptibility to neurodevelopmental diseases [1]. Much of our current understanding of brain development and function is based upon a long history of observational and functional studies in a variety of animal models. These foundational studies have revealed general features of vertebrate and mammalian brain development that are shared across taxa, especially early events of brain patterning and neuron generation. However, specific features of human brain development and disease are much less understood [2].
The original experiments of cellular reprogramming, led by the researcher Shinya Yamanaka, surprised the scientific community by break down the dogma that specialized cells of the human body would have a lifelong identity. The forced expression of a group of transcription factors, pluripotent-related genes, has the ability to redirect the identity of specialized cells and represents an extraordinary way of demonstrating cell flexibility. This induced return to the embryonic stage pluripotent was baptized of iPSC (Induced Pluripotent Stem Cells) [3,4]. Recent progress with in vitro models of various organ systems has demonstrated the enormous self-organizing capacity for pluripotent stem cells to form whole tissue [5]. Yamanaka's experiments allow us to bring to reality the dream of many neuroscientists: to capture the human genome of a patient in pluripotent stem cells and to use them for the unlimited production of specialized cells of the nervous system [3,6].
The development of such 3D in vitro cultures, in which cells self-organize into complex structures, has recently brought into usage the term “organoid”, previously an imprecisely defined term used for many different structures [7]. Cerebral organoids are three dimensional in vitro cultures that recapitulate key characteristics of human brain development including brain regional specification, the formation of progenitor layers and the generation of diverse types of functional neurons [5,8]. Thus far, organoid models have been applied to study events of neural progenitor dysfunction that occur during early stages of brain development, including microcephaly-associated phenotypes and progenitor abnormalities resulting from Zika virus infections. Organoids generated from patients with severe idiopathic autism spectrum disorder have also been used to implicate progenitor over proliferation and generation of excessive GABAergic (γ - aminobutyric-acid-releasing) neurons in this complex disease. However, difficulties remain that preclude the broader application of brain organoids to disease modelling [9].
Diseases that can be modelled by cellular reprogramming can be rare, monogenetic or in the broad spectrum of sporadic or multifactorial diseases. To date, there is no scientific publication demonstrating the usefulness of iPSC technology to model this last group of complex diseases. Possibly, it has been complicated to obtain conclusive results from this type of complex disease because of the different genetic backgrounds and influences of the environment. It is worth noting that even monogenetic diseases can also present a great phenotypic variability. It will be necessary to determine whether genotype-phenotype variation observed in patients will be reproduced by neurons generated from patients' iPSCs or whether reprogramming will eliminate environmental or epigenetic "noise" [3].
The general conclusion from recent organoid studies is that stem cells have a remarkable ability to reproduce in culture what they do in the organism. This promises a gateway to create the functional cell types that adherent or liquid cultures cannot produce but, at present, the experimenter has little or no input into what the cells do when they assemble into organoids [10]. Some differentiation protocols for certain subtypes of neurons already exist, but we still do not know how to differentiate in culture the iPSCs in all cell types of the human brain.
Although cerebral organoid technology is promising, many challenges remain, including rampant batch-to-batch and linetoline variability and irreproducibility; irregularities in the timing of neuronal maturation, laminar architecture, and cell diversification; unwanted differentiation into other tissue types; and a paucity of direct comparisons of the organoids to native human tissue [11]. A final major limitation, for 3D models specifically, is the inadequate supply of nutrients and oxygen to the central regions of the tissue. Because cells farther than 200–400 mm from the surface of brain tissue fail to receive enough nutrients through diffusion, healthy tissue is limited to the surface of organoids. This has effects on everything from overall tissue patterning to later expansion of individual brain regions [2,5,12].
Because organoids shape the development and maintenance of a human organ, they have the potential to revolutionize biomedical research and change the drug discovery process. Organoids derived from patients offer possibilities to mimic pathologies of human genetic disorders in a dish and develop a personalized treatment, whether for hereditary disease or cancer [13]. We find that over 80% of genes implicated in neocortex disease or evolution and are differentially expressed along the fetal cortex lineage have similar expression profiles in organoid and fetal cerebral cortex. In addition, cultured organoids have great potential to replace damaged tissues or even whole organs, a potential that has already been demonstrated in animal models. Thus, despite its relatively immature nature, organoid research has already reached the stage of commercialization and medical application, attributing specific responsibilities to scientists working in this field. Biologists working in basic science may not always be used to an immediate medical impact of their work, whereas medical doctors may underestimate the experimental nature of most organoid research.
Combining organoid cultures with recent developments in live imaging will allow us, for the first time, to visualise early events in human development in real time. It will be possible to track cells and to study, for example, how the human cortical plate develops from the very early neural progenitors to the final mature neural cells. It will also be possible to assess the impact of exchanging a single growth factor or of Extracellular Matrix (ECM) modifications on cellular behaviour. Also, as new synthetic ECM components are developed, organoids will provide a platform for determining how physical forces and cell shapes influence tissue differentiation or organ shape. In summary, organoid cultures combined with novel developments in live imaging, genetic engineering and biomaterials represent a tour de force that will influence, in the very near future, how we study human development and how we treat human disease. In our view, the combined emergence of these new technologies raises strong hopes for the development of novel therapies and fundamental improvements in the drug discovery process [14].
Global discussions involving the analysis of the use of organoids as a way of understanding diseases in the last eight years are extremely pertinent and frequent in the scientific world, mainly due to the revolutions they can bring. Faced with this evolution, it is essential to study the iPSC (Induced pluripotent stem cells) and the formation of organoids applied in the diagnosis and treatment of diseases, so that the benefits of this strategy can be fully utilized.
Literature Review
This is a systematic review with meta-analysis using the PRISMA protocol (http://prisma-statement.org/) to prepare the review. During study search, some steps were adopted, such as the research line and eligibility of articles, analysis of the findings to establish which articles would be included, and data interpretation based on the study orientation.
The guiding question followed the PICO acronym, in which P are the induced pluripotent stem cells (iPSC); I is the formation of organoids; C are diseases; and O are diagnostics and treatments.
The guiding question was punctuated in high impact in scientific circles and in the media of the changes that the use of iPSC and Organoids can bring mainly in the diagnosis and treatment of various diseases.
Researches about the subject, related to the period from 2010 to 2020, were included, with registrations found in English and Portuguese that approached iPSC, organoids and scientific ethics (Figure 1). The study period was chosen because the deeper knowledge production is recent, restricting the record of previous years, as new findings on the subject are often held (Figure 1).
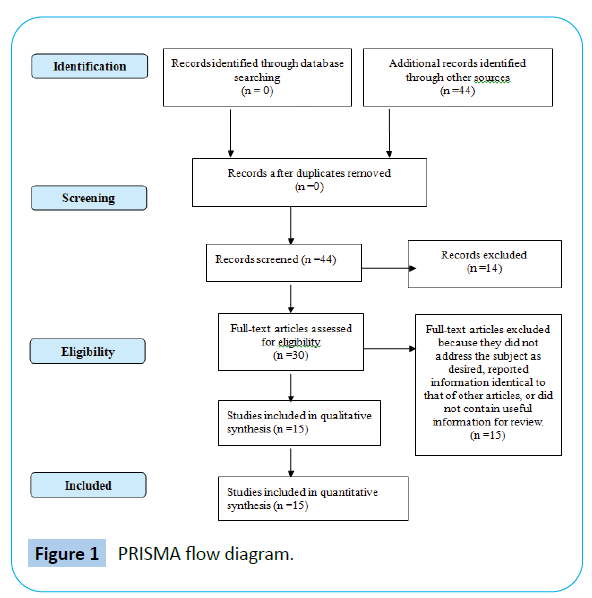
Figure 1: PRISMA flow diagram.
Studies referring to organoids that did not present the desired approach, which provided information already existent in other articles or that analyzed phenomena occurred in the organoids that are not of interest of the research were excluded (Table 1). “Organoids”, “iPSC”, “Ethic” and “Evidence-based medicine” were the keywords used separately and later combined with the Boolean operator “AND”.
| Authors |
Sample Characteristics |
Journal |
Main Findings |
Limitations |
Conclusions |
| Camp et al. (2015) |
They use single-cell RNA sequencing (scRNA-seq) to dissect and compare cell composition and progenitor-to-neuron lineage relationships in human cerebral organoids and fetal neocortex. |
PNAS |
They find that, with some exceptions, the same genes used to build cortical tissue in vivo characterize corticogenesis in vitro. Their data thus show that genetic features underlying human cortical development can be accurately studied in organoid culture systems. |
The extent to which in vitro organoid systems recapitulate neural progenitor cell proliferation and neuronal differentiation programs observed in vivo remains unclear. |
They concluded that these organoid cortical cells use gene expression programs remarkably similar to those of the fetal tissue to organize into cerebral cortex-like regions. |
| Kelava and Lancaster (2016b) |
They describe advances in the development of these methods, focusing on neural rosette and organoid approaches, and compare their relative capabilities and limitations. They also discuss current technical hurdles for recreating the cell-type complexity and spatial architecture of the brain in culture and offer potential solutions. |
Cell stem cell |
They find that the combination of higher throughput with dual-SMAD inhibition leads to reproducible forebrain organoids that hold great promise for future therapeutic avenues. |
While all three approaches methods are feasible in most tissue culture laboratories, some require more specialized equipment or complicated culture conditions. |
They concluded that extraordinary progress has been made in recent years in the development of in vitro models of human brain development. |
| Muotri (2010) |
He presents a critical view on the recent advances obtained from disease modeling using human pluripotent stem cells. The focus on cellular reprogramming as tool to generate patient-specific induced pluripotent stem cells is justified by the great experimental potential, not only for disease modeling, but also as a biotecnological tool for future drug-screening platforms and personalized medicine. |
Estudos avançados |
They find that the focus on cellular reprogramming as tool to generate patient-specific induced pluripotent stem cells is justified by the great experimental potential, not only for disease modeling, but also as a biotecnological tool for future drug-screening platforms and personalized medicine. |
The article analyzes future perspectives of the application of the iPSC and, therefore, it is difficult to predict and to determine its uses and utilities of forceful form. |
They concluded that the potential for cellular reprogramming seems to be even limited by human creative ability and ethical principles defined by society. |
| Lancaster et al. (2013) |
They have developed a human pluripotent stem cell-derived three-dimensional organoid culture system, termed cerebral organoids, that develop various discrete, although interdependent, brain regions. These include a cerebral cortex containing progenitor populations that organize and produce mature cortical neuron subtypes. |
Nature |
They demonstrate premature neuronal differentiation in patient organoids, a defect that could help to explain the disease phenotype. |
Although considerable progress has been made forin vitro models of whole-organ developmentfor other systems, such as intestine, pituitary and retina, a three-dimensional culture model of the developing brain as a whole has not been established. |
They concluded that, together, these data show that three-dimensional organoids can recapitulate development and disease even in this most complex human tissue. |
| Kelava and Lancaster (2016b) |
They focus on the similarities of current organoid methods to in vivo brain development, discuss their limitations and potential improvements, and explore the future venues of brain organoid research. |
Elsevier |
They find that the development of protocols for different mammalian species will deepen our insight into evolutionary aspects of neurogenesis. |
Understandably, in vitro organoid culture takes place without the normally present embryonic surrounding. |
They concluded that although tremendous advances have been made in improving the in vitro culture of developing neural tissues, these methods are not without their faults and limitations. |
| Luo et al. (2016) |
They compared epigenomic and regulatory features in cerebral organoids and human fetal brain, using genome-wide, base resolution DNA methylome and transcriptome sequencing. |
Cell reports |
They find that molecular markers can be designed from hypo-DMR block regions and facilitate the screening of CO culture conditions that eliminate or reduce the pericentromeric demethylation, which may contribute to greater long-term genomic stability of CO culture. |
Organoids derived from human pluripotent stem cells recapitulate the early three-dimensional organization of the human brain, but whether they establish the epigenomic and transcriptional programs essential for brain development is unknown. |
They concluded that early non-CG methylation accumulation at superenhancers in both fetal brain and organoids marks forthcoming transcriptional repression in the fully developed brain. |
| Quadrato et al. (2017) |
They analyse gene expression in over 80,000 individual cells isolated from 31 human brain organoids. We find that organoids can generate a broad diversity of cells, which are related to endogenous classes, including cells from the cerebral cortex and the retina |
Nature |
They find that 3D brain organoids have the potential to model higher-order functions of the human brain, such as cellular interactions and neural circuit dysfunctions related to neurodevelopmental and neuropsychiatric pathologies. |
The cells generated within organoids and the extent to which they recapitulate the regional complexity, cellular diversity and circuit functionality of the brain remain undefined. |
They concluded that neuronal activity within organoids could be controlled using light stimulation of photosensitive cells, which may offer a way to probe the functionality of human neuronal circuits using physiological sensory stimuli. |
| Huch et al. (2017) |
In this Spotlight article, Meritxell Huch and Juergen Knoblich begin by discussing the exciting promise of organoid technology and give concrete examples of how this promise is starting to be realised. In the second part, Matthias Lutolf and Alfonso Martinez-Arias offer a careful and considered view of the state of the organoid field and its current limitations, and lay out the approach they feel is necessary to maximise the potential of organoid technology. |
The company of biologists |
They find that bioreactor technology or engineered blood vessel systems may be employed to address the major problem of nutrient availability in growing organoids and thereby allow proper longterm growth of complex systems. |
There is no doubt that this technology opens up a world of possibilities for scientific discovery in developmental biology as well as in translational research, but whether organoids can truly live up to this challenge is, for some, still an open question. |
They concluded that organoids have revealed what developmental biologists have suspected for years: that cells have amazing self-organising abilities, the regulation of which is only just beginning to emerge. |
| Watanabe et al. (2017) |
They describe optimized organoid culture methods that efficiently and reliably produce cortical and basal ganglia structures similar to those in the human fetal brain in vivo. |
Cell reports |
They find that even though infants exposed to ZIKV might escape structural brain defects, their risk for neurodevelopmental and neuropsychiatric disorders may be significantly elevated. |
However, many organoid differentiation protocols are inefficient and display marked variability in their ability to recapitulate the three-dimensional architecture and course of neurogenesis in the developing human brain. |
They concluded that although the predictive value of the organoid system requires further validation, their studies demonstrate its power in singling out therapeutic candidates meriting future investigations. |
| Bredenoord et al. (2017) |
They describe the current state of research and discuss the ethical implications of organoid technology |
Science |
They find that only by engaging in constructive interdisciplinary dialog around these issues, involving not only scientists but also patients, policy-makers, clinicians, ethicists, and the public, can we ensure responsible innovation and long-term acceptance of this exciting technology. |
Organoid research also raises additional ethical questions that require reexamination and potential recalibration of ethical and legal policies. |
They concluded that organoids face several layers of complexity, not only technologically but also with regard to their ethical introduction in research, clinical care, and society. |
| Lancaster and Knoblich (2014) |
They describe a recently established protocol for generating 3D brain tissue, so-called cerebral organoids, which closely mimics the endogenous developmental program. This method can easily be implemented in a standard tissue culture room and can give rise to developing cerebral cortex, ventral telencephalon, choroid plexus and retinal identities, among others, within 1–2 months. |
Nature |
They find that this system is perhaps most suited to examining neurodevelopmental disorders, as it best recapitulates the early developing brain (first trimester, on the basis of histological comparisons). |
As in all in vitro systems, the method lacks surrounding embryonic tissues that are important for the interplay of neural and non-neural tissue cross-talk. |
They concluded that as organoids can be maintained for more than 1 year in long-term culture, they also have the potential to model later events such as neuronal maturation and survival. |
| Sloan et al. (2017) |
They present an approach for generating astrocyte lineage cells in a three-dimensional (3D) cytoarchitecture using human cerebral cortical spheroids (hCSs) derived from pluripotent stem cells. |
Neuron |
They find that because many of the genes involved in synaptogenic and synapse pruning pathways are tightly correlated with astrocyte maturation state, it is possible that the development of abnormal neural circuits in various neurodevelopmental disorders may be related to the inappropriate timing and/or degree of astrocyte maturation . |
Because of technical limitations, human astrocytes have received particularly little study |
They concluded that hCS-derived glia closely resemble primary human fetal astrocytes and that, over time in vitro, they transition from a predominantly fetal to an increasingly mature astrocyte state. |
| Clevers (2016) |
They present that organoid technology can therefore be used to model human organ development and various human pathologies ‘in a dish.’’ Additionally, patient-derived organoids hold promise to predict drug response in a personalized fashion. |
Cell |
They find that from a basic science perspective, PSC-based organoids will by their very nature play a key role in understanding the developmental biology of organs and will thus complement the long tradition of in vivo studies in this field. |
The current versions of organoids have clear limitations,e.g., innervation, blood vessels, and immune cells are absent, and as a consequence, disease processes are only partially recapitulated. |
They concluded that organoids open up new avenues for regenerative medicine and, in combination with editing technology, for gene therapy. The many potential applications of this technology are only beginning to be explored. |
| Zhu et al (2017) |
They propose a human induced pluripotent stem cell (hiPSC)-based 3D brain organoid model, and explore the mechanisms underlying neural dysfunctions in prenatal alcohol exposure (PAE) in vitro. |
Integrative biology |
They find that lthough the molecular mechanism underlying the modulation of the excitatory–inhibitory balance is not yet known, their results indicate that ethanol may disturb neuronal subtypes by altering the neuronal response to signals involved in neural terminal differentiation. |
A comprehensive understanding of fetal brain development under ethanol exposure is challenging due to the limitations of animal models. |
They concluded that with ethanol exposure, the brain organoids displayed attenuated neurite outgrowth and skewed neural maturation. |
| Jo et al (2016) |
They developed a method to differentiate human pluripotent stem cells into a large multicellular organoid-like structure that contains distinct layers of neuronal cells expressing characteristic markers of human midbrain. Importantly, we detected electrically active and functionally mature mDA neurons and dopamine production in our 3D midbrain-like organoids (MLOs). |
Cell stem cell |
They find that mDA neurons within the hMLOs produced DA, exhibited mature neuronal properties, and were able to form synapses with other neurons within the hMLOs. |
A 3D organoid model of the midbrain containing functional midbrain dopaminergic (mDA) neurons has not been reported. |
They concluded that MLOs bearing features of the human midbrain may provide a tractable in vitro system to study the human midbrain and its related diseases. |
Table 1 Main findings.
1. “Organoids”,
2. “iPSC”,
3. “Ethic” and
4. “Evidence-based medicine”
Results and Discussion
In total, 44 evidences were found. With the subsequent application of the inclusion and exclusion criteria, 15 studies were included for qualitative synthesis. Figure 1 and Figure 2 summarize the main methodological features for inclusion or exclusion of searched studies.
Figure 2: Global discussions involving the analysis of the use of organoids.
Cerebral organoids represent a novel system to interrogate the mechanisms of human neurological conditions that have been difficult or impossible to examine in mice and other model organisms. We have used brain organoids to examine the cell biological basis of a form of microcephaly, a disorder involving small brain size. Similarly, a variety of neurological disorders could be examined in cerebral organoids [15]. Human Induced Pluripotent Stem Cells (iPSCs) provide a unique platform to investigate neural development in vitro [16], since neurons in cerebral organoids have electrophysiological properties that closely match the profiles of neurons recorded from midgestational stages of human fetal cortex, consistent with our immunohistochemical, molecular, and bioinformatics analyses.
Although none of the currently available organoid models recapitulate the complete physiology of a human organ, organoids have already been used successfully for disease modelling and drug research e.g. for the development of individualized human cancer models and for the patient-specific evaluation of the therapeutic efficacies of cystic fibrosis drugs. The possible uses of these systems are boundless and have the potential to overcome the frequently observed lack of translation from animal studies. In addition, there is a further benefit with regard to ethical considerations of using animals where there is the potential to limit the numbers of animals needed for neurodevelopmental studies.
Since organoids-unlike cell lines-ideally represent all cellular components of a given organ, they are theoretically well suited for infectious disease studies, particularly of pathogens that are restricted to man and are dependent on specialized cell types. In addition organoids can be used to study and model organ-specific monogenic hereditary diseases [17].
In principle, the adult stem cell-based organoid technology allows rapid ex vivo testing of drug responses on the affected tissue of individual patients. Personalized medicine would mean that clinicians and researchers would need to obtain cells from a patient, grow brain organoids on a high throughput scale and test the effectiveness of a large set of drugs, finding the ones most appropriate for the patient.
Proof-of-concept studies have demonstrated the feasibility of expanding organoids from adult stem cells followed by safe transplantation into animals. Moreover the possibility to grow human organoids representative of the main targets for drug related toxicity (gut, liver, and kidney) opens up theoretical avenues to complement animal-based toxicology with assays performed directly on these vulnerable human tissues.
Once culturing protocols for human aSC-based organoids were established, we have shown the feasibility of growing organoids from primary colon, prostate, and pancreatic cancers. These cancer organoids provide the unique opportunity for functional testing (e.g. for drug sensitivity) and for correlating such data with the genetic make-up of individual tumors.
In diseases context, brain organoids have been a powerful tool for the rapid analysis of the effects of Zika on human brain development, providing insight in an extremely short time period. Several very recent studies reported an effect of Zika on neural stem cells and on brain organoids. Another neurodevelopmental disorder recently studied in organoids is Autism Spectrum Disorder (ASD). Mariani et al. [18] used iPS cell-derived organoids from patients with idiopathic ASD to study the processes taking place during neocortical neurogenesis that may contribute to the described complex pathologies. ASD-derived organoids, and their comparison to wild type organoids, showed that in the patients the early neural progenitors had a decreased cell cycle length, resulting in their over-proliferation. An additional feature of ASD organoids was that the production of GABAergic neurons was increased, due to the increase in expression of FOXG1, a gene involved in the production of early cortical neurons and in some ASDs with prenatal microcephaly. The ASD organoids also exhibited overgrowth of neurites and an increase in the number of synapses, which is one of the characteristics found in some post-mortem studies of ASD patients [19-27].
It is worth mentioning that extensive research has focused on generating midbrain dopaminergic (mDA) neurons from hPSCs in recent years particularly because the selective loss of mDA neurons is a key pathological feature of Parkinson's disease (PD) [28,29].
In addition to these shortcomings Schizophrenia represents another debilitating disorder, originsof which are thought to come partly from a disruption of neurodevelopment. Some regions of the genome show particular association with an increased risk of schizophrenia, but the underlying mechanisms remain elusive. Yoon et al. [30] used iPS cells-derived from patients with a deletion in one of the regions implicated in increased schizophrenia risk (15q11.2) to derive neural rosettes which model the behaviour of early cortical neural progenitors [31,32].
The use of organoids is also able to demonstrate influences suffered before birth. Research has shown that when exposed to ethanol, these organoids exhibited significantly impaired neurogenesis in comparison with controls, including neurite outgrowth and neural maturation. RNA-sequencing analysis was used to identify a series of new genetic and molecular pathways that were significantly altered with ethanol exposure, indicating the utility of this model for the investigation of the underlying mechanisms of various pathological features in individuals with prenatal alcohol exposure [33].
The ethical challenges of organoid bio banking are not new, but the storage and use of organoids in biobanks constitute an area of complex converging technology which several ethical discussions come together. When we talk about clinical trials, organoid technology may be viewed as the long-awaited alternative to animal testing. Although animal research will never become entirely obsolete, organoid technology does affect the ethics of animal experimentation. The use of organoids is complementary to, rather than in competition with, this classical research methodology. However, the onus of proof for rationalizing the use of animals might justifiably shift further toward a “comply or explain” paradigm: Either one uses organoids, or one explains why animal experimentation is needed. Justification for the use of animal experiments over organoid models might become necessary on a case-by-case basis. Both the ethical paradigm and legislation of animal research may need ongoing critical scrutiny.
From a basic science perspective, pluripotent stem cell-based organoids will by their very nature play a key role in understanding the developmental biology of organs and will thus complement the long tradition of in vivo studies in this field. From the same perspective, adult stem cell (aSCs)-based organoids provide basic insights into the processes that allow aSCs to maintain and repair established tissues. Yet, because of the ease of production and the close resemblance to human organs in health and disease, organoids hold great appeal for translational research and invite an almost immediate application into the clinic.
We find that upon maturation organoids acquire structural traits of mature neurons, including dendritic spine-like structures, which have been difficult to generate by in vitro directed differentiation. This offers the opportunity to study a new set of developmental processes, such as human synaptic pruning and active spine refinement, which could not previously be modelled in vitro. The diversity and maturation of cell types generated, the robustness of the neuronal networks, the presence of structural traits of mature neurons and the possibility of using sensory experience to modulate neuronal activity collectively suggest that, beyond modelling early events of progenitor biology, these 3D brain organoids have the potential to model higher-order functions of the human brain, such as cellular interactions and neural circuit dysfunctions related to neurodevelopmental and neuropsychiatric pathologies. The problem of heterogeneity of whole-brain organoids might be solved by a combination of the accumulation of knowledge about stem cells and further technological improvements. New research about stem cells will bring about a deeper understanding of the starting material and the organoids produced thereof.
Organoids face several layers of complexity, not only technologically but also with regard to their ethical introduction in research, clinical care, and society. Only by engaging in constructive interdisciplinary dialog around these issues, involving not only scientists but also patients, policy-makers, clinicians, ethicists, and the public, can we ensure responsible innovation and long-term acceptance of this exciting technology. What is clear is that, although tremendous advances have been made in improving the in vitro culture of developing neural tissues, these methods are not without their faults and limitations. Improvements in the techniques will allow for more complex processes to be studied, including intricate cell-cell interactions and migration in the developing brain. Furthermore, diseases other than severe, early neurodevelopmental disorders could be modeled, with the potential to model more common, but also more subtle, disorders.
We believe that interactions with engineers will be central for achieving robust and reproducible organoid cultures, particularly in the case of PSCs where the challenge is most obvious at the moment. Further progress is keenly anticipated: engineers have developed powerful tools that will allow the culture of organoids in 3D contexts that provide more defined environments. Thus, instead of growing organoids without any spatial constraints in ill-defined 3D matrices, well-defined biomaterials and micro technology may be applied to guide in vitro development through geometric and/or mechanical inputs that mimic the embryo environment. It should be possible to utilise engineering technology, for instance microfluidics and photochemistry, to deliver morphogens in a highly controlled manner – both spatiotemporally and in terms of dosage. Such controlled, systematic approaches will provide insight into the positional information that may be necessary to overcome the stochasticity in symmetry breaking in current organoid systems. Finally, bioreactor technology or engineered blood vessel systems may be employed to address the major problem of nutrient availability in growing organoids and thereby allow proper long-term growth of complex systems [10]. Organoids of the future, which model the physiology of ageing neurons, might be able to provide further insight into cellular and molecular mechanisms of pathology and aid in developing drugs and treatments for the prevention and alleviation of disease symptoms [7].
Organoids have revealed what developmental biologists have suspected for years: that cells have amazing self-organising abilities, the regulation of which is only just beginning to emerge. It is now time to harness control of this phenomenon for our own benefit. Rigour, self-criticism and, sometimes, a slow pace will be essential in this process and are prices worth paying when the stakes are as high and as exciting as they are here.
Conclusion
The use of organoids derived from iPSC as a means to understand and subsequently treat certain diseases has been extremely encouraging in the last eight years. Like all new technology, it is undeniable that organoids still have certain obstacles to be overcome, but it is extremely exciting to note that in such a short period of time their immense ability to resolve previously unknown pathologies of treatment has been verified. There are many indications about the functional capacity of these organoids, as can be seen in this systematic review.
Some examples of diseases that can be resolved and better studied with the use of iPSC and organoids are diabetes, autism, schizophrenia, microcephaly, coronary dysfunctions, Parkinson's disease, among many others. That is, the enormous power that technology has to positively influence the lives of people, not only patients but also their families, is undeniable. Like all emerging technologies, the questions about organoids go far beyond the technological aspect; they enter the ethical sphere, which, as everyone knows, involves very delicate aspects that over time need to be discussed.
It is therefore necessary to state that expectations for the coming years are the best possible. Perhaps we are witnessing a revolution that changes the history of medicine. In fact, not just the history of medicine, but the world. The changes that the organoids can generate go far beyond the medical field, the scientific field, they penetrate the immense social field. If the expectations come true, we can witness a great change that certainly did not happen by chance, but it was the result of a lot of work by many professionals.
This is what inspires us: to know that as science evolves and more research is done on this new technology, many engineers and biochemists are mainly investing their energies and resources to enhance the development of organoids. Of course, if these different professionals come together, it is science and the millions of patients who can benefit from the use of this innovative technology. Remarkable to note that an organoid, something so small, can change so many lives, something so great..
38466
References
- Camp JG, Badsha F, Florio M, Kanton S, Gerber T, et al. (2015) Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A 112: 15672-15677.
- Kelava I, Lancaster MA (2016a) Stem cell models of human brain development. Cell Stem Cell 18: 736-748.
- Muotri AR (2010) Células-tronco pluripotentes e doenças neurológicas. estudos avançados 24: 71-79.
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861-872.
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS (2013) Cerebral organoids model human brain development and microcephaly. Nature 501: 373.
- Muotri AR (2009) Modeling epilepsy with pluripotent human cells. Epilepsy Behav 14: 81-85.
- Kelava I, Lancaster MA (2016b) Dishing out mini-brains: current progress and future prospects in brain organoid research. Dev Biol 420: 199-209.
- Luo C, Lancaster MA, Castanon R, Nery JR, Knoblich JA, et al. (2016) Cerebral organoids recapitulate epigenomic signatures of the human fetal brain. Cell Rep 17: 3369-3384.
- Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Yang SM, et al. (2017) Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545: 1-16.
- Huch M, Knoblich JA, Lutolf MP, Martinez-Arias A (2017) The hope and the hype of organoid research. Dev 144: 938-941.
- Watanabe M, Buth JE, Vishlaghi N, de la Torre-Ubieta L, Taxidis J, et al. (2017) Self-organized cerebral organoids with human-specific features predict effective drugs to combat Zika virus infection. Cell Rep 21: 517-532.
- Rambani K, Vukasinovic J, Glezer A, Potter SM (2009) Culturing thick brain slices: an interstitial 3D microperfusion system for enhanced viability. J Neurosci Methods 180: 243-254.
- Bredenoord AL, Clevers H, Knoblich, JA (2017) Human tissues in a dish: the research and ethical implications of organoid technology. Sci 355: eaaf9414.
- Pampaloni F, Chang BJ, Stelzer EH (2015) Light sheet-based fluorescence microscopy (LSFM) for the quantitative imaging of cells and tissues. Cell Tissue Res 360: 129-141.
- Lancaster MA, Knoblich JA (2014) Generation of cerebral organoids from human pluripotent stem cells. Nat protocols 9: 2329.
- Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, et al. (2017). Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95: 779-790.
- Clevers H (2016) Modeling development and disease with organoids. Cell 165:1586-1597.
- Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, et al. (2015) FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162: 375-390.
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, et al. (2016) Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18: 587-590.
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JLM, et al. (2016) The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534: 267.
- Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, et al. (2016) Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19: 258-265.
- Garcez PP, Loiola EC, Costa RM, Higa LM, Trindade P, et al. (2016) Zika virus impairs growth in human neurospheres and brain organoids. Sci 352: 816-818.
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, et al. (2016). Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165: 1238-1254.
- Hanashima C, Li SC, Shen L, Lai E, Fishell G (2004) Foxg1 suppresses early cortical cell fate. Science 303: 56-59
- Jacob FD, Ramaswamy V, Andersen J, Bolduc FV (2009) Atypical Rett syndrome with selective FOXG1 deletion detected by comparative genomic hybridization: case report and review of literature. Eur J Hum Genet 17: 1577.
- Hutsler JJ, Zhang H (2010) Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res 1309: 83-94.
- Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran H, et al. (2016). Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19: 248-257.
- Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, et al. (2014) Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell 15: 653-665.
- Yoon KJ, Nguyen HN, Ursini G, Zhang F, Kim NS, et al. (2014) Modeling a genetic risk for schizophrenia in iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell15: 79-91.
- Rapoport JL, Giedd JN, Gogtay N (2012) Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry 17: 1228.
- Malhotra D, Sebat J (2012) CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell 148: 1223-1241.
- Zhu Y, Wang L, Yin F, Yu Y, Wang Y, et al. (2017) Probing impaired neurogenesis in human brain organoids exposed to alcohol. Integr Biol (Camb) 9: 968-978.








