Zunaira Alina1, Sumaira Noreen1, Ramsha Abbas1, Shahneel Kousar1, Laiba Riaz1, Hira Naeem1,Abida Niazi3, Hira Ijaz1, Syeda Fiza Raza Bukhari2, Sadia Ashraf1, Muhammad Shoaib4,5 and Faiza Naseer2,5
1Institute of Pharmacy, Physiology and Pharmacology, University of Agriculture, Faisalabad, Pakistan
2Department of Pharmacy, Government College University, Faisalabad, Pakistan
3University of Veterinary and Animal Sciences Lahore, Pakistan
4Department of Biochemistry, University of Arid Agriculture Rawalpindi, Pakistan
5College of Medical Technology, Shifa Tamer e Millat University Islamabad, Pakistan
*Corresponding Author:
Faiza Naseer
College of Medical Technology
Shifa Tamer e Millat University Islamabad, Pakistan
Tel: +92 51 8464215
E-mail: faiza.naseer@ymail.com
Received date: October 22, 2016; Accepted date: November 25, 2016; Published date: December 02, 2016
Citation: Alina Z, Noreen S, Abbas R, et al. Therapeutic Effects of Albendazole on Kidney Functions and Urinary Excretion of Florfenicol in Goats. J Biomedical Sci. 2016, 6:1. doi:10.21767/2254-609X.100050
Copyright: © 2016 Alina Z. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Polypharmacy; Cytochrome; Post-medication; Centrifugation; Glomerular filtration rate; Renal clearance; Urinary excretion
Introduction
Concurrent use of more than one drugs or herbs along with a drug may cause drug interactions. The interacting drugs or herbs may mimic, potentiate or counter the effect of other pharmacological agent. Drug-drug interactions are common in patients being exposed to polypharmacy or using multiple drugs. The possibility that drug-drug interactions will occur, increases with age, polypharmacy and number of physicians visited by the patient. As the number of drugs prescribed increased, possibility of occurrence of drug interactions increased. Critically ill patients receive complex therapies which include the use of various pharmacological agents of different classes, risk for drug interactions increased in such cases. Pharmacological properties and pharmacokinetic data are also contributing agents for the occurrence of drug interaction. Consequences of drug interactions are ranging from treatment failure to serious health hazards [1].
Drug-drug interactions are classified as unidirectional and bidirectional. When pharmacological effect of one drug is magnified by another drug the interaction is called unidirectional. When one drug opposed or diminished the action of other drug the interaction is called bidirectional. Drug interactions can also be classified as Pharmacodynamics interactions and Pharmacokinetic interactions [2]. In pharmacodynamics interactions one drug altered the action of another drug without any change in plasma concentration of that drug. Pharmacodynamics interactions involve the modification of specific effect of one drug on target organ by another drug without altering absorption, distribution, metabolism and excretion of that drug. The interacting drug may interfere with receptor activity, signal transduction mechanism or may produce antagonic physiological response. Pharmacodynamics interactions have four possible outcomes that include synergistic effect, additive effect, potentiation effect and antagonistic effect [3].
In pharmacokinetic interactions effect of one drug is altered by another drug by modifying its absorption, distribution, metabolism or excretion. These interactions involved alterations in absorption from target site, plasma protein binding, carrier transport, liver enzymes and kidney function. The mechanism most commonly involved in pharmacokinetic drug-drug interactions is induction or inhibition of drug metabolizing enzymes [4]. Most of the drugs are metabolized by hepatic cytochrome P450 enzymatic family. When an inducer or inhibitor of CYP450- is administered concomitantly with another drug that is metabolized by this enzyme the possibility of drug interaction exist. If the interacting drug is an enzyme inducer it enhances the metabolism of other drug and thus reduces pharmacological effect of that drug and leads to pharmacotherapy failure, On the other hand if interacting drug is enzyme inhibitor, cause toxicity of other drug [5].
Florfenicol is a C-3 fluorinated synthetic analogue of thiamphenicol which is structurally related to chloramphenicol. The drug has known bacteriostatic activity against micro-organisms that are chloramphenicol and thiamphenicol resistant. Florfenicol is resistant to inactivation by bacterial acetyl-transferase because it has lesser exposed sites for bacterial acetylation as compared to chloramphenicol and thiamphenicol. The drug has broad spectrum of activity and is used worldwide for the treatment of infections caused by bacteria specially infections of respiratory tract, it is also used to treat in infections caused by Pasteurella species, Actinobacillus species, Mycoplasma mycoides, Staphylococcus aureus, Salmonella species and E.coli in food animals [6]. Florfenicol produce its bacteriostatic action by inhibiting protein synthesis in bacteria. The drug is used extensively in animals due to good absorption, high volume of distribution, high bioavailability and less side effects. Unlike other fenicols florfenicol does not cause reversible and dose dependent suppression of bone marrow due to inhibition of synthesis of mitochondrial protein. It also lacks the ability to cause aplastic anemia, which is observed with chloramphenicol that is why safer to treat infections in food producing animals [7].
Materials and Methods
The present study was conducted to investigate the effect of albendazole on the kidney functions and urinary excretion of commercially available preparation of florfenicol in ten healthy goats after single intramuscular administration.
Experimental design
The study was conducted in ten clinically healthy adult non pregnant non-lactating goats of beetle breed, between 12- 36 months of age and 29.5-33 kg body weight. The goats were housed in animal shed with concrete floor, Department of Clinical Medicine and Surgery, University of Agriculture Faisalabad. All animals were maintained on fresh green fodder and water ad lib. Experiments were performed during the month of January 2015. The body weight and age of each animal was recorded. The study protocol was approved by the Ethical Review Committee, University of Agriculture Faisalabad, Pakistan.
The study was conducted in two phases. In the first phase of study, after restraining the animals from food, a single dose 20 mg Kg-1 body weight of florfenicol (Naflor® 30%, Nawan Laboratories Pvt Ltd., Karachi, Pakistan) was administered intramuscularly, blood and urine sample were collected at different time intervals to study the kidney functions and urinary excretion of drug. After providing a wash out period of 10 days, in the second phase of study, the same preparation of florfenicol (Naflor® 30%, Nawan Laboratories (Pvt) Ltd., Karachi, Pakistan) at the dose rate of 20 mg Kg-1 body weight and albendazole suspension (Albamax® 10%, Mylab (Pvt) Ltd, Pakistan) at the dose rate of 5 mg Kg-1 body weight were administered concurrently for the investigation of effect of albendazole on kidney functions and urinary excretion of florfenicol.
Drugs
The following commercial preparations of florfenicol and albendazole were used for the pharmacokinetics evaluation and effects;
• Florfenicol: Naflor® 30%, Nawan Laboratories (Pvt) Ltd., Karachi, Pakistan.
• Albendazole: Albamax® 10%, Mylab (Pvt) Ltd, Pakistan.
Collection of samples
Blood and urine samples under different time course intervals were taken for the determination of kidney functions and urinary excretion of florfenicol in goats with and without albendazole.
Collection of blood samples
Blood samples were collected from the jugular vein under aseptic conditions by using swabs of methylated spirit. The blood was collected by direct pricking of vein with needle and then poured into serum tubes. Every time new syringe is used for the collection of blood samples. Prior to the drug administration a control/blank sample was also collected from each goat. After drug administration the blood samples were collected at 0.5, 1, 1.5 and 2 hours intervals. The blood samples were allowed to clot for at least 1 hour at room temperature then samples were centrifuged at 4000 rpm for 10 minutes at room temperature. Serum was separated in eppendorf and was kept at -20º? until analysis.
Collection of urine samples
A sterile disposable balloon catheter was inserted into urinary bladder through urethra of each animal after lubrication with paraffin gel. The external opening of catheter was connected through rubber tubing to a urine-collecting reservoir in which all the voided urine was collected. A control urine sample was collected before the drug administration. Urinary bladder of each animal was washed with 20 ml of distilled water after 45 minutes of drug administration. Other samples were collected at 0.75, 1.25, 1.75, 2.25, 2.75, 4, 5, 6, 8, 10 and 12 hours intervals. After collection of samples pH and volume of urine was measured for each sample. Samples were stored at -20°C until analysis.
Florfenicol analysis
Concentration of florfenicol in plasma and urine was determined by reverse phase high performance liquid chromatography with UV detector at wavelength 224 nm [8,9].
Chemicals and solvents
Following chemicals/reagents of HPLC grade were purchased from reagent grade suppliers.
Florfenicol standard was 99 % pure, Acetonitrile, Deionized water, Methanol, 0.1M pH 7.0 Phosphate buffer, Ethylene Acetate and Ethyl Acetate.
Instrumentation and Chromatographic Conditions
Instrumentation
Apparatus used include: Analytical balance (Sartorius, Germany), Centrifugation machine (Z 233 M-2), (Hermle Germany), Centrifugation machine (80-2, China), Filtration assembly (42 Millipore), Micropipettes 10 μL and 1000 μL (Oxford, Ireland), Sonication apparatus (Oqawa Seiki Co, Japan), ANG Nitrogen air generator (2381HC, Clind, Italy), Liquid Chromatographic pump Sykam S1122, System controller unit Sykam, Column oven Lab Alliance, UV- Visible detector Sykam S3210, Column C-18 thermohypersil, 75 mm×4.6 nm, 3.5 μm and sample Injector Sykam S5111.
Chromatographic conditions
• Mobile Phase Acetonitrile: Deionized water (18:82)
• Flow Rate 1.5 mLmin-1
• Wavelength 224 nm
• Pressure 20 kg/cm2
• Injection Volume 20 μL
• Column C-18 thermohypersil (75 mm×4.6 nm, 3.5 μm)
• Temperature 20°C
• Detector UV-Visible Detector
Stock solutions and standards
Calibration standards for blood samples were prepared by dissolving 200 mg of drug in 2 ml of HPLC-grade methanol (100 mg/ml), it was further diluted to 1.0, 0.1 and 0.01 mg/ml in methanol. The appropriate volumes of each dilution were added in 1.0 ml of blank serum to prepare serum florfenicol standards that covered the range of dilution from 0.025-10.0 μg mL-1.
Serum sample preparation
Serum samples were separately extracted in ethylene acetate (1 ml: 2.5 ml). The tubes were rotated for 10 min and then centrifuged at 2000 RPM for 10 min as well. 2 ml of the organic layer was aspirated and evaporated under nitrogen. Each of the residues was dissolved in 0.375 ml of the solvent mixture of acetonitrile: water (1:3, v/v), vortexed, and then centrifuged again at 2000 RPM for 20 min at 4°C. The supernatant was separated, ?ltered through a 0.45-μm nylon ? lter. The drug containing solvent was dried under a stream of dry nitrogen at 40ºC and each sample was reconstituted in 1 ml of mobile phase. These prepared samples were vortex mixed for 1 minute, sonicated for 2 minutes, centrifuged at 1000 RPM for 10 minutes. Aliquots of each sample (20 μL) were injected in injection port of HPLC.
Urine sample preparation
Urine samples were prepared by extracting the drug in ethyl acetate. First of all urine samples were diluted in double distilled water in ratio urine: double distilled water 1:10. Then 1 ml of these diluted urine samples along with 1 ml of 0.1 M pH 7.0 phosphate buffer and 4 ml of ethyl acetate were added to screw capped tubes. The tubes were vortex mixed for 10 minutes and then centrifuged at 2000 RPM for 10 minutes. Three ml of organic layer was aspirated and evaporated under nitrogen. The extracted sample was mixed with 100 μl of mobile phase. After that these prepared samples were vortex mixed for one minute, sonicated for two minutes and a 20-μl injection was made onto the chromatograph.
Standard curve
Working standards having florfenicol concentrations 10, 3.5, 3.0, 2.5, 1.25, 1.0, 0.1 and 0.025 μg/ml in serum and water were prepared. These working standards were analyzed by using HPLC. Concentration versus peak area data was plotted on a graph to construct the calibration curve. The representative calibration curve is shown in Figure 1.
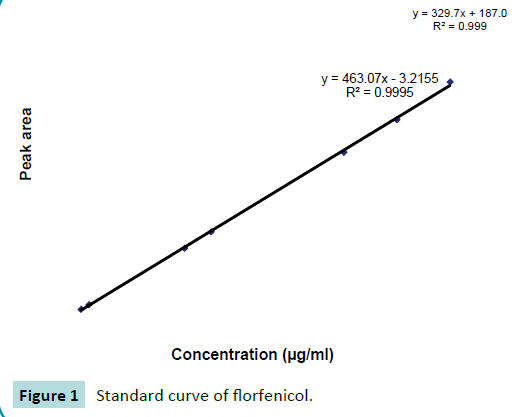
Figure 1: Standard curve of florfenicol.
Determination of florfenicol in sample
The concentrations of florfenicol in samples were calculated by comparison with peak area obtained from the standard solutions. These concentrations of drug were determined by using the following regression equation:
Y=a + bx
Y=Peak area for unknown concentration of florfenicol
a=Intercept, b=Slope of regression line and x=known concentration of florfenicol.
Results and Discussion
Urine concentration
The comparison was made between Mean ± SEM values of urine concentrations (μg/mL) of florfenicol 20 mg Kg-1 following its intramuscular administration alone and with orally administered albendazole 5 mg Kg-1 to ten healthy adult goats and values were exhibited in Table 1. The comparison of Mean ± SEM urine concentrations were also graphically presented in Figure 2. The maximum urine concentration of florfenicol were 121.2 ± 10.58 μg mL-1 obtained at 1.75 hours after single intramuscular administration increased up to 129.4 ± 10.26 μg mL-1 at 1.75 hours when administered with albendazole. This reflected increase in the urine concentration of florfenicol due to albendazole co-administration. Moreover, this increase in florfenicol urine concentration was persistent up to 10 hours of post medication.
| Time in minutes |
Florfenicol alone
(µg mL-1) |
Florfenicol with Albendazole
(µgmL-1) |
| 45 |
115.3 ± 5.72 |
122.4 ± 5.07ns |
| 75 |
178.1 ± 9.96 |
185.2 ± 10.14ns |
| 105 |
194 ± 5.15 |
201.2 ± 5.34* |
| 135 |
121.2 ± 10.58 |
129.4 ± 10.26* |
| 165 |
105 ± 4.61 |
112.4 ± 4.6* |
| 240 |
130.9 ± 8.43 |
138.4 ± 8.63* |
| 300 |
98.3 ± 3.21 |
105 ± 3.16* |
| 360 |
100.3 ± 5.196 |
107.3 ± 5.32* |
| 480 |
132.3 ± 3.55 |
139 ± 3.68ns |
| 600 |
85.9 ± 6.72 |
92.8 ± 7.17* |
| 720 |
84 ± 6.58 |
84.1 ± 6.62ns |
Data are mean values (± SEM)
*= Significant p<0.05 difference from respective value
Table 1 Mean ± SEM urine concentration (μg mL-1) of florfenicol 20 mg Kg-1IM dose alone and along with albendazole 5 mg Kg-1 in ten healthy adult goats.
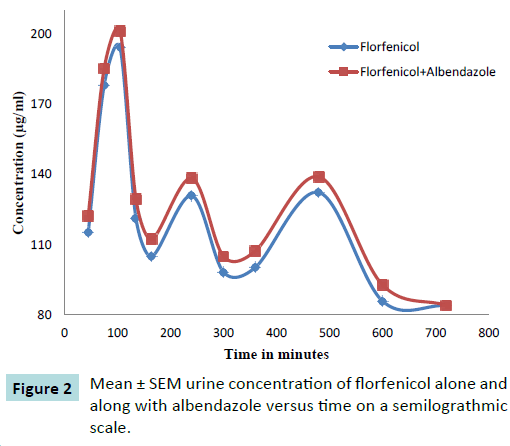
Figure 2: Mean ± SEM urine concentration of florfenicol alone and along with albendazole versus time on a semilograthmic scale.
Urinary excretion
The renal excretion of florfenicol was measured in ten healthy adult goats (Figure 3) following administration of 20 mg Kg-1 IM dose of florfenicol (Figure 4) alone as well as after administration of 20 mg Kg-1 IM dose of florfenicol along with orally administered albendazole 5 mg per kg. The urine samples were collected till 12 hours post drug administration. The florfenicol concentration in urine was measured by HPLC method. The amount of dose excreted in urine at different time intervals is given in the Table 2.
| Goat No. |
Florfenicol alone |
Florfenicol with albendazole |
| 1 |
18.91±3.88 |
19.23 ± 3.93 |
| 2 |
22.67 ± 3.40 |
23.78 ± 3.42 |
| 3 |
15.87 ± 1.76 |
17.1 ± 1.67 |
| 4 |
16.98 ± 1.81 |
17.65 ± 1.88 |
| 5 |
29.84 ± 4.45 |
31.26 ± 4.5 |
| 6 |
19.23 ± 3.80 |
20.41 ± 3.7 |
| 7 |
21.56 ± 2.99 |
22.68 ± 2.9 |
| 8 |
16.29 ± 1.43 |
16.81 ± 1.25 |
| 9 |
17.25 ± 1.74 |
18.08 ± 1.74 |
| 10 |
16.76 ± 1.59 |
17.43 ± 1.72 |
Table 2 Milligram dose of Florfenicol excreted in urine following administration of florfenicol alone and along with albendazole in ten healthy adult goats.
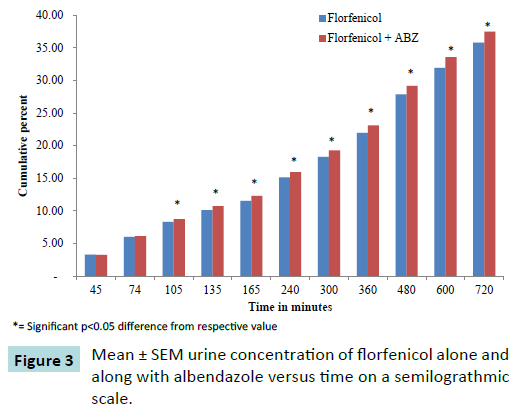
Figure 3: Mean ± SEM urine concentration of florfenicol alone and along with albendazole versus time on a semilograthmic scale.
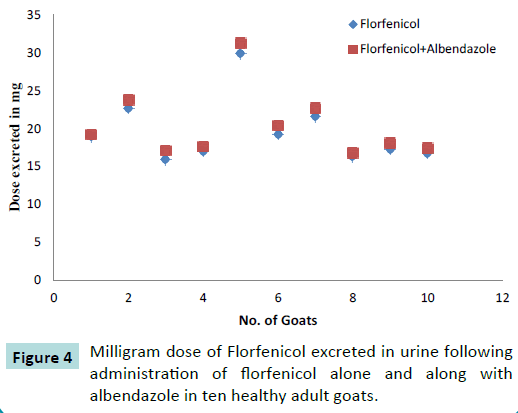
Figure 4: Milligram dose of Florfenicol excreted in urine following administration of florfenicol alone and along with albendazole in ten healthy adult goats.
Conclusion
There is significant drug-drug interaction between florfenicol and albendazole. Albendazole increased the renal clearance and urinary excretion of florfenicol up to 10%. Therefore, this increase in urinary excretion of florfenicol suggests that there is a decrease in its serum concentration. Present study suggests that if it is necessary to administer albendazole and florfenicol concomitantly, dose adjustment of florfenicol is required.
The change in kidney functions and urinary excretion is due to rapid or induced metabolic elimination of florfenicol when given concurrently with albendazole to healthy adult goats. This is probably due to induction of CYP1A1 or CYP1A2 isoenzyme by albendazole, florfenicol is the substrate of this enzymatic family. The results of this study are in agreement with previous finding in which it has been suggested that albendazole is the potent inducer of CYP1A1 isoenzyme which are responsible for metabolism of florfenicol (Tables 3 and 4). The potential of albendazole significantly enhance the rate of elimination and total body clearance of florfenicol and decreased the serum concentration of florfenicol in the body of goats when administered concurrently. So, dose adjustment as well as drug monitoring of florfenicol may be required when both the drugs are given concurrently (Table 5).
| Time in minutes |
Florfenicol alone
(µg mL-1) |
Florfenicol with Albendazole
(µgmL-1) |
| 45 |
3.27 ± 0.55 |
3.26 ± 0.54ns |
| 75 |
2.73 ± 0.24 |
2.85 ± 0.25ns |
| 105 |
2.33 ± 0.29 |
2.64 ± 0.26* |
| 135 |
1.79 ± 0.14 |
1.99 ± 0.15* |
| 165 |
1.44 ± 0.10 |
1.58 ± 0.9* |
| 240 |
3.58 ± 0.33 |
3.62 ± 0.39ns |
| 300 |
3.17 ± 0.37 |
3.33 ± 0.34* |
| 360 |
3.64 ± 0.53 |
3.84 ± 0.53* |
| 480 |
5.91 ± 0.59 |
6.08 ± 0.60ns |
| 600 |
4.07 ± 0.54 |
4.42 ± 0.57* |
| 720 |
3.89 ± 0.29 |
3.89 ± 0.34ns |
Table 3 Percentage dose of Florfenicol excreted in urine following administration of florfenicol alone and along with albendazole in ten healthy adult goats.
| Time in minutes |
Florfenicol alone
(µg mL-1) |
Florfenicol with Albendazole
(µgmL-1) |
| 45 |
3.27 ± 0.55 |
3.26 ± 0.54ns |
| 75 |
6.0 ± 0.71 |
6.11 ± 0.72ns |
| 105 |
8.32 ± 0.9 |
8.75 ± 0.86* |
| 135 |
10.11 ± 0.97 |
10.74 ± 0.94* |
| 165 |
11.55 ± 1.01 |
12.32 ± 0.98* |
| 240 |
15.13 ± 1.30 |
15.94 ± 1.27* |
| 300 |
18.29 ± 1.35 |
19.26 ± 1.35* |
| 360 |
21.94 ± 1.45 |
23.10 ± 1.48* |
| 480 |
27.85 ± 1.88 |
29.18 ± 1.93* |
| 600 |
31.92 ± 2.32 |
33.59 ± 2.40* |
| 720 |
35.81 ± 2.47 |
37.48 ± 2.60* |
Data are mean values (± SEM)
*= Significant p<0.05 difference from respective value
Table 4 Cumulative percentage of dose of Florfenicol excreted in urine following administration of florfenicol alone and along with albendazole in ten healthy adult goats.
| Parameters |
Florfenicol 600mg |
Florfenicol with albendazole |
| Diuresis (ml/min/kg) |
0.087±0.00 |
0.085±0.00 ns |
| pH |
8.4±0.04 |
8.38±0.03ns |
| Concentration (µg/ml) |
126±1.9 |
129.3±2.6 * |
| Creatinine Clearance |
0.82±0.063 |
0.82±0.06 ns |
| Drug Clearance |
3.32±0.60 |
3.9±0.52 * |
| Clearance Ratio |
4.04±0.38 |
4.8±0.33* |
| Dose excreted in mg |
19.53±1.2 |
20.44±0.97* |
| % dose excreted |
3.26±0.29 |
3.41±0.3* |
| Cumulative dose excreted |
35.8±2.4 |
37.5±2.6* |
Data are mean values (± SEM)* = Significant p<0.05 difference from respective value
Table 5 Mean ± SE comparison of parameters following administration of florfenicol alone and along with albendazole in ten healthy adult goats.
Mechanism of Interaction
Cure and prevention of various diseases is greatly affected by the interaction between prescribed drugs. The pharmacokinetic behavior of florfenicol when administered along with MAD (maduramycin), MON (monensin) and SAL (salinomycin) like ionophore antibiotics in broiler chicken was investigated. Florfenicol was administered at the dose of 30 mg per kg of body weight to the broiler chickens either orally or intravenously. Samples of blood were collected till 24 hours after administration of drug. The concentration of florfenicol in plasma was analyzed by HPLC method. When florfenicol is administered with SAL, MAD and MON via oral or IV route, the concentration of florfenicol in plasma decreased significantly. This decrease in plasma concentration can be explained as drug-drug interaction (Figure 5). It is suggested that whenever florfenicol is prescribed to broiler chicken to treat bacterial infection, its interaction with ionophore antibiotics should kept in mind [10].
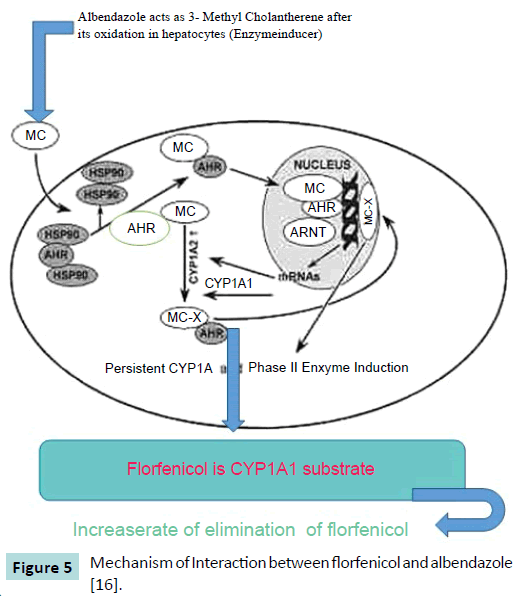
Figure 5: Mechanism of Interaction between florfenicol and albendazole [16].
AHR (Aryl Hydrocarbon receptor) is a transcription factor which is activated when a ligand aryl hydrocarbon e.g. benzimidazole attached to it. Aryl hydrocarbon receptors present in cytoplasm in an inactivated form attached with heat shock proteins 90 [11,12]. The mechanism of interaction between albendazole and florfenicol is best elaborated by the findings of Asteinza et al. [13] who elaborated the enzyme induction property of albendazole in rats and Gleizes et al. [14], they concluded that oxfendazole and albendazole act as 3-methylcholanthrene (3 MC) type inducer. By keeping these findings in mind, Goodman and Gilman [15] suggested that the hepatic enzyme induction cause decreased availability and increased renal clearance of the drug (florfenicol) by increasing the rate of metabolism by hepatocytes. The lower serum level and higher excretion of florfenicol in goats pretreated with anthelmintic drug like albendazole than the behavior of other drugs can be well described by its induction effect on microsomal enzymes of liver. CYP1A1 in liver converted 3-methylcolanthrene to 3-methylcholanthrene-X which is a reactive metabolite. These metabolic products reached the nucleus of the cell and make a complex with ArhNT (aryl hydrocarbon receptor nuclear trans locator), then this complex covalently binds to CYP1A1 and 2 promoters and enhance the transcription of genes responsible for production of CYP1A1 and 2 [16].
Funding Source
The analyses were performed by using an HPLC chromatograph in Department of Pharmacy, Physiology and Pharmacology, University of Agriculture, Faisalabad, Pakistan. Department has provided instruments and chemicals. Sample collection was author’s research.
Conflict of Interest
The authors declare that there is no conflict of interest.
17650
References
- Silvana MDA, Cinthia GS, Nelson A(2007) Prevalence and classification of drug drug interactions in intensive care patients. Einstein 5: 347-351.
- Williams D, Feely J (2002) Pharmacokinetic pharmacodynamic drug interactions with HMG CoA reductase inhibitors. ClinPharmacokinet 41: 343-370.
- Thomas M, Boggs AA, Di-Paula B,Shadhida S(2010) Adverse drug reactions in hospitalized psychiatric patients. Ann Pharmacother 44:819-825.
- Seymour RM, Routledge PA(1998)Important drug-drug interactions in the elderly. Drugs Aging 12: 85-494.
- Lin JH, Anthony YH(1998) Inhibition and Induction of Cytochrome P450 and the clinical implications. ClinPharmacokinet 35: 361-390.
- Tohamy MA, Rady AM(2008) Bioavailability and pharmacokinetics of florfenicolin healthy foals. J Egypt SocPharmacolExpTher 29: 529-537.
- Yunis AA (1988)Chloramphenicol: relation of structure to activity and toxicity.Annu Rev PharmacolToxicol 28:83-100.
- Alcorn JP, Woodbury DM, Killeen R(2004) Pharmacokinetics of florfenicolin north American elk (Cervuselaphus). J Vet PharmacolTher 27: 289-292.
- Rebecca LP, Niehaus AJ, Werle NA, Lakritz J(2013) Pharmacokinetics of florfenicol after intravenous and intramuscular dosing in llamas. Res Vet Sci95: 594- 595.
- Wang L, He X, Szklarz GD, Bi Y,Rojanasakul Y, et al. (2013) The aryl hydrocarbon receptors interact with nuclear factor erythroid 2 related factor 2 to mediate induction of NADPH:quinoneoxidoreductase 1 by 2, 3, 7, 8, tetrachlorodibenzo-p-dioxin. Arch. BiochemBiophys 537: 31-8.
- Denis M,Cuthill S,Wikström AC,Poellinger L,Gustafsson JA(1988) Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor.BiochemBiophys ResCommun155: 801-807.
- Perdew GH(1988) Association of the Ah receptor with the 90-kDa heat shock protein. J BiolChem263: 13802-13805.
- Asteinza J, Carranza C, Reyes R,Gomzales D, Aguirre E (2000) Induction of cytochrome P450 enzymes by Albendazole treatment in the rat. Environ ToxicolPharmacol9: 31-37.
- Gleizes C,Eeckhoutte C,Pineau T,Alvinerie M,Galtier P(1991) Inducing effect of oxfendazole on cytochrome p-450 IA2 in rabbit liver. Consequences oncytochromeP-450 dependent monooxygenases. BiochemPharmacol 41: 1813-1820.
- Goodman M,Gilmans AG (1995)The pharmacological basis of therapeutics. 9thedition, McGraw – Hill Companies pp: 14-15.
- Kondraganti SR, Jiang W, Jaiswal AK,Moorthy B(2008) Persistent induction of hepatic and pulmonary phase II enzymes by 3-methylcholanthrene in rats. ToxicolSci 102: 337-344.










